ABSTRACT
Background: We evaluated bacterial nasopharyngeal carriage (NPC) prevalence and cumulative acquisition following 7-valent pneumococcal conjugate vaccine (PCV7) or pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) administration.
Methods: Participants were children from two clinical trials in a South African center who received PCV7 (n = 250) or PHiD-CV (n = 100) at ~6 weeks, ~14 weeks, and ~9–10 months of age, and were enrolled between Dec2009-Apr2010 and Mar2009-May2010 in the PCV7 and PHiD-CV studies, respectively. Sample collection, most microbiological assessments, and data re-analysis methods were identical.
Results: NPC prevalence of any pneumococcal serotype was 18.5% and 17.0% at pre-vaccination, and 63.1% and 67.3% in 24–27 month-old children among PCV7 and PHiD-CV recipients, respectively. In 24–27 month-old children, 96.1% and 99.0% of PCV7 and PHiD-CV recipients had acquired ≥1 pneumococcal serotype, 53.7% and 62.9% ≥1 PCV7 serotype, 1.5%, and 3.1% ≥1 of serotypes 1, 5 or 7F, 23.2% and 19.6% serotype 6A, 23.2% and 21.7% serotype 19A, 88.7%, and 91.0% H. influenzae, and 50.3% and 62.9% Staphylococcus aureus, respectively.
Conclusions: This analysis of two concurrent clinical trials did not reveal differences in bacterial NPC prevalence or acquisition in PCV7- and PHiD-CV-vaccinated children.
Trial registration: South African National Clinical Trial Register (NHREC DOH-27-0511-299); ClinicalTrials.gov (NCT00829010).
1. Introduction
Asymptomatic nasopharyngeal carriage (NPC) precedes pneumococcal disease and plays an essential role in transmission of Streptococcus pneumoniae [Citation1]. Therefore, the effect of pneumococcal conjugate vaccines (PCVs) on NPC can be used as a surrogate for evaluating their potential to induce herd effects [Citation2–5]. Furthermore, while carriage studies are not a direct measure of reduction in disease burden, they are important for monitoring impact of PCVs in terms of pneumococcal disease control at the population level [Citation3–5], especially in settings where no significant microbiology laboratory infrastructure and no large surveillance networks are available [Citation6,Citation7].
The 7-valent pneumococcal conjugate vaccine (PCV7) [Citation8–10] and the pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV) [Citation11–18] were shown to reduce NPC of pneumococcal serotypes included in the vaccines. The introduction of PCVs in infant immunization programs also led to a reduction in invasive pneumococcal disease rates in unvaccinated individuals due to their indirect effects on S. pneumoniae acquisition and NPC rates [Citation19]. However, by reducing NPC of vaccine-type pneumococci, PCVs lead to serotype replacement and may create ecological niches for colonization by other respiratory pathogens such as Staphylococcus aureus and H. influenzae [Citation20–26].
The World Health Organization (WHO) currently recommends two alternative schedules for childhood pneumococcal vaccination: 3-dose primary vaccination (3 + 0 schedule) or 2-dose primary vaccination followed by a booster dose (2 + 1 schedule) [Citation27]. In South Africa, PCVs are included in the immunization program according to a 2 + 1 vaccination schedule given at the age of 6 weeks, 14 weeks, and 9 months [Citation28]. Compared to the 3 + 0 schedule, the 2 + 1 schedule may help to ensure longer protection [Citation29,Citation30], particularly against serotype 1 invasive disease which is highly prevalent in African children [Citation31]. The timing of the booster dose administration at 9 months of age has been supported by the WHO recommendation [Citation32] and allows co-administration with the first dose of measles vaccine as implemented in many low- and middle-income settings.
PCV7 was introduced in the South African National Immunization Program (NIP) in April 2009 for children aged >6 weeks without a catch-up campaign. PCV7 was replaced by the 13-valent PCV (PCV13) in May 2011 along with a limited catch-up campaign for children <30 months of age [Citation33]. PHiD-CV was licensed in South Africa in June 2010. Based on the WHO vaccine preventable diseases monitoring system, the estimated coverage of the third dose of pneumococcal vaccine in South Africa increased from 10% in 2009 to 58% in 2010 and remained above 79% from 2012 onwards [Citation34].
Here, we describe the prevalence of pneumococcal colonization and cumulative acquisition of various pneumococcal serotypes, H. influenzae and S. aureus following infant vaccination with PCV7 or PHiD-CV according to the 2 + 1 vaccination schedule in two clinical trials conducted in the same location, population, and settings in South Africa [Citation35,Citation36]. Both trials were performed during the same period (2009–2012): infants in the PCV7 study were enrolled from December 2009 and received PCV7 as part of the NIP, while the PHiD-CV study started enrolling infants in February 2009, just before PCV7 introduction in the NIP.
2. Patients and methods
2.1. Study design and participants
The PCV7 study was a single-arm, prospective study registered in the South African National Clinical Trial Register (NHREC DOH-27-0511-299) [Citation37]. The immunogenicity of PCV7 (Prevenar/Prevnar, Pfizer, USA), the prevalence of bacterial colonization, and the rate of new pneumococcal serotype acquisition following vaccination were evaluated in 250 young children born to human immunodeficiency virus (HIV)-uninfected women [Citation35,Citation37]. In the current analysis, we included all study participants enrolled between December 2009 and April 2010, who received PCV7 according to a 2 + 1 vaccination schedule.
The PHiD-CV study was a phase III, open, controlled, single-center, partially randomized study registered at www.clinicaltrials.gov (NCT00829010). The immunogenicity and safety of PHiD-CV (Synflorix, GSK, Belgium), and the prevalence of NPC following PHiD-CV vaccination in infants were evaluated according to HIV status (3 + 1 series) or dosing schedule (3 + 1, 3 + 0, 2 + 1 series in HIV-unexposed-uninfected children) [Citation36,Citation38,Citation39]. In the current analysis, we included only one of the study groups, consisting of 100 HIV-unexposed-uninfected children who received the vaccine according to the 2 + 1 schedule, and were enrolled between March 2009 and May 2010.
Both trials were approved by an independent ethics committee (Wits Human Research Ethics Committee) and written informed consent was obtained from the parent(s) or legally acceptable representative(s) of each child before any study procedure. Both trials were carried out under the supervision of the same investigator and in the same urban setting of Soweto, Johannesburg, South Africa.
Eligibility criteria were similar for children participating in the PCV7 trial and for the children from the PHiD-CV study who were included in this analysis. In the PCV7 study, healthy infants aged 6–8 weeks, born at term to mothers documented as being HIV-uninfected during the last trimester of pregnancy were eligible for participation [Citation37]. Children with a known immunosuppressive condition or those whose mother received any pneumococcal vaccines were excluded from participation in the PCV7 study. In the PHiD-CV study, 6–10-week-old infants, born to HIV-uninfected mothers were eligible for participation. Exclusion criteria have been presented elsewhere [Citation38].
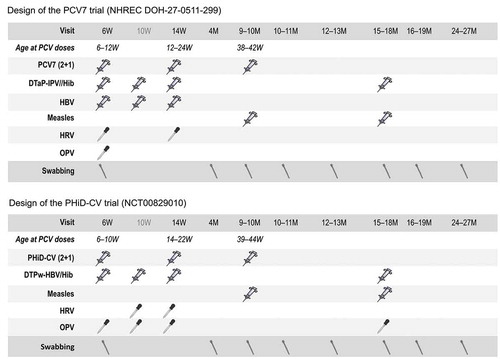
In both trials, children received the two primary doses of PCV at approximately 6 and 14 weeks of age, and a booster dose at 9–10 months of age [Citation37,Citation39]. An overview of the designs of the two trials, including co-administered vaccines, is presented in .
Figure 1. Study designs
2.2. Microbiological assessment
In both trials, nasopharyngeal swabs were collected before and after administration of the primary vaccination series, and before and at five subsequent visits following booster vaccination. Children were 2 years old at the time of the last visit. Dacron-tipped swabs on a flexible aluminum shaft (Cat# 151D, MedicalWire Equipment Co. Ltd.; Wiltshire) were used to collect samples through a nostril and then inoculated into skim milk-tryptone-glucose-glycerol transport medium and stored at −70°C until processing [Citation35,Citation36].
Microbiological assessments of the nasopharyngeal swabs were performed at the same bacteriology laboratory (National Institute for Communicable Diseases, Johannesburg, South Africa) using the same methods in both trials. Conventional bacteriological methods were used for the culture and identification of S. pneumoniae, H. influenzae and S. aureus isolates [Citation35,Citation36]. For serotyping of S. pneumoniae isolates, the Quellung reaction was used in both trials [Citation40]. Pneumococcal serotypes 6C and 6D were differentiated from serotypes 6A and 6B by the Quellung reaction. H. influenzae confirmation was performed by polymerase chain reaction (PCR), and serotyping by slide agglutination and PCR. NTHi confirmation by PCR was performed at the National Institute for Communicable Diseases (NICD; Johannesburg, South Africa) for the PCV7 trial and at DDL Diagnostic Laboratory (DDL; Rijswijk, the Netherlands) for the PHiD-CV trial. No comparison was performed between the PCR assays used to confirm the identification of NTHi isolates at NICD (targeting the genes bexA/hpd) and at DDL (targeting the genes lgtC/P6).
2.3. Statistical analyses
Prevalence was defined as the percentage of children with a given pathogen/serotype identified in the nasopharyngeal sample at a given timepoint. Cumulative acquisition of new bacterial pathogens/serotypes in the nasopharynx was defined as the occurrence of bacterial pathogens/serotypes not detected at any of the previous sampling timepoints for which a test result was available. A new pathogen/serotype led to a positive result once identified in a child, even if at the next timepoints the child did not carry this serotype anymore and/or acquired a new pathogen/serotype.
The primary analysis of NPC prevalence was based on the total vaccinated cohort, including all children with at least one vaccine dose administered and available NPC data. For the analysis of cumulative acquisition, if a visit was missed or no swab sample was available for a visit, the participant was excluded from that visit onwards.
Prevalence of NPC was tabulated at each swabbing timepoint, while cumulative acquisition rates were calculated for swabbing timepoints from the age of 4 months onwards, i.e. after the administration of the second dose of PCV7 or PHiD-CV. Age at the time of visit was described using means with standard deviations (SD). Kaplan-Meier estimates of the percentage of children who were never found colonized with a pathogen/serotype among PCV7 and PHiD-CV recipients were calculated from the age of 4 months onwards and were restricted to children with samples available and cultured at the respective visit and previous visit(s). Assessments of NPC of S. pneumoniae, H. influenzae, and S. aureus identified in the nasopharynx were descriptive. Potential similarities (or differences) between groups were assessed based on the overlap of the 95% confidence intervals (CIs).
The statistical analyses, using raw data from both trials, were performed using the STATA version 13.1.
3. Results
3.1. Study groups
Demographic characteristics of PCV7- and PHiD-CV-vaccinated children were comparable in terms of age at first vaccination (), sex, and ethnicity. Among PCV7 and PHiD-CV recipients, 43% and 47% of participants were female, respectively. All children in both studies were of Black-African heritage [Citation37,Citation39].
Table 1. Age at each study visit (total vaccinated cohort)
The age of children at the time of swab collection was similar in the PCV7 and PHiD-CV trials, except for two timepoints: the 10–11 and 15–18 months of age visits (). This is due to slight differences in design between the two trials, e.g. for the 10–11 months of age visit, swabbing occurred 1–3 weeks following the third PCV dose in the PCV7 trial while swabbing was performed 4–6 weeks after the third PCV dose in the PHiD-CV trial [Citation35,Citation36].
3.2. Prevalence of S. pneumoniae
The NPC prevalence of any pneumococcal serotype was similar in PCV7 and PHiD-CV recipients across all timepoints. Colonization rates with any pneumococcal serotype were 18.5% and 17.0% at 6 weeks, 76.8% and 71.4% at 10–11 months, and 63.1% and 67.3% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively ().
Figure 2. Colonization rates with Streptococcus pneumoniae at each visit in children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule (total vaccinated cohort)
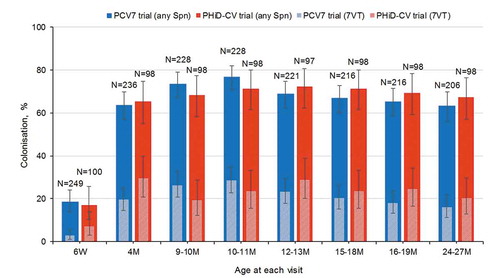
The NPC prevalence of any of the seven pneumococcal serotypes included in PCV7 (7VT) was 2.8% and 7.0% at 6 weeks, 28.5% and 23.5% at 10–11 months, and 16.0% and 20.4% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (). NPC prevalence of any of the 10 pneumococcal serotypes included in PHiD-CV was similar to that observed for 7VT (Figure S1).
3.3. Acquisition of S. pneumoniae
Cumulative acquisition rates of at least one pneumococcal serotype were 54.7% and 57.1% at 4 months, 84.1% and 83.7% at 10–11 months, and 96.1% and 99.0% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (). At least one of the 7VT was acquired by 17.4% of PCV7 and 24.5% PHiD-CV recipients by the age of 4 months. This proportion was 36.1% and 40.8% at 10–11 months, and 53.7% and 62.9% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively. Cumulative acquisition rates of at least one non-7VT serotype in PCV7 and PHiD-CV recipients, respectively, were 38.1% and 33.7% at 4 months, 68.3% and 67.3% at 10–11 months, and 90.1% and 88.7% at 24–27 months of age (). The cumulative acquisition rates of pneumococcal serotypes 1, 5, and 7F (combined) reached 1.5% and 3.1% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively ().
Table 2. Cumulative acquisition of Streptococcus pneumoniae serotypes (any, 7VT, non-7VT serotypes, [1, 5, 7F], 6B, 9V, 14, 19F, 23F, vaccine-related 6A and 19A serotypes) in children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule (total vaccinated cohort)
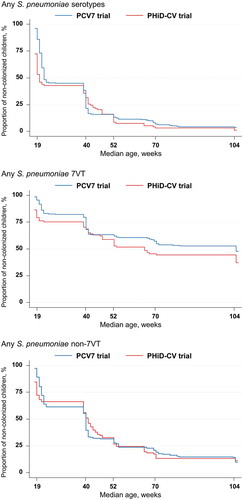
The percentages of children that were never found colonized by any pneumococcal serotype, any 7VT, or any non-7VT serotype were in the same ranges in both trials ().
Figure 3. Kaplan-Meier estimates of the percentages of children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule and were never found colonized with Streptococcus pneumoniae (total vaccinated cohort)
Since serotypes 4 and 18C were each acquired by less than 5 PCV7 or PHiD-CV recipients by the age of 24–27 months, the acquisition of these serotypes was included in the combined analyses of 7VT, but their acquisition rates are not presented individually. The cumulative acquisition rates of the five remaining individual pneumococcal serotypes included in PCV7 were in similar ranges in PCV7 and PHiD-CV recipients across visits ().
Cumulative acquisition rates of the vaccine-related serotype 6A were 6.4% and 0.0% at 4 months, 13.7% and 5.1% at 10–11 months, and 23.2% and 19.6% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively. Vaccine-related serotype 19A was acquired by 3.0% of PCV7 and 3.1% PHiD-CV recipients by 4 months of age. This proportion was 10.1% and 6.1% at 10–11 months and 23.2% and 21.7% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (). Finally, across visits, serotype 6C was only detected in one PCV7 and one PHiD-CV recipient, while serotype 6D only in one PHiD-CV recipient.
3.4. Acquisition of H. influenzae and S. aureus
Cumulative acquisition rates of H. influenzae were 39.0% and 31.3% at 4 months, 71.4% and 59.4% at 10–11 months, and 88.7% and 91.0% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (Table S1). In each study, acquisition rates of H. influenzae and NTHi were similar, suggesting that most H. influenzae isolates were NTHi (Table S1).
S. aureus was acquired by 8.9% of PCV7 and 11.2% of PHiD-CV recipients by 4 months of age. This proportion was 20.3% and 32.7% at 10–11 months and 50.3% and 62.9% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (Table S1).
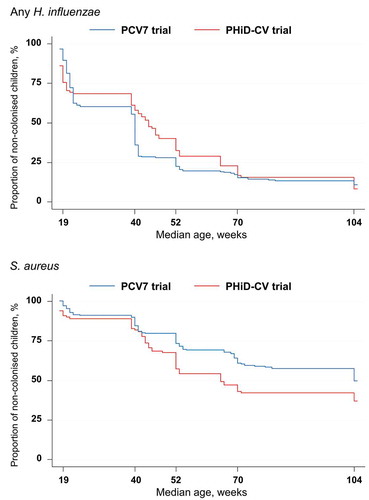
The percentages of PCV7- and PHiD-CV-vaccinated children that were never found colonized by H. influenzae or S. aureus are presented in .
Figure 4. Kaplan-Meier estimates of the percentages children who received PCV7 or PHiD-CV according to a 2 + 1 vaccination schedule and were never found colonized with Haemophilus influenzae or Staphylococcus aureus (total vaccinated cohort)
3.5. Co-acquisition rates
Cumulative co-acquisition rates of at least one pneumococcal serotype with H. influenzae were 25.0% and 24.0% at 4 months, 62.6% and 54.2% at 10–11 months, and 85.7% and 89.7% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (Table S2).
At least one of the 7VT was cumulatively acquired with H. influenzae by 9.8% of PCV7 and 9.4% of PHiD-CV recipients by 4 months of age. This proportion was 26.0% and 31.3% at 10–11 months and 48.3% and 60.3% at 24–27 months of age in PCV7 and PHiD-CV recipients, respectively (Table S2).
4. Discussion
This analysis of bacterial NPC in children enrolled in two clinical trials conducted concurrently in South Africa in the same clinical setting did not reveal differences based on overlapping 95% CIs in prevalence of NPC or acquisition of pneumococcal serotypes between children vaccinated according to a 2 + 1 dosing schedule with PCV7 [Citation37] or PHiD-CV [Citation39]. Acquisition rates of H. influenzae and S. aureus tended also to be similar between PCV7- and PHiD-CV-vaccinated children, even if small differences were observed.
In this analysis, the prevalence of NPC of any pneumococcal serotype was similar and relatively high in both PCV7- and PHiD-CV-vaccinated children, reaching approximately 65% by 4 months of age. This is in line with carriage rates observed for children under 5 years of age in other studies conducted in African countries [Citation17,Citation41–44], but contrasts with observations made in developed country settings where colonization rates are usually lower and do not exceed 40–50%, especially in countries where PCVs are implemented in the NIP [Citation45]. In our analysis, the prevalence of 7VT and non-7VT NPC was also comparable between PCV7- and PHiD-CV-vaccinated children across all timepoints.
Another analysis of the data from the PHiD-CV trial showed that PHiD-CV had a similar impact on the prevalence of NPC of S. pneumoniae, NTHi, and S. aureus when administered according to a 3 + 1 or a 2 + 1 vaccination schedule in HIV-unexposed-uninfected children, even if immune responses after primary vaccination tended to be higher when the 3 + 1 schedule was used [Citation36,Citation39]. NPC prevalence of any pneumococcal serotype, 7VT, non-7VT, and of S. aureus were also found to be similar when comparing PCV7 given using the 2 + 1 vaccination schedule with an historical cohort of children vaccinated with PCV7 according to a 3 + 1 vaccination schedule [Citation35]. Both studies showed the importance of the booster dose when the 2 + 1 vaccination schedule is used [Citation35,Citation36].
In our analysis, the cumulative acquisition rates of at least one pneumococcal serotype exceeded 90% in the second year of life and were similar across all timepoints in PCV7- and PHiD-CV-vaccinated children. This contrasts with findings from the FinIP trial in Finland that enrolled children from about 18 months before and until PHiD-CV implementation in the NIP [Citation14]. In the FinIP trial, lower cumulative acquisition rates of at least one pneumococcal serotype were detected, with maximum rates of 50.8% in PHiD-CV-vaccinated (2 + 1 schedule) and 58.4% in unvaccinated children from 6 months of age through the end of the second year of life [Citation14], taking into account lower swabbing frequency (four swabs). In our study, by the age of 2 years, more than half of the children had acquired at least one 7VT, and more than 88% had acquired at least one non-7VT. The highly invasive serotypes 1, 5, and 7F were detected in very few (<3.1%) of the swabs collected from either PCV7- or PHiD-CV-vaccinated children, which was an expected finding because these serotypes are not frequently found as colonizers of the nasopharynx [Citation43,Citation46–48]. No differences based on overlapping 95% CIs between PCV7 and PHiD-CV recipients were detected in terms of cumulative acquisition rates of any of the serotypes included in PHiD-CV, or serotypes 6A and 19A. Cumulative acquisition rates of H. influenzae, as well as cumulative co-colonization rates of any pneumococcal serotype with H. influenzae were high. In line with this finding, a previous study conducted in the same settings showed a synergistic association between S. pneumoniae and H. influenzae colonization in vaccine-naïve children [Citation49]. Cumulative acquisition rates of NTHi tended to be lower in PHiD-CV- than in PCV7-vaccinated children at most timepoints (except at 24–27 months of age), although 95% CIs overlapped. Similarly, previous efficacy studies have shown that PHiD-CV had no or only a transient impact on NTHi NPC [Citation12–16].
Overall, we did not detect differences based on overlapping 95% CIs in terms of acquisition rates of S. pneumoniae, H. influenzae, and S. aureus between children vaccinated with PCV7 or PHiD-CV. These results are consistent with those of a previous randomized controlled trial initiated approximately 2 years after the introduction of PCV7 in the Dutch NIP that found similar colonization rates of S. pneumoniae, H. influenzae and S. aureus in healthy Dutch children vaccinated with PCV7 or PHiD-CV [Citation12].
Our results should be interpreted with caution in the light of the limitations of analyses describing two groups of children who were not randomized in the same clinical trial. Nonetheless, in both trials, NPC was assessed in the same population and center by the same laboratory using identical microbiological assessment methods. Moreover, investigator teams were the same in both trials, and data were re-analyzed using the same statistical methods. The limitations of our analysis included the differences in enrollment season between the two groups (summer and fall for the PCV7 trial, and fall and winter for the PHiD-CV trial), which might impact carriage, as the prevalence of respiratory pathogen carriage is greater in the cold season [Citation50–52]. In addition, samples were collected every few months, which was frequent enough to describe NPC prevalence rates but not sufficient to accurately describe acquisition. In other settings and studies, swabbing for the assessment of nasopharyngeal carriage of S. pneumoniae and other bacteria was performed every 2–6 weeks [Citation52–54]. As the number of children included in both studies was low, no additional analyses with seasonality adjustments between groups were performed. Also, acquisition could not be directly compared for each visit due to differences in swabbing timepoints between PCV7 and PHiD-CV recipients, but this should not have affected the cumulative acquisition rates presented here. Another limitation was the difference in terms of PCV coverage rates in the NIP between the enrollment periods of the two trials: two-thirds of the children vaccinated with PHiD-CV were enrolled before or concomitantly (between March and July 2009) to the introduction of PCV7 in the NIP in April 2009, while all children in the PCV7 trial were enrolled between December 2009 and April 2010, 8–12 months after introduction of PCV7 in routine immunization. Nevertheless, one-third of children in the PHiD-CV trial were also enrolled 10–13 months after introduction of PCV7 in the NIP (between February 2010 and May 2010). Although it seems unlikely to observe clinically relevant impact of the introduction of PCV7 in the NIP on bacterial NPC after 6 to 12 months, this difference in PCV coverage rates might have impacted the results if some level of herd protection and non-VT replacement had occurred within the 9 months that elapsed between the start of enrollment in the PHiD-CV and the PCV7 studies. This was suggested by a previous study showing almost 50% reduction in VT carriage and 30% increase in non-VT carriage in <2-year-old children between 2009 and 2011 in South Africa [Citation55,Citation56]. Other factors may also be perceived as limitations to our analyses. Neither of the studies included an unvaccinated control group because South Africa was a region with high risk for pneumococcal disease, and a 2 + 1 PCV7 schedule became the standard of care during the PHiD-CV study. Still, a reduction of VT colonization was observed in the PCV7 study when comparing to a historical cohort of unvaccinated children [Citation35]. The number of participants was small (250 and 100), and although no formal sample size or detection limit calculations were performed, the sample size was considered adequate for an exploratory analysis. Also, the PCR methods used in the PCV7 and the PHiD-CV trials to confirm the identification of NTHi isolates by microbiological methods were different. This should however have little impact on our findings, as most cultured H. influenzae was NTHi. A final drawback of our analysis was the inability to describe NPC of S. pneumoniae in children vaccinated with PCV13 because this vaccine was not licensed when both trials started. However, a recent study indicated that PHiD-CV and PCV13 had similar impact on S. pneumoniae carriage [Citation17].
A plain language summary contextualizing the results and potential clinical research relevance and impact is displayed in .
5. Conclusions
Overall, this analysis of two clinical trials conducted concurrently in the same population did not detect major differences in terms of prevalence of NPC or acquisition of pneumococcal serotypes, H. influenzae or S. aureus between South African children vaccinated according to a 2 + 1 vaccination schedule with PCV7 or PHiD-CV. Thus, a similar protection against 7VT invasive pneumococcal disease could be expected for both vaccines.
Author contributions
J P Yarzabal, L Schuerman, M Moreira, N François and S A Madhi designed the study. A Koen, C L Cutland, L Jose, M C Nunes, N van Niekerk and S A Madhi acquired the data. C L Cutland, D Borys, J P Yarzabal, J Ruiz-Guiñazú, L Jose, L Schuerman, M Moreira, M C Nunes, N François, N van Niekerk and S A Madhi analyzed the data. A Koen, C L Cutland, D Borys, J P Yarzabal, L Jose, L Schuerman, M Moreira, N van Niekerk, S A Madhi and S Schoonbroodt contributed to the conduct of the study. All authors participated in the interpretation of the data. All reviewed and revised the manuscript, and approved the final manuscript as submitted.
Declaration of interest
S A Madhi’s institution received grants from the Bill & Melinda Gates Foundation, the GSK group of companies, Novartis and Minervax and personal consulting fees for advisory boards and/or speaker’s bureaus from the GSK group of companies, Medimmune, Pfizer and Sanofi Pasteur. M Moreira and J Ruiz-Guiñazú were each employees of the GSK group of companies and own shares of the GSK group of companies. N François is an employee of the GSK group of companies. S Schoonbroodt, J P Yarzabal, D Borys and L Schuerman are each employees of the GSK group of companies and own shares of the GSK group of companies. Writing assistance was utilized in the production of this manuscript; Claire Verbelen and Alpár Pöllnitz (Modis c/o GSK) provided writing support; Stéphanie Deroo (Modis c/o GSK) provided manuscript coordination; and funded by GlaxoSmithKline Biological SA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials.
Reviewer disclosures
A reviewer on this manuscript has disclosed that within the last 5 years they have had research funding and non-financial support (supply of reagents) from vaccine company GlaxoSmithKline. Another reviewer of this manuscript disclosed that they were one of three PI’s for an extensive study on pneumococcal conjugate vaccines in Iceland. The study was an investigator-initiated study but received funding from GlaxoSmithKline Biologicals SA, which ended in 2019. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Trademark statement
Prevenar/Prevnar is a trademark of Pfizer Inc. Synflorix is a trademark of the GSK group of companies.
Supplemental Material
Download MS Word (64 KB)Acknowledgments
The authors would like to thank Dr Stephanie Jones, Dr Michelle Groome, Dr Anne von Gottberg and Locadiah Kuwanda for their conduct of the PCV7 trial; Dr Peter Adrian and Linda de Gouveia for their conduct of the PHiD-CV trial; DDL Diagnostic Laboratory (Rijswijk, the Netherlands) for their contribution to the study assays; the GSK Clinical Laboratory Sciences teams for their contribution to the PHiD-CV trial; Sudheer Ravula (GSK) for statistical analysis; Mireille Venken (GSK), Janice Beck, Kristel Vercauteren, Domenica Majorino (Modis c/o GSK) and Ann Dhoest (freelance for GSK) for study protocol and clinical report writing; and Catena Lauria (GSK) and Katleen Van Hoefs (Keyrus Biopharma c/o GSK) for global study management.
Data availability statement
Anonymized individual participant data and study documents from PHiD-CV study (NCT00829010) can be requested for further research from www.clinicalstudydatarequest.com.
For the PCV7 study, data are available upon request to Wits Health Consortium and after they have signed a material transfer agreement.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Simell B, Auranen K, Kayhty H, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–1190.
- Principi N, Esposito S. Serological criteria and carriage measurement for evaluation of new pneumococcal vaccines. Hum Vaccin Immunother. 2015;11(6):1494–1500.
- Weinberger DM, Bruden DT, Grant LR, et al. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. Am J Epidemiol. 2013;178(9):1488–1495.
- Flasche S, Givon-Lavi N, Dagan R. Using pneumococcal carriage data to monitor postvaccination changes in the incidence of pneumococcal otitis media. Am J Epidemiol. 2016;184(9):652–659.
- Devine VT, Cleary DW, Jefferies JM, et al. The rise and fall of pneumococcal serotypes carried in the PCV era. Vaccine. 2017;35(9):1293–1298.
- Nzenze SA, Madhi SA, Shiri T, et al. Imputing the direct and indirect effectiveness of childhood pneumococcal conjugate vaccine against invasive pneumococcal disease by surveying temporal changes in nasopharyngeal pneumococcal colonization. Am J Epidemiol. 2017;186(4):435–444.
- Satzke C, Turner P, Virolainen-Julkunen A, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 32(1): 165–179. 2013.
- Dagan R, Givon-Lavi N, Porat N, et al. The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine. 2012;30(34):5132–5140.
- Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;33(Suppl 2):S152–160.
- Spijkerman J, van Gils EJ, Veenhoven RH, et al. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerg Infect Dis. 2011;17(4):584–591.
- Prymula R, Hanovcova I, Splino M, et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine. 2011;29(10):1959–1967.
- van den Bergh MR, Spijkerman J, Swinnen KM, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013;56(3):e30–e39.
- Hammitt LL, Ojal J, Bashraheil M, et al. Immunogenicity, impact on carriage and reactogenicity of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in Kenyan children aged 1-4 years: a randomized controlled trial. PLoS One. 2014;9(1):e85459.
- Vesikari T, Forsten A, Seppa I, et al. Effectiveness of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugated vaccine (PHiD-CV) against carriage and acute otitis media-a double-blind randomized clinical trial in Finland. J Pediatric Infect Dis Soc. 2016;5(3):237–248.
- Sáez-Llorens X, Rowley S, Wong D, et al. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children – A randomized controlled trial. Hum Vaccin Immunother. 2017;13(6):1213–1228.
- Brandileone MC, Zanella RC, Almeida SC, et al. Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in Sao Paulo, Brazil. Vaccine. 2016;34(46):5604–5611.
- Odutola A, Ota MOC, Antonio M, et al. Efficacy of a novel, protein-based pneumococcal vaccine against nasopharyngeal carriage of Streptococcus pneumoniae in infants: A phase 2, randomized, controlled, observer-blind study. Vaccine. 2017;35(19):2531–2542.
- Andrade AL, Ternes YM, Vieira MA, et al. Direct effect of 10-valent conjugate pneumococcal vaccination on pneumococcal carriage in children Brazil. PLoS One. 2014;9(6):e98128.
- Davis SM, Deloria-Knoll M, Kassa HT, et al. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. 2013;32(1):133–145.
- Spijkerman J, Prevaes SM, van Gils EJ, et al. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One. 2012;7(6):e39730.
- Bosch A, van Houten MA, Bruin JP, et al. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the pneumococcal conjugate vaccine in the Netherlands. Vaccine. 2016;34(4):531–539.
- Dunne EM, Satzke C, Ratu FT, et al. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. Lancet Glob Health. 2018;6(12):e1375–e1385.
- Quirk SJ, Haraldsson G, Erlendsdóttir H, et al. Effect of vaccination on pneumococci isolated from the nasopharynx of healthy children and the middle ear of children with otitis media in Iceland. J Clin Microbiol. 2018;56:12.
- Vissers M, Wijmenga-Monsuur AJ, Knol MJ, et al. Increased carriage of non-vaccine serotypes with low invasive disease potential four years after switching to the 10-valent pneumococcal conjugate vaccine in The Netherlands. PLoS One. 2018;13(3):e0194823.
- Navne JE, Koch A, Slotved HC, et al. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal carriage by respiratory pathogens among Greenlandic children. Int J Circumpolar Health. 2017;76(1):1309504.
- Reiss-Mandel A, Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: myth or reality? Hum Vaccin Immunother. 2016;12(2):351–357.
- Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine. 2012;30(32):4717–4718.
- Expanded Programme on Immunisation in South Africa. Vaccinator’s manual. [ cited 2020 Mar 18]. Available from: https://www.health-e.org.za/wp-content/uploads/2014/03/Vaccinators_Manual_Final.pdf
- Madhi SA, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine. 2012;30(Suppl 3):C21–27.
- Hamaluba M, Kandasamy R, Upreti SR, et al. Comparison of two-dose priming plus 9-month booster with a standard three-dose priming schedule for a ten-valent pneumococcal conjugate vaccine in Nepalese infants: a randomised, controlled, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(4):405–414.
- Klugman KP, Madhi SA, Adegbola RA, et al. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine. 2011;29(18):3372–3373.
- WHO. Summary of WHO position papers - recommended routine immunizations for children. [ cited 2020 Mar 18]. Available from: http://www.who.int/immunization/policy/Immunization_routine_table2.pdf
- Madhi SA, Nunes MC. The potential impact of pneumococcal conjugate vaccine in Africa: considerations and early lessons learned from the South African experience. Hum Vaccin Immunother. 2016;12(2):314–325.
- WHO. Vaccine-preventable diseases: coverage time series for South Africa. [ cited 2020 Mar 18]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/coverages?c=ZAF
- Nunes MC, Jones SA, Groome MJ, et al. Acquisition of Streptococcus pneumoniae in South African children vaccinated with 7-valent pneumococcal conjugate vaccine at 6, 14 and 40 weeks of age. Vaccine. 2015;33(5):628–634.
- Madhi SA, Moreira M, Koen A, et al. Impact of HIV status and vaccination schedule on bacterial nasopharyngeal carriage following infant immunisation with the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in South Africa. Vaccine. 2020;38(10):2350–2360.
- Jones SA, Groome M, Koen A, et al. Immunogenicity of seven-valent pneumococcal conjugate vaccine administered at 6, 14 and 40 weeks of age in South African infants. PLoS One. 2013;8(8):e72794.
- Madhi SA, Koen A, Jose L, et al. Vaccination with 10-valent pneumococcal conjugate vaccine in infants according to HIV status. Medicine (Baltimore). 2017;96(2):e5881.
- Madhi SA, Koen A, Jose L, et al. Immunization with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) according to different schedules in infants in South Africa: a phase III trial. Expert Rev Vaccines. 2017;16(6):641–656.
- Neufeld F. Über die Agglutination der Pneumokokken und über die Theorieen der Agglutination. Z Hyg Infektionskr. 1902;40:54–72.
- Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2(7):e397–405.
- Roca A, Hill PC, Townend J, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med. 2011;8(10):e1001107.
- Usuf E, Bottomley C, Adegbola RA, et al. Pneumococcal carriage in sub-Saharan Africa–a systematic review. PLoS One. 2014;9(1):e85001.
- Sime WT, Aseffa A, Woldeamanuel Y, et al. Serotype and molecular diversity of nasopharyngeal Streptococcus pneumoniae isolates from children before and after vaccination with the ten-valent pneumococcal conjugate vaccine (PCV10) in Ethiopia. BMC Infect Dis. 2019;19(1):409.
- K L O, Dagan R. Nasopharyngeal carriage. Pneumococcal vaccines. Washington, DC: American Society for Microbiology press; 2008.
- Olwagen CP, Adrian PV, Nunes MC, et al. Use of multiplex quantitative PCR to evaluate the impact of pneumococcal conjugate vaccine on nasopharyngeal pneumococcal colonization in African children. mSphere. 2017;2:6.
- Abdullahi O, Karani A, Tigoi CC, et al. Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. J Infect Dis. 2012;206(7):1020–1029.
- Adegbola RA, DeAntonio R, Hill PC, et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS One. 2014;9(8):e103293.
- Shiri T, Nunes MC, Adrian PV, et al. Interrelationship of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus colonization within and between pneumococcal-vaccine naive mother-child dyads. BMC Infect Dis. 2013;13:483.
- Bojang A, Jafali J, Egere UE, et al. Seasonality of pneumococcal nasopharyngeal carriage in rural gambia determined within the context of a cluster randomized pneumococcal vaccine trial. PLoS One. 2015;10(7):e0129649.
- Boyles TH, Brink A, Calligaro GL, et al. South African guideline for the management of community-acquired pneumonia in adults. J Thorac Dis. 2017;9(6):1469–1502.
- Heinsbroek E, Tafatatha T, Chisambo C, et al. Pneumococcal acquisition among infants exposed to HIV in rural Malawi: a longitudinal household study. Am J Epidemiol. 2016;183(1):70–78.
- Dube FS, Ramjith J, Gardner-Lubbe S, et al. Longitudinal characterization of nasopharyngeal colonization with Streptococcus pneumoniae in a South African birth cohort post 13-valent pneumococcal conjugate vaccine implementation. Sci Rep. 2018;8(1):12497.
- Salter SJ, Turner C, Watthanaworawit W, et al. A longitudinal study of the infant nasopharyngeal microbiota: the effects of age, illness and antibiotic use in a cohort of South East Asian children. PLoS Negl Trop Dis. 2017;11(10):e0005975.
- Nzenze SA, Shiri T, Nunes MC, et al. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J. 2013;32(11):1270–1278.
- Nzenze SA, Shiri T, Nunes MC, et al. Temporal association of infant immunisation with pneumococcal conjugate vaccine on the ecology of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonisation in a rural South African community. Vaccine. 2014;32(42):5520–5530.

