Abstract
Recurrent type I endometrial cancer (EC) has poor prognosis and demands novel therapeutic approaches. Bevacizumab, a VEGF-A neutralizing monoclonal antibody, has shown clinical activity in this setting. To our knowledge, however, although some diabetic cancer patients treated with bevacizumab may also take metformin, whether metformin modulates response to anti-VEGF therapy has not yet been investigated. Here, we report the case of a patient with advanced EC treated, among other drugs, with bevacizumab in combination with metformin. The patient affected by relapsed EC G3 type 1, presented in march 2010 with liver, lungs and mediastinic metastases. After six cycles of paclitaxel and cisplatin she underwent partial response. Later on, she had disease progression notwithstanding administration of multiple lines of chemotherapy. In march 2013, due to brain metastases with coma, she began steroid therapy with development of secondary diabetes. At this time, administration of Bevacizumab plus Metformin improved her performance status. CT scans performed in this time window showed reduced radiologic density of the lung and mediastinic lesions and of liver disease, suggestive of increased tumor necrosis. Strong 18F-FDG uptake by PET imaging along with high levels of monocarboxylate transporter 4 and lack of liver kinase B1 expression in liver metastasis, highlighted metabolic features previously associated with response to anti-VEGF therapy and phenformin in preclinical models. However, clinical benefit was transitory and was followed by rapid and fatal disease progression. These findings—albeit limited to a single case—suggest that tumors lacking LKB1 expression and/or endowed with an highly glycolytic phenotype might develop large necrotic areas following combined treatment with metformin plus bevacizumab. As metformin is widely used among diabetes patients as well as in ongoing clinical trials in cancer patients, these results deserve further clinical investigation.
Abbreviations
| AMPK | = | AMP-activated protein kinase |
| CT | = | computed tomography |
| EC | = | endometrial cancer |
| LKB1 | = | liver kinase B1 |
| MCT4 | = | monocarboxylate transporter 4 |
| OS | = | overall survival |
| PFS | = | progression free survival |
| TACE | = | trans-catheter arterial chemoembolization |
| VEGF-A | = | vascular endothelial growth factor A |
Introduction
Retrospective studies suggested that metformin is associated with better survival in patients with endometrial cancer (EC). Metformin - a biguanide widely used for the treatment of type II diabetes - is currently under investigation for possible therapeutic applications in cancer patients in combination with chemotherapy.Citation1 In a 10-year retrospective study, Nevadunsky et al. reported improved overall survival (OS) in diabetic patients with non-endometrioid EC who used metformin compared with other groups, including both diabetic patients who did not use metformin and EC in non-diabetic patients.Citation2 On the other hand, Ko et al. conducted a multicentric retrospective study comparing patients suffering from type 2 diabetes mellitus using or not metformin at time of diagnosis. This study demonstrated a 1.8 times worse Recurrence-Free Survival in non-metformin users (95% CI: 1.1–2.9, p = 0.02 ) and 2.3 times worse OS (95% CI: 1.3–4.2, p = 0.005) but no significant difference in Time to Recurrence. Metformin was associated with better survival in all-cause mortality but its impact in cancer-related outcome was unclear, as overall health status of patients and glycemic control were not investigated.Citation3 In any case, other studies have shown that metformin is associated with improved cancer specific survival in some types of cancer.Citation4
Bevacizumab, a recombinant humanized monoclonal antibody which binds and neutralizes all active isoforms of vascular endothelial growth factor A (VEGF-A) is currently the leading anti-angiogenic drug for cancer patients. Based on the positive outcome of several phase III clinical trials, in Europe bevacizumab was approved by the European Medicines Agency for 5 types of metastatic or locally advanced cancer in combination with other drugs.Citation5 In recurrent or persistent EC, clinical activity of bevacizumab was assessed in a phase II trial.Citation6 Bevacizumab was given as a single drug (15 mg/Kg every 21 days) to patients who had received one to 2 prior chemotherapic regimens; clinical response was observed in 7 of 52 patients (13.5 %, 1 CR and 6 PR) and 40.4% patients remained progression free at least 6 months. Progression free survival (PFS) and OS were 4.2 and 10.5 months, respectively.
To our knowledge, although some diabetic cancer patients treated with bevacizumab may also take metformin, whether metformin modulates response to anti-VEGF therapy has not yet been explored. Here, we report the case of a patient who was diagnosed with endometrial cancer and was treated, among other drugs, with bevacizumab in combination with metformin. As presented below, we observed an interesting serological and radiological response to single agent bevacizumab and metformin. Notably, when metformin dosage was reduced due to gastro-enteric disturbances, rapid and fatal disease progression was observed.
Case Report
Clinical data
In October 2008, a 63-year-old woman underwent laparoscopic hysterectomy, bilateral annessiectomy and pelvic lymphadenectomy at our center for endometrioid adenocarcinoma of the uterine body. Pathological examination reported a G3 pT1cN0M0 stage, with estrogen receptor positivity 15% and progesterone receptor positivity 50%. Post-operative follow-up was complicated by bladder perforation. No further medical treatment was performed until April 2010, when a planned CT (CT) scan revealed liver, lung e mediastinal metastases. Hepatic needle biopsy showed metastasis of endometrial origin: Histologic grading 3, progesterone receptor positivity 90%, Estrogen Receptor negative. From April to August 2010, the patient received first line chemotherapy with a platinum-based regimen (6 cycles, Cumulative Doses: Paclitaxel 1050 mg/m2 - cisplatin (DDP) 430 mg/m2), achieving partial response according to RECIST criteria. Hormonal Therapy with Medroxyprogesteron (320 mg/die) was prescribed after chemotherapy discontinuation. In December 2010, routine CT imaging showed marked tumor progression in the liver. Hepatic lesions in the right lobe were promptly treated with laparoscopic wedge resection (6th-7th segment) and micro-wave thermal ablation (3rd segment). From February to May 2011, the patient underwent 2nd line therapy with 4 cycles Pegylated Liposomal Doxorubicin (PLD) and Carboplatin (JM8) (Cumulative Dose: PLD 140 mg/m2 – JM8 AUC5 2110 mg); follow-up CT scan in June 2011 revealed pulmonary progression, stable mediastinal lymphoadenomegaly and 4 hepatic nodules. In July 2011, the patient began bevacizumab (7.5 mg/Kg) - increased after 3 cycles at 15 mg/kg 4 - along with Tamoxifen, which was administered until July 2012. In November 2011 - due to rising CEA and liver progression - she underwent hepatic trans-catheter arterial chemoembolization (TACE) with doxorubicin. In July 2012, weekly Paclitaxel-Carboplatin therapy Citation7 was added to Bevacizumab while discontinuing Tamoxifen, due to multi-organ progression. In November 2012, following various episodes of sinus tachycardia, bevacizumab was temporary discontinued, to prevent cardiac adverse events, which were reported by Aghajanian et al..Citation4 Weekly Taxol-Carboplatin schedule was also stopped in January 2013, due to rising CEA titer. In February 2013 a lung CT scan showed progression of known pulmonary and mediastinic lesions and the patient was symptomatic (cough). Gemcitabine-Carboplatin (cumulative dose: Gemcitabine 5800 mg/m2 - JM8 100 mg/m2) was given until March 2013 (2 cycles) when a brain MRI showed 2 metastatic lesions of the frontal lobe, both of them <2 cm. Bevacizumab was given in combination with steroids as salvage therapy for brain metastasis and the patient experienced worsening of blood sugar levels. At that time (March 2013) performance status of the patient was poor, coping with epilepsy episodes and dyspnea (3 ECOG). Moreover, CEA levels sharply increased, compared to previous measurements (). To control glycemia, it was initially employed insulin, replaced since March 2013 by metformin, (850 mg twice a day), along with 3 cycles of bevacizumab (7.5 mg/Kg - 15 mg/kg). While receiving bevacizumab and metformin with no other drugs that could affect cancer progression we observed a full recovery of the Performance Status (1 ECOG). CT scans performed at the end of this window of clinical improvement showed reduced radiologic density of the lung and mediastinic lesions, as well as of the hepatic metastasis, compared with previous scans (). The volume of the lung and hepatic metastasis, however, was not reduced and new secondary lesions appeared in the liver; moreover, a moderate increase in biomarkers of liver injury (including AST, ALT, ALP and γGT) was measured, whereas renal function was normal. Peri-lesional brain metastases edema was reduced (not shown). Serum CEA slightly increased, compared to pre-treatment values (). The patient did exceptionally well for 2 months. At the end of May 2013, however, the patient began complaining abdominal discomfort (a known side effect of biguanides) and metformin was partially (850 × 1) replaced by insulin therapy, whereas bevacizumab was continued. In June 2013 the patient's conditions worsened, due to epileptic seizures and rising hepatocellular injury markers. At the end of June, she was admitted to hospital with jaundice. Abdomen US showed massive metastatic infiltration of the liver and eventually the patient died after a short time.
Figure 1. Timeline of drug administrations and CEA levels. Top panel represents the different drug combinations - including both chemotherapy, bevacizumab and metformin - received by the patient in 2012–2013. Bottom panel shows serum CEA levels in the same time window.
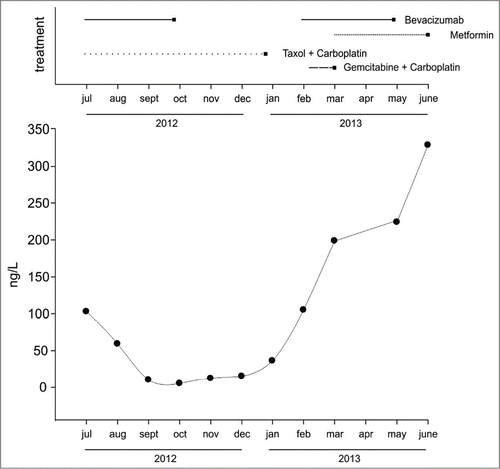
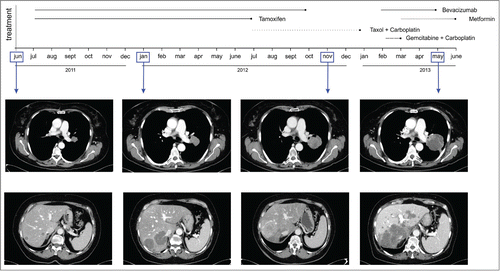
Figure 2. Timeline of morphologic changes in lung and liver metastasis by CT. Representative CT scans of lung (top panels) and liver metastasis (bottom panels) showing marked attenuation of radiologic density following combined administration of bevacizumab plus metformin in a patient with metastatic endometrial cancer.
Molecular features
Recently, preclinical studies disclosed that anti-VEGF therapy causes marked impairment in ATP and glucose levels into tumors, showing that highly glycolytic tumors become largely necrotic following VEGF blockade.Citation8 These findings suggested that certain metabolic features of tumor cells could modulate response to anti-angiogenic therapy. To investigate the metabolic traits of the tumor of this patient, we analyzed by immunohistochemistry (IHC) expression of monocarboxylate transporter 4 (MCT4), a lactate transporter whose expression is associated with highly glycolytic cells.Citation9 Intriguingly, we found strong MCT4 expression in the liver metastases (), suggesting presence of highly glycolytic tumor cells. In keeping with these findings, strong 18F-FDG uptake was observed in liver metastasis by PET imaging ().
Figure 3. Assessment of metabolic features of liver metastasis. Panel A shows IHC staining of monocarboxylate transporter 4 (MCT4) and liver kinase B1 (LKB1) in the liver metastasis of the patient. Magnifications x100 and x200 were used. This sample is strongly positive for the lactate transporter MCT4 and negative for LKB1. Panel B: PET imaging performed in May 2012 shows strong 18F-FDG uptake in the liver metastasis.
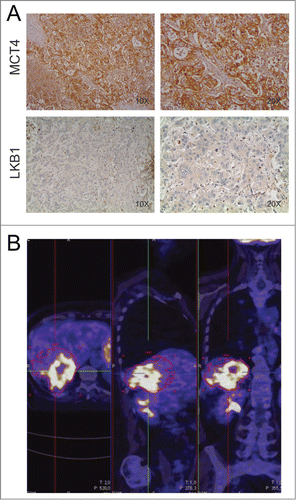
Metformin, a weak inhibitor of respiratory complex I, could also cause energy stress in tumors.Citation1 When combined with anti-angiogenic therapy, which appears to increase tumor hypoxia,Citation10 metformin might further compromise mitochondrial respiration. Although clinical data are lacking, in pre-clinical models of melanoma metformin has shown some beneficial effects when combined with anti-VEGF therapy.Citation11 Recently, increased tumor responses to phenformin - a biguanide related to metformin - were reported in non-small cell lung cancer models bearing genetic inactivation of liver kinase B1 (LKB1), the major upstream kinase activating the energy-sensing kinase AMP-activated protein kinase (AMPK).Citation12 Intrigued by this study and by our previous observation linking defects in LKB1/AMPK pathway to increased tumor necrosis following anti-VEGF therapy,Citation8 we investigated by IHC expression of LKB1 in liver metastasis from this patient. We found that liver metastasis of this patient lacked LKB1 expression (). In contrast, LKB1 was readily expressed in control tumors that were stained in parallel (not shown). Loss of LKB1 has also been shown to promote metabolic reprogramming in tumor cells by an HIF-1α-dependent mechanism,Citation13 suggesting that this might in part contribute to the highly glycolytic phenotype of liver metastasis.
These findings - albeit limited to a single case - are in keeping with results of pre-clinical studies and suggest that lack of LKB1 and an highly glycolytic phenotype might associate with induction of large necrotic areas following combined treatment with metformin plus bevacizumab in patients.
Conclusions
In this clinical case, simultaneous administration of metformin and bevacizumab led to increased tumor necrosis at CT scan and improved performance status in a terminally-ill patient. As the patient received 2 cycles of gemcitabine plus carboplatin shortly before beginning bevacizumab plus metformin, a contribution of chemotherapy to this transient clinical benefit cannot be ruled out. Moreover, it is unclear whether subsequent tumor progression was due to reduction of metformin dosage or was the natural course of the cancer. Despite these intrinsic limitations, this clinical observation suggests that metformin could modulate bevacizumab activity in highly glycolytic tumors and deserves further validation in clinical studies.
Ethical Statement
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal upon request.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant IG14295 from AIRC to S.I.
References
- Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012; 2:778-90; PMID:22926251; http://dx.doi.org/10.1158/2159-8290.CD-12-0263
- Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, Conroy E, Goldberg GL, Einstein MH. Metformin use and endometrial cancer survival. Gynecol Oncol 2014; 132:236-40; PMID:24189334; http://dx.doi.org/10.1016/j.ygyno.2013.10.026
- Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C, Havrilesky LJ, Secord AA, Moore DT, Gehrig PA, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol 2014; 132:438-42; PMID:24269517; http://dx.doi.org/10.1016/j.ygyno.2013.11.021
- Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist 2013; 18:1248-55; PMID:24258613; http://dx.doi.org/10.1634/theoncologist.2013-0111
- Feliz LR, Tsimberidou AM. Anti-vascular endothelial growth factor therapy in the era of personalized medicine. Cancer Chemother Pharmacol 2013; 72:1-12; PMID:23463481; http://dx.doi.org/10.1007/s00280-013-2124-y
- Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, Rotmensch J, Barnes MN, Hanjani P, Leslie KK. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol 2011; 29:2259-65; PMID:21537039; http://dx.doi.org/10.1200/JCO.2010.32.6397
- Vandenput I, Vergote I, Leunen K, Berteloot P, Neven P, Amant F. Leuven dose-dense paclitaxel/carboplatin regimen in patients with primary advanced or recurrent endometrial carcinoma. Int J Gynecol Cancer 2009; 19:1147-51; PMID:19820384; http://dx.doi.org/10.1111/IGC.0b013e3181ad3dcb
- Nardo G, Favaro E, Curtarello M, Moserle L, Zulato E, Persano L, Rossi E, Esposito G, Crescenzi M, Casanovas O, et al. Glycolytic phenotype and AMP kinase modify the pathologic response of tumor xenografts to VEGF neutralization. Cancer Res 2011; 71:4214-25; PMID:21546569; http://dx.doi.org/10.1158/0008-5472.CAN-11-0242
- Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol 2011; 2:49; PMID:21904528; http://dx.doi.org/10.3389/fphar.2011.00049
- Quintieri L, Selmy M, Indraccolo S. Metabolic effects of antiangiogenic drugs in tumors: Therapeutic implications. Biochem Pharmacol 2014; 89: 162-70; PMID:24607274; http://dx.doi.org/10.1016/j.bcp.2014.02.018
- Martin MJ, Hayward R, Viros A, Marais R. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov 2012; 2:344-55; PMID:22576211; http://dx.doi.org/10.1158/2159-8290.CD-11-0280
- Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013; 23:143-58; PMID:23352126; http://dx.doi.org/10.1016/j.ccr.2012.12.008
- Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, Mamer OA, Avizonis D, Shackelford DB, Shaw RJ, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1alpha. Proc Natl Acad Sci U S A 2014; 111:2554-9; PMID:24550282; http://dx.doi.org/10.1073/pnas.1312570111
