ABSTRACT
Putative gender differences in bladder cancer (BCa) have been proposed to result from sex hormone influence. Aromatase is the key enzyme catalyzing the conversion of androgens to estrogens which may result in an intratumoral microenviroment with increased estrogen production. In this study, we investigated the expression pattern of aromatase and its association with BCa progression. Tissue samples from 88 BCa patients who underwent cystectomy were obtained. Using immunohistochemistry (IHC), expression of aromatase in tumor epithelium (TE) and tumor related stroma (TS) were evaluated separately, and the association of aromatase expression status with pathologic variables and overall survival (OS) outcome was examined. High aromatase expression was found in 33/88 (37.5%) of TE and in 65/88 (73.9%) of TS. Increased aromatase expression in TE had a trend to correlate with male gender. Increased aromatase in TS was significantly associated with adverse pathologic variables including higher pathologic pT, positive lymph node metastasis (pN), lymphovascular invasion (LVI), and distant metastasis. In univariate analysis, high aromatase expression in TS was significantly associated with poorer overall survival (p = 0.014), but this association was not significant (p = 0.163) in multivariate cox analysis adjusted for independent factors including age at surgery and pN. These results demonstrate that aromatase expression in TS but not TE may play a critical role in BCa progression. Our findings provide direct evidence of aromatase involvement in BCa and suggest endocrine therapy may have a potential role in the treatment of BCa.
Introduction
n the United States, BCa was the 4th most common cancer in men (estimated 60,490 new cases) and 12th most common in women (estimated 18, 540 new cases) in 2017.Citation1 This three to four fold gender difference is still present after controlling for potential environmental or lifestyle factors.Citation1-4 Moreover, men had a 25% faster increase in the rate of new cases of BCa than women between 1988 and 2008 in the USA.Citation5 Epidemiological and clinical data showed that although men had a higher BCa incidence rate, most of the tumors in men showed favorable pathologic factors and better survival. In contrast, women had a lower BCa incidence rate, but tended to have higher risk of disease recurrence, progression, and mortality.Citation6 These findings suggest that hormonal axis may influence bladder tumorigenesis and progression.Citation7,Citation8 Preclinical studies investigating the role of androgen and estrogen receptors (AR and ER) as well as their cognate hormones in bladder cancer have shown there is involvement of sex hormone signaling pathways in initiation and progression of BCa.Citation9
Aromatase (estrogen synthetase, encoded by the CYP19A1 gene) is the unique enzyme that catalyzes a critical, irreversible step in the synthesis of estrogens through mediating the conversion of androstenedione and testosterone to estrone and estradiol.Citation10 Aromatase is found in the ovary, placenta, hypothalamus, liver, muscle, adipose tissue, and breastCitation11 as well as normal human bladder tissue.Citation12,Citation13 In extragonadal tissue, aromatase can generate higher local estrogen concentration than circulating serum levels.Citation10 Moreover, several studies have shown that expression of aromatase is correlated with tumorigenesis and/or progression in gender-related tumor types including breast cancer,Citation14 endometrial cancer,Citation15 cervical cancer,Citation16 ovarian cancer,Citation17 prostate cancer,Citation18 as well as tumors which may be sex hormone-related types including lung cancer,Citation19 colon cancerCitation20 and BCa.Citation21 The prognostic role of aromatase expression in TE has been discussed in breast cancer, endometrial cancer, lung cancer, BCa and prostate cancer, however this remains controversial.Citation18, Citation21-24 In addition, studies also highlighted the predictive and prognostic importance of intratumoral aromatase expression in TS in breast cancer, endometrial cancer and prostate cancer.Citation18,Citation22,Citation25 Aromatase inhibitor (AI), which can abolish tumor estrogen production and enhance androgenic activity, is customarily used as first-line treatment for advanced breast cancer.Citation26
A recent study revealed that aromatase expression in TE was positively correlated with pathologic stage and inversely correlated with cancer-specific survival in a cohort of BCa patients.Citation21 However, limited knowledge of aromatase activity in BCa was understood. In the present study, we aimed to examine the expression pattern of aromatase in BCa tissues, and to investigate its clinical significance in BCa malignancy.
Materials and methods
Patients and tissue specimens
A total of eighty-eight BCa specimens were obtained from patients who underwent cystectomy from 2002 to 2010 through the tumor bank database of the Department of Pathology and Urology at Massachusetts General Hospital with approval by the Institutional Review Board. Patient demographics, clinicopathologic features, and follow up information were obtained from clinical records (). These patients included 68 (77.3%) males and 20 (22.7%) females with a median age of 71 years (Interquartile Range (IQR) 62–79years), and median overall survival was 21 months (IQR 11–58 months). The pathology of all primary cancers was reviewed by two of the authors (SW and CW) and tumors were staged and graded according to the American Joint Committee on Cancer (TNM classification, 7th edition)Citation27 and the 2016 World Health Organization (WHO) classification of tumor of the bladder.Citation28
Table 1. Association of aromatase expression in tumor epithelium and tumor related stroma with clinicopathologic characteristics in 88 BCa patients who underwent cystectomy between 2002 and 2010.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed on formalin-fixed, paraffin-embedded 5 µm sections. Briefly, after deparaffinization in xylene and rehydration with graded concentrations of alcohol to distilled water, the specimens were washed in Tris-buffered saline with 0.1% Tween 20 (TBST), and antigen retrieval was performed in R-buffer A (Electron Microscopy Science) using a pressure cooker. Endogenous peroxidase activity was blocked with 3% H2O2. Nonspecific binding was blocked with 5% goat serum for 30 minutes. Slides were then incubated overnight with the primary polyclonal rabbit anti-Aromatase antibody (ab18995; abcam, Cambridge, MA) at a dilution of 1: 300 at 4°C. After washing three times in TBST, slides were incubated with anti-rabbit secondary antibody (Vector Labs, Burlingame, CA) at a dilution of 1: 300.The peroxidase reaction was developed using the DakoCytomation Liquid DAB plus Substrate Chromogen System (DakoCytomation). Placental tissue was used for a positive control and rabbit isotype IgG was used instead of primary antibody as a negative control.
IHC scoring
Immunostaining was independently evaluated by two authors (SW and CW) blinded to patient characteristics and clinical features of the neoplasm. Scores assigned by these two investigators were compared and discrepant scores were reevaluated by re-examination of the staining by both authors to achieve a consensus score. TE and TS were evaluated separately. Aromatase expression was evaluated according to staining intensity (0 (negative), 1 (weak), 2 (moderate), and 3 (strong)) and the percentage of positive cells. Since TE staining was in general weaker than TS staining, therefore, different cutoff value was used in our study. For TE positive, staining intensity showed marked variation from weak to strong, hence high expression group was defined as 10% or more of cells positive (staining ≥ weak) for aromatase in the region of interest (cancer tissue and surrounding tissues appearing in the same section).Citation22 Staining for TS showed a distinct pattern, hence high expression group was defined as 20% or more of cells positive (staining ≥ moderate) for aromatase in the region of interest.
Statistical methods
All statistical analysis was performed with Stata14 (College Station, TX, USA). The Mann-Whitney U test and Chi-squared test or Fisher's exact test were used to evaluate the association of clinicopathologic variables and aromatase expression. Survival rates were calculated with the Kaplan-Meier method and comparison was made with the log rank test. Univariable analysis was performed to evaluate the association of aromatase expression and overall survival, and variables which showed statistical significance were further fitted into multivariable Cox regression models. Relative risks of dying were expressed as adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). All statistical tests were two-sided and p < 0.05 was considered statistically significant.
Results
Aromatase protein expression in BCa
The expression pattern of aromatase protein was assessed by IHC in tissue specimens from 88 patients with BCa. Aromatase staining was found in the cytoplasm of both TE cells and TS cells as shown in , but weak aromatase staining was also detectable in adjacent normal muscle as reported previously.Citation11 TE and TS staining were evaluated separately, and all specimens were placed into low or high aromatase expression groups for TE or TS. In all 88 cases examined, high aromatase expression was present in 37.5% and 73.9% of TE and TS respectively ().
Figure 1. Aromatase expression in BCa. A, B Aromatase staining is negative in both TE and TS. C, D Aromatase expression is strong in TE (High), but weak in TS (Low). E, F Aromatase expression is weak in TE (Low), but strong in TS (High). G, H Aromatase expression is strong in both TE (High) and TS (High). (A,C,E,G 20 ×; B,D,F,H 40 ×) (TE: tumor epithelium, TS: tumor related stroma).
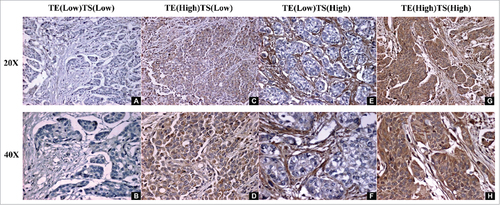
In TE, high aromatase expression was found more frequently in males than in females with a marginal statistical significance (42.7% vs 20.0%, p = 0.073). There was no other significant association of aromatase status with clinicopathologic features ().
In TS, there was no significant association of aromatase status with gender (p = 0.386). However, high expression of aromatase was significantly associated with adverse pathologic variables including higher pTstage (83.9% vs 50.0%, p = 0.003), positive pN (96.4% vs 56.9%, p<0.001), LVI (89.2% vs 62.7%, p = 0.007), and distant metastases (86.1% vs 65.4%, p = 0.047). There was no significant association of aromatase expression with age at surgery, perineural invasion (PNI), or soft tissue surgical margins (STSM) ().
Correlation of overall survival and aromatase status in BCa patients
Kaplan-Meier curves shown in demonstrate that overall survival in BCa patients with high TE aromatase expression did not differ from that of patients showing low aromatase expression over the entire cohort (A, p = 0.479) or when stratified by gender (A1, p = 0.419 in males and A2, p = 0.951 in females). However, patients with high TS aromatase expression had significantly worse overall survival than those with low aromatase expression (B, p = 0.010). When TS aromatase expression was evaluated between male and female groups, we found such significant worse in overall survival was only observed in male patients (B1, p = 0.045), but was lost in female patients (B2, p = 0.120). In addition, univariate analysis showed that age at surgery (p = 0.029), pT stage (p = 0.006), pN (p<0.001), LVI (p = 0.006), STSM (p = 0.002), and TS high aromatase expression (p = 0.014) were significantly associated with shorter overall survival of BCa patients (). However, when all variables which showed significance in univariate analysis were fitted into multivariate analysis based on the Cox proportional hazard model, only age at surgery (p = 0.003), and pN (p = 0.009) were found to be independent prognostic indicators, and TS aromatase expression did not reach statistical significance (HR = 1.72; 95% CI 0.80–3.71; p = 0.163) ().
Figure 2. Kaplan-Meier curves representing the overall survival of patients treated with cystectomy for bladder cancer stratified by aromatase status shown by TE staining (A, p = 0.479 in entire cohort; A1, p = 0.419 in male; A2, p = 0.951 in female) and TS staining (B, p = 0.010 in entire cohort; B1, p = 0.045 in male; B2, p = 0.120 in female). (TE: tumor epithelium, TS: tumor related stroma).
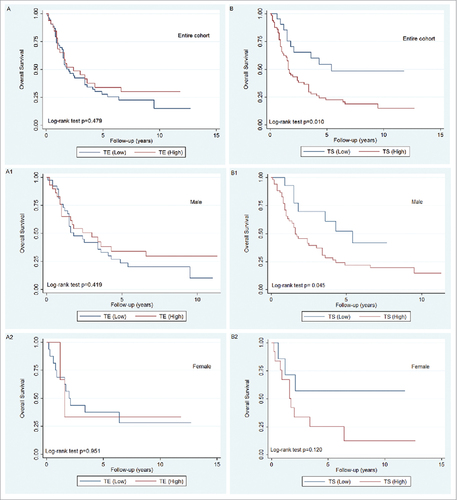
Table 2. Univearitate and Multivariable analysis of clinical and pathologic factors with overall survival. Multivariable Cox regression models fitted with factors that showed significance in univariable analysis.
Discussion
The involvement of the sex hormone signaling pathway in tumorigenesis and progression of BCa has been established in studies investigating the observed gender differences in both the incidence and severity of the disease.Citation9,Citation29 However, findings regarding expression of AR/ER in BCa have been inconsistent and contradictory. In a review paper on androgen and estrogen receptors in BCa, Godoy et al.Citation9 mentioned that most studies showed a positive correlation of ERβ expression with pathologic stage and grade, whereas only a few studies showed a negative correlation. On the other hand, AR expression and aggressiveness of BCa were inversely related in most studies, and only a single study reported a positive correlation. The conflicting prognostic roles of the AR and/or ERβ expression were observed in 7 of 9 studies with two separated studies reporting no significant correlation between AR and BCa prognosis. Moreover, AR or ER expression was not significantly different between male and female BCa patients.Citation8,Citation30 These inconsistent findings regarding AR/ER in BCa raise question as to the mechanism by which AR and ER are balanced in BCa progression. Understanding the role of aromatase which is responsible for the conversion of androstenedione to estrone and testosterone to estradiol.Citation10 is critical for addressing this question.
In this study, we found that aromatase is expressed in both TE and TS in BCa. The expression of aromatase was high in TS, and it correlated with factors reflecting aggressive tumor behavior including advanced pT stage, positive pN, LVI and distant metastases. Overall survival analysis showed that high aromatase expression in TS was associated with significantly worse overall survival. When stratified by gender, similar survival curves were found in males and females, but this significance was lost in multivariate analysis adjusted for independent predictors including age at surgery and pN.
The role of aromatase in TS has also been discussed in other tumors. Segawa et al.Citation22 found an expression of aromatase 36% in TS and 58% in TE in endometrial cancer, and immunoreactivity in TS correlated positively with advanced surgical stage and poor overall survival, whereas the aromatase expression in TE was not correlated with disease progression. These results are similar to our findings and suggest aromatase in TS may have an important role in tumor progression in BCa. However, conflicting prognostic results were found in a study of prostate cancer,Citation18 which showed that higher aromatase expression in TS has a protective role for biochemical recurrence free survival, but no such role was showed in clinical failure free survival and prostate cancer death free survival. The discrepancy in TS aromatase expression and its prognostic correlation between prostate cancer and BCa suggests the role of sex hormones might be different in these two cancers. This is consistent with the concept that AR might play a different role in prostate cancer than in BCa.Citation31
Positive aromatase staining of TE in BCa showed marked variation from weak to strong in which most of cases were weak to moderate staining. This finding is consisted with the description of TE expression pattern in previous aromatase study in BCa.Citation21 There was no significant association of TE staining with pT stage or pN stage in our study. In addition, we did not find a significant association of high aromatase expression in TE with overall survival. However, we observed a trend of higher frequency of aromatase expression in male patients when compared it with female patients, which was not founded in the previous study.Citation21 This trend in gender of aromatase expression in TE raised the possibility that high levels of androgen/AR may play a role in driving gender disparity in BCa. Furthermore, in the Kaplan-Meier plot, the group with high aromatase expression in TE shows better survival than that in the group with low aromatase expression. When stratified by gender, a similar pattern of survival was found in males, but there was no survival distinction observed between high and low TE aromatase expression in females. The difference of gender frequency and gender prognosis of aromatase expression in TE is consistent with the generally accepted concept that males with BCa have a higher incidence and better survival outcomes than females.Citation6 Grindstad et al.Citation18 also reported that higher aromatase expression in TE of prostate cancer was associated with significantly better clinical failure free survival and, with marginally better prostate cancer death-free survival. These results suggest that aromatase expression in TE (especially in males) could be a protective factor in the context of genitourinary malignancy.
Expression of aromatase in tumor stroma has also been discussed in previous studies. Suzuki et al.Citation14 found aromatase expression in different cells in breast cancer, including stroma cells, carcinoma cells, normal ductal epithelium and adipose cells. Miki et al.Citation32 showed intratumoral aromatase is expressed in both stroma and neoplastic epithelium in breast carcinoma. Singer et al.Citation25 confirmed that aromatase expression was significantly more common in tumoral stroma when compared with peritumoral and distant breast stroma. Liao et al.Citation33 used a murine breast cancer model to show that aromatase positive stromal cells promote invasion and metastasis.Citation33 These findings regarding high expression of aromatase in stroma raise the possibility that tumor growth is modulated by paracrine, autocrine and intracrine mechanisms.Citation14,Citation34
Our study provided the evidence indicating the relevance of the hormonal axis in bladder tumor development and progression. Understanding the involvement of aromatase in BCa may result in better understanding of the current controversial findings regarding AR and ERβ. Our results suggest that the aromatase in TS is involved in BCa progression and may be a prognostic factor. In addition, two phase II clinical trials on the effect of tamoxifen (an ER agonist) for BCa have been initiated.Citation35,Citation36 Further investigation of the mechanisms underlying aromatase involvement in BCa is warranted. Understand the role of aromatase in BCa will provide basis for using aromatase expression as predictive biomarker for disease progression and helping the development of aromatase inhibitor treatment for BCa patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262.
- Hemelt M, Yamamoto H, Cheng KK, Zeegers MP. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer. 2009;124:412–9. doi:10.1002/ijc.23856.
- Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, Crowder JS, et al. Gender- and smoking-related bladder cancer risk. J Natl Cancer Inst. 2001;93:538–45. doi:10.1093/jnci/93.7.538.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi:10.3322/CA.2007.0010.
- Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi:10.1002/cncr.23986.
- Lucca I, Klatte T, Fajkovic H, de Martino M, Shariat SF. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat Rev Urol. 2015;12:653. doi:10.1038/nrurol.2015.257.
- Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69:300–10. doi:10.1016/j.eururo.2015.08.037.
- Godoy G, Gakis G, Smith CL, Fahmy O. Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bladder Cancer. 2016;2:127–37. doi:10.3233/BLC-160052.
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology. 2001;142:4589–94. doi:10.1210/endo.142.11.8547.
- Santen RJ, Harvey HA. Use of aromatase inhibitors in breast carcinoma. Endocr Relat Cancer. 1999;6:75–92. doi:10.1677/erc.0.0060075.
- Chavalmane AK, Comeglio P, Morelli A, Filippi S, Fibbi B, Vignozzi L, et al. Sex steroid receptors in male human bladder: expression and biological function. J Sex Med. 2010;7:2698–713. doi:10.1111/j.1743-6109.2010.01811.x.
- Strauss L, Rantakari P, Sjogren K, Salminen A, Lauren E, Kallio J, et al. Seminal vesicles and urinary bladder as sites of aromatization of androgens in men, evidenced by a CYP19A1-driven luciferase reporter mouse and human tissue specimens. FASEB J. 2013;27:1342–50. doi:10.1096/fj.12-219048.
- Suzuki T, Miki Y, Akahira J, Moriya T, Ohuchi N, Sasano H. Aromatase in human breast carcinoma as a key regulator of intratumoral sex steroid concentrations. Endocr J. 2008;55:455–63. doi:10.1507/endocrj.K07E-053.
- Jarzabek K, Koda M, Walentowicz-Sadlecka M, Grabiec M, Laudanski P, Wolczynski S. Altered expression of ERs, aromatase, and COX2 connected to estrogen action in type 1 endometrial cancer biology. Tumour Biol. 2013;34:4007–16. doi:10.1007/s13277-013-0991-9.
- Veerapaneni P, Kirma N, Nair HB, Hammes LS, Hall KL, Tekmal RR. Elevated aromatase expression correlates with cervical carcinoma progression. Gynecol Oncol. 2009;114:496–500. doi:10.1016/j.ygyno.2009.05.041.
- Cunat S, Rabenoelina F, Daures JP, Katsaros D, Sasano H, Miller WR, et al. Aromatase expression in ovarian epithelial cancers. J Steroid Biochem Mol Biol. 2005;93:15–24. doi:10.1016/j.jsbmb.2004.10.021.
- Grindstad T, Skjefstad K, Andersen S, Ness N, Nordby Y, Al-Saad S, et al. Estrogen receptors alpha and beta and aromatase as independent predictors for prostate cancer outcome. Sci Rep. 2016;6:33114. doi:10.1038/srep33114.
- Verma MK, Miki Y, Sasano H. Aromatase in human lung carcinoma. Steroids. 2011;76:759–64. doi:10.1016/j.steroids.2011.02.020.
- Sato R, Suzuki T, Katayose Y, Miura K, Shiiba K, Miki Y, et al. Aromatase in colon carcinoma. Anticancer Res. 2012;32:3069–75.
- Nguyen DP, O'Malley P, Al Hussein Al Awamlh B, Furrer MA, Mongan NP, Robinson BD, et al. Association of Aromatase With Bladder Cancer Stage and Long-Term Survival: New Insights Into the Hormonal Paradigm in Bladder Cancer. Clin Genitourin Cancer. 2017;15(2):256–262.e1. doi:10.1016/j.clgc.2016.05.017. PubMed PMID: 27324053.
- Segawa T, Shozu M, Murakami K, Kasai T, Shinohara K, Nomura K, et al. Aromatase expression in stromal cells of endometrioid endometrial cancer correlates with poor survival. Clin Cancer Res. 2005;11:2188–94. doi:10.1158/1078-0432.CCR-04-1859.
- Mah V, Marquez D, Alavi M, Maresh EL, Zhang L, Yoon N, et al. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer. 2011;74:318–25. doi:10.1016/j.lungcan.2011.03.009.
- Bollet MA, Savignoni A, De Koning L, Tran-Perennou C, Barbaroux C, Degeorges A, et al. Tumor aromatase expression as a prognostic factor for local control in young breast cancer patients after breast-conserving treatment. Breast Cancer Res. 2009;11:R54. doi:10.1186/bcr2343.
- Singer CF, Fink-Retter A, Gschwantler-Kaulich D, Thalhammer T, Hudelist G, Mueller R, et al. Selective spatial upregulation of intratumoral stromal aromatase in breast cancer patients: evidence for imbalance of local estrogen metabolism. Endocr Relat Cancer. 2006;13:1101–7. doi:10.1677/erc.1.01230.
- Riemsma R, Forbes CA, Kessels A, Lykopoulos K, Amonkar MM, Rea DW, et al. Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer. Breast Cancer Res Treat. 2010;123:9–24. doi:10.1007/s10549-010-0974-0.
- Edge SB, American Joint Committee on Cancer. AJCC cancer staging manual. New York; London: Springer, 2010.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106–19. doi:10.1016/j.eururo.2016.02.028.
- Ide H, Miyamoto H. Steroid Hormone Receptor Signals as Prognosticators for Urothelial Tumor. Dis Markers. 2015;2015:840640. doi:10.1155/2015/840640.
- Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol. 2012;42:569–77. doi:10.1093/jjco/hys072.
- Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. 2014;33:3225–34. doi:10.1038/onc.2013.274.
- Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, et al. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007;67:3945–54. doi:10.1158/0008-5472.CAN-06-3105.
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi:10.1371/journal.pone.0007965.
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
- US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT00589017?term=NCT00589017&rank=1.
- US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT02197897?term=NCT02197897&rank=1.
