ABSTRACT
Gastric cancer (GC) has been a major health burden all over the world but there are fewer promising chemotherapeutic drugs due to its multidrug resistance. It has been reported that WYC-209 suppresses the growth and metastasis of tumor-repopulating cells but the effect on GC was not explored. MTT, colony formation, and transwell assays were performed to examine the effects of WYC-209 on the proliferation, colony growth, and mobility of GC cells. Western blotting and qRT-PCR were used to detect the expression of proteins and mRNA. RNA-seq and enrichment analyses were conducted for the differentially expressed genes and enriched biological processes and pathways. The rescue experiments were carried out for further validation. Besides, we constructed xenograft model to confirm the effect of WYC-209 in vivo. The dual-luciferase reporter and Chromatin immunoprecipitation were implemented to confirm the underlying mechanism. WYC-209 exerted excellent anti-cancer effects both in vitro and in vivo. Based on RNA-seq and enrichment analyses, we found that Wnt family member 4 (WNT4) was significantly down-regulated. More importantly, WNT4 overexpression breached the inhibitory effect of WYC-209 on GC progression. Mechanically, WYC-209 significantly promoted the binding between retinoic acid receptor α (RARα) and WNT4 promoter. WYC-209 exerts anti-tumor effects in GC by down-regulating the expression of WNT4 via RARα.
KEYWORDS:
Introduction
Known as the second leading reason of cancer death following lung cancer,Citation1 gastric cancer (GC) has a miserable prognosis which can be exemplified by the 5-y survival rate below 20%.Citation2 Nowadays, the incidence of GC has declined apparently due to the increased emphasis on a healthy diet and improved hygiene standards.Citation3 But worryingly, the therapy of GC remains non-guaranteed, for there are fewer promising chemotherapeutic drugs.Citation4 So the exploration of effective anti-tumor therapy or drugs for GC is still an imminent endeavor.
Retinoids are a collection of vitamin A and its derivatives (natural and synthetic analogues). Accumulating studies have proved that retinoids are engaged in multiple biological processes, including cell proliferation, differentiation, embryogenesis, and metabolism.Citation5 In the last two decades, the application of retinoids in anti-cancer therapy has progressed a lot. For example, the promising anti-tumor efficacy of all-trans retinoic acid (ATRA), 13-cis-RA, and 9-cis-RA have been widely reported in vivo .Citation6–8 The function of retinoids is addressed through hormone receptors (NRs), including the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs).Citation9
Although retinoids were promising for normal or malignant diseases, the low water solubility based on their structure and the high toxicity at high doses were an obstacle to developing new drugs.Citation10,Citation11 Fortunately, synthetic retinoids have introduced an amide linkage that reduces lipophilicity and this is helpful for their affinity for RAR, especially RARα.Citation12 Moreover, it seems that synthetic retinoids exert stronger anti-cancer activity with fewer adverse events compared with natural retinoids, for example, ST1926 (also named adarotene), has been reported to have better effects on the cell growth MCF-7 and MDA-MB-231 cells compared with that of ATRA.Citation13 WYC-209 studied in this work, is a novel synthetic retinoid analogue designed from the class III retinoid Tazarotene. It has been proved that WYC-209 has negligible toxicity in non-cancerous cells but inspiringly inhibited the proliferation of tumor-repopulating cells (TRCs),Citation14 which is potentially tumorigenic and indispensable in tumor development.Citation15 Given that WYC-209 exhibited inspiring capacity in antitumor therapy, we tried to investigate the effect of WYC-209 on GC and wondered about the mechanisms it works.
Results
WYC-209 exerted prospective anti-cancer effects in vitro
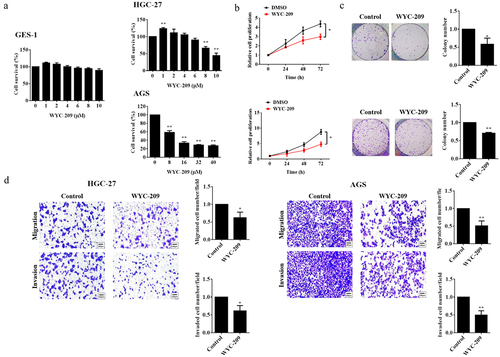
It has been reported that retinoids have already been utilized as prospective cancer therapeutic agents because of the reported anti-proliferative, pro-apoptotic as well as antioxidant roles.Citation16 So in this study, we wondered about the effects of WYC-209, a novel synthetic retinoid analogue, on GC. Preliminarily, we examined the toxicity of the drug on non-cancerous cells (GES-1) and two human gastric cancer cell lines. As shown in , 8 μM of WYC-209 treatment significantly inhibited cell survival, and its inhibitory effect was positively related to concentration (**P < .01), yet it didn’t affect the cell survival of GES-1. Based on this result, 8 μM of WYC-209 treatment was applied for the following experiments. Furthermore, WYC-209 demonstrated its provocative suppression of cell proliferation, colony-forming ability, migration as well as invasion (, **P < .01, *P < .05).
Figure 1. WYC-209 inhibited the cell survival, proliferation, colony growth, and motility of GC cells.
Furthermore, we found that WYC-209 treatment significantly up-regulated the expression of epithelial biomarker E-cadherin and down-regulated the expression of mesenchymal biomarkers N-cadherin and vimentin. Besides, the expression levels of the Snail and Slug, were also distinctly subdued due to WYC-209, revealing that this agent played an indispensable role in inhibiting EMT (epithelial-mesenchymal transition) process (, **P < .01, *P < .05), which was further illustrated by the results from subsequent qRT-PCR experiments (, **P < .01, *P < .05).
Figure 2. WYC-209 suppressed EMT and tumor progression.
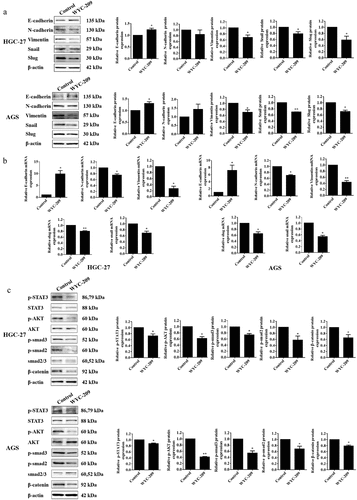
Besides, we examined the phosphorylation of STAT3, AKT, smad2, and smad3 as well as β-catenin, which were always activated in tumorigenesis and progression.Citation17–20 As shown in , after WYC-209 treatment, the expression levels of p-STAT3, p-AKT, p-smad2, p-smad3, and β-catenin were all impeded significantly (**P < .01, *P < .05), further pointed out that WYC-209 could suppress tumor progression and was potential for antitumor therapy.
WYC-209 induced the down-regulation of WNT4 in GC
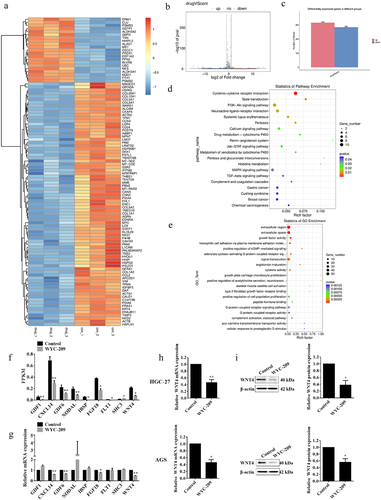
To further investigate the underlying mechanism of WYC-209 functions, we performed RNA-seq in HGC-27 cells with or without WYC-209 treated for 24 h to detect DEGs. The sequencing outcomes told us that the agent led to 316 up-regulated and 284 down-regulated genes (). Furthermore, enrichment analyses underscored the PI3K-Akt, Jak-STAT pathway, and the biological processes like growth factor activity, signal transduction, and the regulation of cell proliferation (). Subsequently, according to the fold change of DEGs and enrichment analysis results, we selected 9 genes for the Fragments per Kilobase Million (FPKM) analysis and qRT-PCR validation (, **P < .01, *P < .05). Interestingly, the results revealed that the WNT4 mRNA level was statistically decreased. And the following qRT-PCR and WB experiments further supported that at both mRNA and protein levels (, **P < .01, *P < .05). These data were meaningful for it has been reported that WNT4 was engaged in multiple human diseases and was always up-regulated in tumor tissues.Citation21
Figure 3. WYC-209 induced the heterotypic down-regulation of WNT4.
Overexpressing WNT4 breached the inhibitory effect of WYC-209 on GC progression
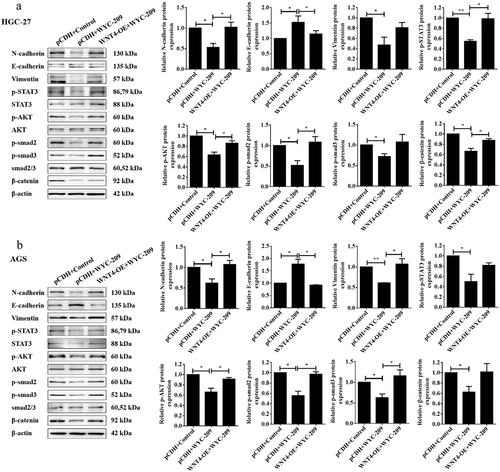
According to the result that WYC-209 induced a significant down-regulation of WNT4 in GC, we next constructed rescue experiments for further validation. As shown in , WNT4-overexpressed GC cell lines were constructed and qRT-PCR was utilized to examine the transfection efficiency. The following MTT and colony formation assays pointed out that the overexpression of WNT4 countervailed the cell proliferation and colony-forming capacity impaired by WYC-209 (**P < .01, *P < .05). Additionally, the WYC-209-decreased cell mobility of HGC-27 and AGS cells was also reversed due to WNT4 overexpression (, *P < .05). More importantly, WNT4 overexpression caused significant reversal in the WYC-209-suppressed tumor progression because, after WNT4 overexpression, the expression of E-cadherin was decreased while the expression of N-cadherin and Vimentin were increased, while STAT3, AKT, smad2/3 as well as β-catenin were re-activated (, **P < .01, *P < .05). In aggregate, we could probably conclude that the inhibitory effect of WYC-209 on GC cells may be accomplished by down-regulating WNT4.
Figure 4. Overexpressing WNT4 breached the inhibitory effect of WYC-209 on cell proliferation, colony-forming, and motility.
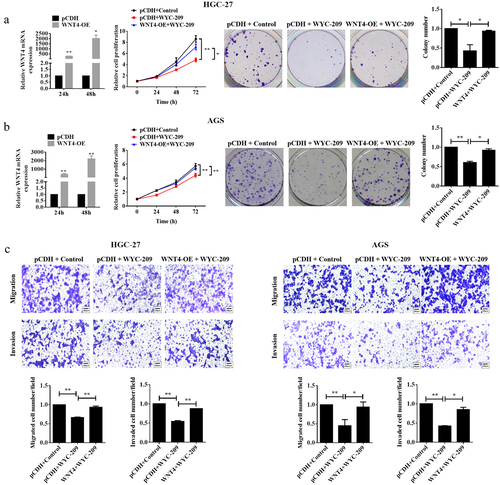
Figure 5. EMT and tumor progression inhibited by WYC-209 were abrogated by overexpressing WNT4.
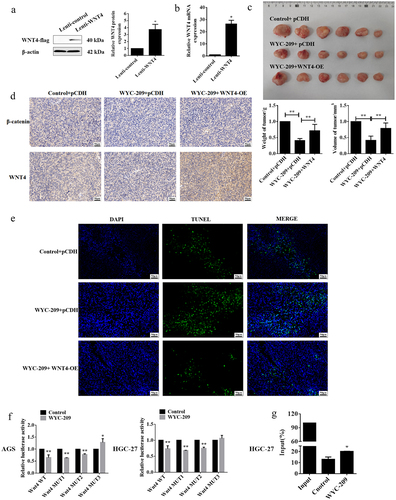
WYC-209 inhibited GC progression by down-regulating WNT4 via RARα
Earlier we preliminarily confirmed the anti-cancer role of WYC-209 on GC cells and this function may be addressed by suppressing WNT4 expression. So for further investigation, xenograft models were constructed to validate the effect of WYC-209 in vivo. The WNT4 stably overexpressed HGC-27 cell line was generated using lentivirus and after WB and qRT-PCR validation for transfection efficiency (, *P < .05), the xenograft tumor models were constructed. Unsurprisingly, WYC-209 treatment indeed attenuated the weight and volume of the xenograft tumors, which could be recovered by overexpressing WNT4 (, **P < .01). And, the WYC-209-inhibited WNT4 and β-catenin protein levels expressed in the xenograft tumor tissues were reversed by WNT4 overexpression (), besides, the facilitated cell apoptosis induced by WYC-209 was also abrogated (). Collectively, we can conclude that WYC-209 played an anti-cancer part in GC both in vitro and in vivo by down-regulating WNT4, at least partially. Last but not least, dual-luciferase reporter and ChIP experiments were implemented for a more detailed mechanism. Primarily, we predicted the binding sites on the WNT4 promoter to RARα, a retinoic acid receptor, by the JASPAR website, and three probably binding sites were exhibited. The following dual-luciferase reporter pointed out that only the mutation of site 3 recovered the luciferase activity both in AGS and HGC-27 cells after WYC-209 treatment (, **P < .01, *P < .05), indicating that WNT4 bond to RARα at site 3, which was further proved by the subsequent ChIP experiment (, *P < .05). In aggregate, WYC-209 may down-regulate the expression of WNT4 via RARα, a retinoic acid receptor, and then decelerate GC progression.
Discussion
Retinoids, including vitamin A and related derivatives, have been proven to own the anti-differentiation and pro-apoptosis capacity and thus can be utilized for cancer therapy,Citation22 but the bleak water solubility and breakdown during intravenous administration greatly limit their efficacy and impede the application.Citation23 Provocatively, synthetic retinoids are superior to endogenous retinoids, because their structures are more chemically stable.Citation13,Citation24 Besides, they always function through a specific RAR that results in minor side effects.Citation25 For example, the synthetic retinoid ST1926 showed an inhibitory effect on the proliferation of MDA-MB-231 and MCF7 cells by attenuating the Wnt/β-catenin signaling pathway, more inspiringly, this inhibition was superior compared with that of ATRA.Citation13 And bexarotene (LGD1069, Targretin) inhibited considerably non-small cell lung cancer when combined with erlotinib.Citation26
In our study, we mainly concentrated on WYC-209, the new synthetic retinoid analogue, which has been reported to have an inhibitory effect on the proliferation of TRCs, including breast cancer, lung cancer, and so on. And importantly, its inhibitory effect on the growth of TRCs did not disappear after the drug washout.Citation14 Furthermore, WYC-209 effectively suppressed multiple tumorigenic features and drug-resistance of cancer stem cells (CSCs) in vitro, and attenuated the tumor growth in vivo, with feeble side effects compared with sorafenib and acyclic retinoid.Citation27 Accordingly, we investigated the effect of WYC-209 on GC and surprisingly, this novel retinoid analogue evidently inhibited the survival of HGC-27 and AGS cells, with a positive correlation to concentration. But notably, 1 μM of WYC-209 increased the survival of HGC-27, we guessed that this phenomenon might be related to the reversible functions of some small molecules because of their bleakly immunogenic, more permeable characteristics so that their functions can be tuned by regulating concentrations.Citation28 More importantly, 8 μM WYC-209 suppressed the cell proliferation, colony-forming capacity, migration, and invasion of both HGC-27 and AGS cells, mitigated the EMT progression and the activation of classical oncogenic signaling pathways, such as STAT3, PI3K/AKT, TGF-β/smad2/3, and WNT/β-catenin. RNA-seq and enrichment analysis also underscored that the DEGs were enriched in the PI3K-Akt, Jak-STAT signaling pathway, and biological processes like growth factor activity, signal transduction, and the regulation of cell proliferation. Besides, we observed a significant down-regulation of WNT4 mRNA expression, so we wondered about the role of WNT4 in the WYC-209-induced effect on GC. By further qRT-PCR and WB validation, we confirmed that the expression of WNT4 protein and mRNA both declined significantly after WYC-209 treatment in HGC-27 and AGS cells. It has been proved that WNT4 is usually over-expressed in multiple tumor tissues, like breast cancer,Citation29 colorectal cancer,Citation30 and so on, and this contributes to cancer cell differentiation and tumor progression by activating β-catenin or β-catenin-independent pathways.Citation31 And the activation of the WNT4/β-catenin pathway contributes to EMT progression and then induces enhanced cell motility,Citation30 which further elaborated our results that WYC-209 down-regulated the WNT4 expression, then influenced the expression of β-catenin through WNT4/β-catenin pathway, as such, inhibited EMT process. This conclusion was further indicated because of the recovered WYC-209-induced suppression of GC progression both in vitro and in vivo after WNT4 overexpression treatment.
As mentioned, retinoids play their anti-cancer role via binding RAR. There are two subfamilies of receptors, one is RARs, binding naturally occurring retinoids and another is retinoid X receptors (RXRs). RARs own three isoforms: RARα, RARβ, and RARγ, respectively.Citation32 Of note, Different retinoids may have different affinities to RARs and RXRs. It has been proved that ATRA binds RARs with better affinity,Citation33 yet 9-cis retinoic acid can activate both RARs and RXRs.Citation34 For WYC-209, researchers have indicated that the treatment of WYC-209 led to hyper-expression of RARα mRNA, but RARα-specific siRNAs breached the inhibitory effects of WYC-209 on TRCs, meaning that WYC-209 acts as an agonist of RARα.Citation27 On this basis, we explored further mechanisms by which WYC-209 works. Interestingly enough, we found that there are three probably binding sites of RARα on the WNT4 promoter, but only the mutation of site 3 recovered the luciferase activity both in AGS and HGC-27 cells after WYC-209 treatment, indicating that RARα could mediate the transcription of WNT4 by binding to WNT4 promoter at site 3 and this could be further proved by ChIP experiments. Therefore, we can conclude that WYC-209 inhibited GC malignant progression by down-regulating WNT4 through RARα.
Materials and methods
Cells
In this study, the normal human gastric epithelial cell line GES-1 (CL-0563) was purchased from Procell Bio (Wuhan, China), and the human gastric cancer cell lines HGC-27 (Procell Bio, CL-0107) and AGS (Procell Bio, CL-0022) were selected and cultivated in DMEM (Gibco, Waltham, MA, USA, 02-5062EJ) which containing 10% fetal bovine serum (FBS, Gibco, Waltham, MA, USA, 10099141C) at 37°C with 5% CO2 supplied in an incubator (Thermofisher, Waltham, MA, NO. 311).
Cell proliferation analysis
At first, for dose screening, the cell survival of HGC-27 under 0, 1, 2, 4, 6, 8, 10 μM of WYC-209 treatment and AGS cells under 0, 8, 16, 32, 40 μM of WYC-209 treatment for 24 h was examined by MTT assay. In short, MTT (Sigma-Aldrich, St. Louis, MO, USA, M2128) was dissolved in PBS at 1 mg/ml and added to each well (50 μl per well). After 3 h of reaction, 150 μl of dimethyl sulfoxide (DMSO) was added to dissolve the formed crystals. Finally, the OD value at 570 nm was determined using the microplate reader (Molecular Device, California, USA). Subsequently, HGC-27 and AGS cells with or without 8 μM of WYC-209 treated were allowed to attach at a density of 3000 cells/well in 96-well plates. Cells cultured for 24, 48, 72, and 96 h were collected for MTT assay.
Soft agar formation assay
The colony-forming ability of AGS and HGC-27 cells was measured by Soft agar formation assay. Generally, cells with or without 8 μM of WYC-209 treated were inoculated at 3000 cells/well into 6-well plates for 3 weeks, followed by methanol and crystal violet (0.5%) treatment for fixing and staining. Finally, the cell colonies were photographed and counted under a microscope (100X magnification) (Olympus Corp, Tokyo, Japan, Olympus IX73).
Tanswell experiments
The cell migration and invasion capacities were detected using transwell assay. Generally, cells with or without 8 μM of WYC-209 treated were suspended in Dulbecco’s modified eagle’s medium (DMEM) (serum-free). The suspensions were added into the upper chamber, additionally, the upper chamber was placed into 24-well plates with 500 μl of DMEM, which contained 10% FBS, supplied. 24 h later, 4% paraformaldehyde and 0.5% crystal violet (Sigma-Aldrich, St. Louis Missouri, USA) were applied to fix and stain cells migrating across the membrane successively. Lastly, a microscope (100X magnification) was used to photograph and the migration results were obtained based on three randomly selected fields.
To detect the cell invasion ability, the PET membrane of the transwell chamber was pre-coated with the matrigel (BD, Franklin, New Jersey, USA 356,234) in advance, and the further steps were the same as those of migration experiments.
Bioinformatics
The total RNA of HGC-27 cells was extracted after 8 μM of WYC-209 treatment for 24 h, and then RNA-seq was conducted by LC-BIO (Hangzhou, China) to screen differentially expressed genes (DEGs). The original count matrix was converted into FPKM, the gene expression of GDF1, CXCL14, GDF6, NODAL, IBSP, FGF18, FLT1, SHC3, and WNT4 from the converted FPKM matrix were extracted and conducted unpaired two-tailed Student’s t-test. R version 3.6.3 and R version 4.1.3 were utilized respectively for the heatmap and the volcano plot. According to the sequencing results, GO and KEGG enrichment analyses were performed by DAVID Bioinformatics Resources (https://david.ncifcrf.gov/). JASPAR (https://jaspar.genereg.net/) was utilized for screening the potential binding sites on the RARα promoter to WNT4.
qRT-PCR
The total RNA of cells after treatment was refined and reversely transcribed to cDNA by a cDNA synthesis kit (Takara, Kyoto, Japan, 6215B). PCR system was generated by SYBR (Takara, Kyoto, Japan, RR420L) and the procedure was performed on the ABI-7500 system (Thermo, Waltham, MA, USA 4,351,106). Finally, the mRNA expression levels can be analyzed from the Ct value measured by the 2−ΔΔCt method. β-actin worked as a control. The primer sequences are shown in .
Table 1. Sequences of qRT-PCR primers.
Western blot (WB)
The concentration of extracted proteins was measured by the BCA method.Citation35 WB experiments were operated referring to the published paper.Citation36 Additionally, blocking was performed with 5% skim milk. β-actin was the loading control. After incubated with the primary antibodies (shown in ) at 4 overnight and the following secondary antibodies at 26°C for 2 h, the protein bands were visualized using a chemiluminescent substrate kit (Bio-Rad, California, USA, NO.1705060) and photographed using BioDoc-it Imaging System (UVP, Upland, USA). Besides, the qualification of protein bands was conducted by Image J (National Institutes of Health, Bethesda, Maryland, USA).
Table 2. Information on the primary antibodies.
Transient transfection
Transfection was performed based on the pCDH-CMV-MCS-EF1-Puro vector (YouBio, Hunan, China, VT1480). To construct WNT4 overexpressed cell lines (HGC-27 and AGS), the human WNT4 CDS sequence was synthesized and cloned into the pCDH vector. Next, HGC-27 and AGS cells were transfected with WNT4 overexpressed plasmid by lipofectamine 2000 (Invitrogen, Waltham, USA 11,668–019) as the applier’s protocol.
Construction of WNT4 stably overexpressed cell lines
The WNT4 stably overexpressed cell line was constructed using lentivirus. The WNT4-overexpressed lentivirus and the control lentivirus were obtained from GeneChem (Shanghai, China). Then, the viruses (1.0 × 10Citation8 Tu·ml-1) were co-incubated with HGC-27 cells for 48 h, and puromycin (1 μg/ml, Thermo, A1113802) was used for positive screening.
Animal experiments
The nude mice were purchased from SLAC Laboratory Animal (Shanghai, China). Eighteen 4-week-old nude mice were grouped into three (n = 6) and injected with the WNT4 stably over-expressed HGC-27 cells or their control cells (2 × 107 cells per mouse) subcutaneously. Four weeks of feeding later, combined with WYC-209 treatment, mice were sacrificed (cervical dislocation) following the principles of animal welfare, the tumors were dissected, and the diameter and volume were measured and calculated. The animal experiments were performed following the procedures of the Guiding Principles for the Breeding and Use of Animals in China.
Immunohistochemistry (IHC)
IHC was carried out according to the published description.Citation37 In short, xenograft tumor samples were successively fixed, paraffin-embedded, and sliced thinly (about 5-μm-thick). Next, those sections were deparaffinized by xylene and alcohol, and then a 90%, 80%, and 70% gradient of alcohol was applied to remove xylene. The following steps were antigen retrieval and inactivation of endogenous peroxidase. Subsequently, the primary antibodies against β-catenin and WNT4 were applied separately to co-incubated with tumor sections at 4°C overnight, and then the secondary antibodies were added and incubated for 1 h at 26°C. Finally, after a fresh DAB solution is added, the staining results can be observed and photographed by a microscope.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay
A TUNEL assay kit (Vazyme, Nanjing, China, #A113) was obtained for the TUNEL assay. After those steps same as the previous steps of IHC, xylene was removed, then TUNEL experiments can be performed according to the protocol. DAPI was used for staining nuclei.
Dual-luciferase reporter
The 3’UTR of human WNT4 promoter mRNA with the three potential binding sites of RARα and corresponding mutant sequence were amplified. Next, these sequences were cloned into pGL3 vector (Promega, Madison, WI, USA) for pGL3-WNT4 WT, and pGL3-WNT4 MUT1, pGL3-WNT4 MUT2, and pGL3-WNT4 MUT3. Subsequently, treated with WYC-209, the HGC-27, and AGS cells were respectively transfected with the plasmids above using lipofectamine 2000, and the relative luciferase activity was demonstrated as the ratio of firefly luciferase activity to renilla luciferase activity.
Chromatin immunoprecipitation (ChIP)
A ChIP-Seq High Sensitivity Kit (Abcam, Cambridge, UK, ab185908) was prepared for this part. Generally, the HGC-27 cells were fixed by formaldehyde and lysed to extract the chromatin. After the chromatins were sheared, the RARα antibody was added to generate the immune complex. Next, the complexes were pelleted, and DNA was released and purified. Finally, qRT-PCR was performed for the input results. The sequence of primer was as follows: Site 3Forward: 5’-TAATAGGTGGTTTGAGGGC-3’
Reverse: 5’-TTCAGAAAGCATGCAGGTG-3’
Statistical analysis
GraphPad Prism 6.0 was used for statistical analysis, data in this study were all shown as mean ± standard deviation (S.D.) from three repeatable experiments. Furthermore, t-test and one-way ANOVA were performed separately for difference analysis between two and multiple groups. **P<0.01, *P<0.05.
Conclusion
In summary, our data revealed that WYC-209 showed an anti-tumor effect on GC both in vitro and in vivo by down-regulating the expression of WNT4 via RARα, making it a prospective strategy in antitumor therapy.
Author contributions
F. W and Z. Y provided the idea, F. W conceived and designed the experiments. Z. Q, W. L, X. C, and K. K performed the experiments and analyzed the data. Z. Q prepared and wrote the draft. F. W revised the manuscript. All authors reviewed the manuscript.
Ethical approval statement
The animal experiments were conducted in accordance with following the “Guiding Principles in the Care and Use of Animals” (China) and approved by the Laboratory Animal Ethics Committee of Zhejiang Provincial People’s Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated or analyzed during this study are shown in this published article.
Additional information
Funding
Notes on contributors
Zhenyuan Qian
Zhenyuan Qian received his PhD degree in Surgery from Zhejiang University in 2021. Currently, he is a deputy chief physician and master’s supervisor in the Department of General Surgery, Division of Gastrointestinal and Pancreatic Surgery at Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital of Hangzhou Medical College). His research focuses on the basic research on the pathogenesis and chemotherapy resistance of gastric cancer, the surgical minimally invasive treatment, and the comprehensive treatment of gastric cancer.
Wenfa Lin
Wenfa Lin received his master’s degree in Surgery from Zhejiang Chinese Medical University in 2023. Currently, he is working in the Department of Gastrointestinal Surgery at the Second Affiliated Hospital of Zhejiang Chinese Medical University. His research focuses on the diagnosis and treatment of gastrointestinal tumors.
Xufan Cai
Xufan Cai received his B.S. degree in Clinical Medicine from Qianjiang College of Hangzhou Normal University in 2021. Currently, he is a professional master’s student in Zhejiang Chinese Medical University, majoring in Surgery. His research focuses on the diagnosis and treatment of common diseases in general surgery and related basic research.
Jianzhang Wu
JianZhang Wu received his master degree in Surgery from Fudan University in 2022. Currently, he is working in the Department of Gastrointestinal & Pancreatic Surgery, Zhejiang Provincial People’s Hospital(Affiliated People’s Hospital of Hangzhou Medical College).His research focuses on the laparoscopic minimally invasive treatment of and basic research on drug resistance mechanism of gastrointestinal tumor.
Kun Ke
Kun Ke received his PhD degree in Surgery from the Fujian Medical University in 2021. Now, he is working in the Department of General Surgery, Division of Gastrointestinal and Pancreatic Surgery at Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital of Hangzhou Medical College). His research focuses on the diagnosis and treatment of gastrointestinal tumors and the related basic research.
Zaiyuan Ye
Zaiyuan Ye is a chief physician and doctoral supervisor in the Department of General Surgery, Division of Gastrointestinal and Pancreatic Surgery at Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital of Hangzhou Medical College). His research focuses on the. He is the director of Zhejiang Provincial Key Laboratory of Gastroenterology. His research focuses on the diagnosis and treatment of digestive tract tumors. In particular, he has profound attainments in the surgical treatment of digestive tract tumors.
Fang Wu
Fang Wu received his master’s degree in Surgery from Wenzhou Medical University in 2014. Currently, he is studying for a doctoral degree at Zhejiang University. He is also a resident physician working in the Department of General Surgery, Division of Gastrointestinal and Pancreatic Surgery at Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital of Hangzhou Medical College). His research focuses on the diagnosis and treatment of gastrointestinal tumors and the related basic research.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–12. doi:10.3322/caac.20107.
- Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42(2):211–7. doi:10.1016/j.gtc.2013.01.002.
- Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi:10.2147/CMAR.S149619.
- Oh DY, Bang YJ. Adjuvant and neoadjuvant therapy for gastric cancer. Curr Treat Options Oncol. 2013;14(3):311–20. doi:10.1007/s11864-013-0238-4.
- Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41(1):41–55. doi:10.1016/s1040-8428(01)00144-5.
- Freemantle SJ, Dragnev KH, Dmitrovsky E. The retinoic acid paradox in cancer chemoprevention. J Natl Cancer Inst. 2006;98(7):426–7. doi:10.1093/jnci/djj116.
- Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, Guidez F, De Simone M, Schiavone EM, Grimwade D, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65(19):8754–8765. doi:10.1158/0008-5472.CAN-04-3569.
- Dragnev KH, Petty WJ, Shah SJ, Lewis LD, Black CC, Memoli V, Nugent WC, Hermann T, Negro-Vilar A, Rigas JR, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res. 2007;13(6):1794–1800. doi:10.1158/1078-0432.CCR-06-1836.
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupé V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124(2):313–26. doi:10.1242/dev.124.2.313.
- Prakash R. The acute and chronic toxic effects of vitamin a. Am J Clin Nutr. 2006;84(2):462. author reply 462-3. doi:10.1093/ajcn/84.2.462.
- Nau H. Embryotoxicity and teratogenicity of topical retinoic acid. Skin Pharmacol Physiol. 1993;6(Suppl 1):35–44. doi:10.1159/000211162.
- Barnard JH, Collings JC, Whiting A, Przyborski SA, Marder TB. Synthetic retinoids: structure–activity relationships. Chem A Eur J. 2009;15(43):11430–11442. doi:10.1002/chem.200901952.
- Aouad P, Saikali M, Abdel-Samad R, Fostok S, El-Houjeiri L, Pisano C, Talhouk R, Darwiche N. Antitumor activities of the synthetic retinoid ST1926 in two-dimensional and three-dimensional human breast cancer models. Anticancer Drugs. 2017;28(7):757–770. doi:10.1097/CAD.0000000000000511.
- Chen J, Cao X, An Q, Zhang Y, Li K, Yao W, Shi F, Pan Y, Jia Q, Zhou W, et al., Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat Commun. 2018;9(1):1406. doi:10.1038/s41467-018-03877-7.
- Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, Zhang S, Hong Y, Yi H, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014;5:4619. doi:10.1038/ncomms5619.
- Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62(13):1285–98. doi:10.1016/j.addr.2010.07.003.
- Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19(1):145. doi:10.1186/s12943-020-01258-7.
- Shariati M, Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs. 2019;28(11):977–988. doi:10.1080/13543784.2019.1676726.
- Zhang L, Zhu Z, Yan H, Wang W, Wu Z, Zhang F, Zhang Q, Shi G, Du J, Cai H, et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. 2021;33(6):1111–1123 e4. doi:10.1016/j.cmet.2021.03.009.
- Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu C, Wang C, Ye L. Wnt/β-catenin signaling in cancers and targeted therapies. Sig Transduct Target Ther. 2021;6(1):307. doi:10.1038/s41392-021-00701-5.
- Zhang Q, Pan Y, Ji J, Xu Y, Zhang Q, Qin L. Roles and action mechanisms of WNT4 in cell differentiation and human diseases: a review. Cell Death Discov. 2021;7(1):287. doi:10.1038/s41420-021-00668-w.
- Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol Mech Dis. 2011;6(1):345–364. doi:10.1146/annurev-pathol-011110-130303.
- Muindi J, Frankel SR, Miller WH Jr., Jakubowski A, Scheinberg DA, Young CW, Dmitrovsky E, Warrell RP Jr. Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid “resistance” in patients with acute promyelocytic leukemia. Blood. 1992;79(2):299–303. doi:10.1182/blood.V79.2.299.299.
- Bahmad HF, Samman H, Monzer A, Hadadeh O, Cheaito K, Abdel-Samad R, Hayar B, Pisano C, Msheik H, Liu YN, et al. The synthetic retinoid ST1926 attenuates prostate cancer growth and potentially targets prostate cancer stem-like cells. Mol Carcinog. 2019;58(7):1208–1220. doi:10.1002/mc.23004.
- Borthwick AD, Goncalves MB, Corcoran JPT. Recent advances in the design of RAR alpha and RAR beta agonists as orally bioavailable drugs. A review. Bioorg Med Chem. 2020;28(20):115664. doi:10.1016/j.bmc.2020.115664.
- Dragnev KH, Ma T, Cyrus J, Galimberti F, Memoli V, Busch AM, Tsongalis GJ, Seltzer M, Johnstone D, Erkmen CP, et al. Bexarotene plus erlotinib suppress lung carcinogenesis independent of KRAS mutations in two clinical trials and transgenic models. Cancer Prev Res (Phila). 2011;4(6):818–828. doi:10.1158/1940-6207.CAPR-10-0376.
- Qi F, Qin W, Zhang Y, Luo Y, Niu B, An Q, Yang B, Shi K, Yu Z, Chen J, et al., Sulfarotene, a synthetic retinoid, overcomes stemness and sorafenib resistance of hepatocellular carcinoma via suppressing SOS2-RAS pathway. J Exp Clin Cancer Res. 2021;40(1):280. doi:10.1186/s13046-021-02085-4.
- Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Sci. 2013;341(6146):651–4. doi:10.1126/science.1239278.
- Vouyovitch CM, Perry JK, Liu DX, Bezin L, Vilain E, Diaz JJ, Lobie PE, Mertani HC. WNT4 mediates the autocrine effects of growth hormone in mammary carcinoma cells. Endocr Relat Cancer. 2016;23(7):571–85. doi:10.1530/ERC-15-0528.
- Yang D, Li Q, Shang R, Yao L, Wu L, Zhang M, Zhang L, Xu M, Lu Z, Zhou J, et al. WNT4 secreted by tumor tissues promotes tumor progression in colorectal cancer by activation of the Wnt/beta-catenin signalling pathway. J Exp Clin Cancer Res. 2020;39(1):251. doi:10.1186/s13046-020-01774-w.
- Li X, Li Z, Wang J, Li Z, Cui H, Dai G, Chen S, Zhang M, Zheng Z, Zhan Z, et al. Wnt4 signaling mediates protective effects of melatonin on new bone formation in an inflammatory environment. FASEB J. 2019;33(9):10126–10139. doi:10.1096/fj.201900093RR.
- Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci USA. 1993;90(1):30–34. doi:10.1073/pnas.90.1.30.
- Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330(6149):624–9. doi:10.1038/330624a0.
- Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6(3):329–44. doi:10.1101/gad.6.3.329.
- Rogatsky E. Pandora box of BCA assay. Investigation of the accuracy and linearity of the microplate bicinchoninic protein assay: analytical challenges and method modifications to minimize systematic errors. Anal Biochem. 2021;631:114321. doi:10.1016/j.ab.2021.114321.
- Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. doi:10.1155/2014/361590.
- Magaki S, Hojat SA, Wei B, So A, Yong WH. An introduction to the performance of immunohistochemistry. Methods Mol Biol. 2019;1897:289–298. doi:10.1007/978-1-4939-8935-5_25.