ABSTRACT
Background
CASC21 was reported to be a hotspot gene in cervical cancer. The relationship between CASC21 genetic polymorphisms and cervical cancer has not been reported. Genetic factors influence the occurrence of cervical cancer. Thus, we explored the correlation between CASC21 polymorphisms and cervical cancer.
Methods
A total of 973 participants within 494 cervical cancer cases and 479 healthy controls were recruited. Five single nucleotide polymorphisms (SNPs) in the CASC21 gene were genotyped using the Agena MassARRAY platform. Chi-squared test, logistic regression analysis, odds ratio (OR), multifactor dimensionality reduction (MDR), and 95% confidence interval (95%CI) were used for data analysis.
Results
In the overall analysis, rs16902094 (p = .014, OR = 1.86, 95% CI = 1.12–3.08) and rs16902104 (p = .014, OR = 1.86, 95% CI = 1.12–3.09) had the risk-increasing correlation with the occurrence of cervical cancer. Stratification analysis showed that rs16902094 and rs16902104 were still associated with cervical cancer risk in the subgroups with age > 51, BMI < 24 kg/m2, smokers, and patients with cervical squamous cell carcinoma. MDR analysis displayed that rs16902094 (.49%) and rs16902104 (.52%) were the main influential attribution factor for cervical cancer risk.
Conclusion
Our finding firstly determined that two CASC21 SNPs (rs16902094, rs16902104) were associated with an increased risk of cervical cancer, which adds to our knowledge regarding the effect of CASC21 on cervical carcinogenesis.
1. Introduction
Cervical cancer is one of the most important causes of female deaths worldwide.Citation1 Cervical cancer has a high incidence (8%) compared to other developed countries with a 6.5% incidence rate globally and a low survival rate in China (59.8% Age-standardized 5-year relative survival).Citation1,Citation2 According to the Global Cancer Observatory 2018 database(https://gco.iarc.fr/), China accounts for a third of cervical cancer cases worldwide.Citation3 The most common symptoms of cervical cancer are contact or irregular vaginal bleeding, or increased leucorrhea after menopause.Citation4 The main risk factors for cervical cancer are age, virus infections, sexually transmittable infections, smoking, and other factors.Citation5 It has been shown that the progression of cancer and the occurrence of tumors is related to the polymorphism of gene loci, including cervical cancer.Citation6 IL1R2 and TNF genetic polymorphisms increase the risk of cervical cancer in Uygur women in China.Citation7,Citation8 The polymorphism in the CCR5 promoter region can affect the occurrence of cervical cancer in the Chinese Han population.Citation9 TP53 Codon 72 polymorphism was reported to be also associated with cervical cancer.Citation10 However, the association of a large number of loci with the risk of cervical cancer has not yet been studied.
Cancer susceptibility 21 (CASC21) is a FOXP1-induced long non-coding RNA (lncRNAs) for cancer susceptibility, located on homo sapiens chromosome 8 (8q24.21). There are currently very few studies on it. Abnormal expression of cyclin-CDK is one of the hallmarks of cancer. CASC21 is a hotspot gene for HPV integration in RNA samples of cervical cancer.Citation11 These studies suggested that CASC21 might play a key role in cervical cancer tumorigenesis. Mutations in long non-coding RNAs are closely associated with the development of cancer. Many studies have shown that lncRNA polymorphisms are closely related to the development of cervical cancer.Citation12–14 Therefore, we speculate that CASC21 polymorphisms may be related to the occurrence of cervical cancer. Previously, rs16902094 was associated with susceptibility to prostate cancer in several European populations.Citation15 An association between rs13281615 and rs1562430 polymorphisms and breast cancer susceptibility was reported.Citation16,Citation17 However, no studies have been done on the relationship between CASC21 and the occurrence of cervical cancer.
These five SNPs (rs16902094, rs16902104, rs13281615, rs1562430, and rs2392780) were selected based on the following: 1) minor allele frequency (MAF) > .05 in the Chinese Han population from 1000 Genomes Chinese Han Beijing population and dbSNP database; 2) Hardy-Weinberg equilibrium (HWE) > .05, and the call rate for genotyping > 99.5%; 3) the related literature of CASC21 polymorphisms.Citation15–17 In this study, the aim was to investigate the relationship between CASC21 single nucleotide polymorphisms (SNPs) and the risk of cervical cancer in the Han population from northwest China.
2. Result
2.1. Study population
In this study, 494 patients with cervical cancers and 479 controls were enrolled. The basic information about the cases and controls was displayed in . The mean age of cases and controls was 51.65 ± 9.84 years and 51.54 ± 9.46 years, respectively. There were no differences in age (p = .860), body mass index (BMI, p = .192), smoking (p = .930), and drinking (p = .674) between the two groups. In the case group, there were 197 cases (39.9%) of stage I-II, 196 cases (39.7%) of stage III-IV, and 101 cases (20.4%) of deletion. Of the 494 patients, 171 (34.6%) had squamous cell carcinoma.
Table 1. The information of all participants.
2.2. Association between SNPs in CASC21 and cervical cancer risk
The allele, MAF, and other information of CASC21 polymorphisms (rs16902094, rs16902104, rs13281615, rs1562430, and rs2392780) were shown in . All SNPs were consistent with HWE. The results of genotyping displayed that the genotyping success rate of each SNP was > 99.8%. The allele frequencies of rs16902094-G and rs16902104-T in the case group (.267 and .265) were higher than that in the control group (.225 and .227), and rs16902094-G (p = .033, Odd ratio (OR) = 1.25, 95% confidence interval (CI) = 1.02–1.54) and rs16902104-T (p = .048, OR = 1.23, 95% CI = 1.00–1.52) had the risk-increasing correlation with the occurrence of cervical cancer. HaploReg database displayed that these polymorphisms might be related to promoter/enhancer histone marks, DNAse, motifs changed, NHGRI/EBI GWAS hits, and selected eQTL hits.
Table 2. Basic information and allele frequencies of the five selected SNPs in CASC21.
2.3. Associations between genotype frequencies and cervical cancer
The association of selected SNPs with the risk of cervical cancer was analyzed by four genotype models (). Rs16902094 in CASC21 contributed to the increased risk of cervical cancer in the co-dominant (p = .042, OR = 1.91, 95% CI = 1.14–3.20), recessive (p = .014, OR = 1.86, 95% CI = 1.12–3.08), and log-additive (p = .039, OR = 1.24, 95% CI = 1.01–1.51) models. Rs16902104 GG genotype might have a higher risk of cervical cancer in the codominant (p = .045, OR = 1.90, 95% CI = 1.13–3.18) and recessive (p = .014, OR = 1.86, 95% CI = 1.12–3.09) models. We also analyzed the remaining SNPs (rs13281615, rs1562430, and rs2392780), and found no significant correlation.
Table 3. Genetic model analyses of five selected SNPs in CASC21 and the risk of cervical cancer.
2.4. Stratified analysis of CASC21 polymorphisms and the risk of cervical cancer
We stratified the cases and the control group by age, BMI, smoking, and drinking to eliminate the influence of confounding factors (). In the subgroup (age >51), rs16902094 (p = .018, OR = 2.43) and rs16902104 (p = .018, OR = 2.43) were found to be significantly correlated with the susceptibility of cervical cancer. Among subjects with BMI <24 kg/m2, rs16902094 (codominant: p = .043, OR = 2.16; and recessive: p = .014, OR = 2.23) and rs16902104 (codominant: p = .041, OR = 2.16; and recessive: p = .013, OR = 2.24) might confer to the risk-increasing effect on the occurrence of cervical cancer. Stratified analysis by smoking, rs16902094 and rs16902104 were related to the higher risk of cervical cancer in smokers under the codominant (p = .026, OR = 2.84; and p = .026, OR = 2.84), recessive (p = .012, OR = 2.65; and p = .012, OR = 2.65), and log-additive (p = .017, OR = 1.43; and p = .017, OR = 1.43) models, respectively. Moreover, we also observed the association of rs16902094 with the occurrence of cervical cancer (p = .042, OR = 2.06) in nondrinkers.
Table 4. Risk analysis of CASC21 and cervical cancer in different genetic models according the stratification by age, BMI, smoking, and drinking.
Furthermore, genetic model analyses for five selected SNPs in CASC21 and the risk of cervical squamous cell carcinoma were performed, and the results were shown in . Rs16902094 (p = .024, OR = 1.39) and rs16902104 (p = .041, OR = 1.35) were correlated with the increased susceptibility of cervical squamous cell carcinoma.
Table 5. Genetic model analyses for five selected SNPs in CASC21 and the risk of cervical squamous cell carcinoma.
There was no significant difference between the remaining SNPs and cervical cancer in the stratification analysis (data not shown).
2.5. FPRP analysis
False positive reporting probability (FPRP) analysis () exhibited the positive results of rs16902094 and rs16902104 for cervical cancer susceptibility in the overall analysis with .1 prior probability level and FPRP < .2. The effects of rs16902094 and rs16902104 on cervical cancer risk in smokers. In addition, rs16902094 was also significantly associated with the risk of cervical squamous cell carcinoma with an FPRP value of < .2 despite a prior probability level of .1.
Table 6. False-positive report probability for the associations of CASC21 variants with the risk of cervical cancer.
2.6. The association between CASC21 haplotypes and the risk of cervical cancer
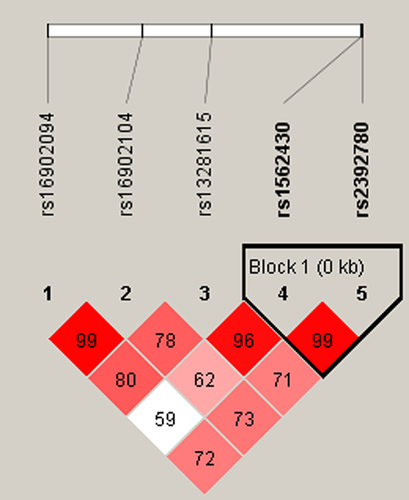
Moreover, haplotype analysis was performed to estimate the association between CASC21 haplotypes and the risk of cervical cancer. As shown in , rs1562430 and rs2392780 are in linkage disequilibrium. The haplotype frequency distribution was shown in . The association between the CASC21 haplotype and cervical cancer susceptibility was investigated; however, there was no significant relationship between these haplotypes and cervical cancer risk (p > .05).
Table 7. Haplotype analysis for the effect of CASC21 haplotypes on the risk of cervical cancer.
2.7. SNP – SNP interaction analysis using MDR
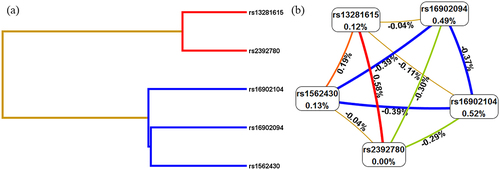
Multifactor dimensionality reduction (MDR)was used to analyze the SNP – SNP interaction between these five SNPs (rs16902094, rs16902104, rs13281615, rs1562430, and rs2392780) in the occurrence of cervical cancer. As shown in , the best model was the combination of rs13281615 and rs2392780 (Bal. Acc. CV testing = .5334, CV consistency = 10/10. p = .0022). The Dendogram plot in and the Fruchterman Rheingold plot in represented the interaction between SNPs. The red color in indicated that there is a synergistic effect between the two SNPs, while the blue color indicates a negative correlation between the two SNPs. The entropy interaction graphical model () revealed that rs13281615 and rs2392780 had significant synergistic interaction (.58%) sharing the positive information gain concerning cervical cancer, whereas rs16902094 (.49%) and rs16902104 (.52%) were the main influential attribution factor for cervical cancer risk.
Table 8. Summary of SNP – SNP interactions on the risk of cervical cancer analyzed by MDR method.
3. Discussion
In our study, we selected five SNPs on the CASC21 gene (rs16902094, rs16902104, rs13281615, rs1562430, and rs2392780) to explore the correlation between these polymorphisms and the risk of cervical cancer. Our results suggested that two SNPs (rs16902094, rs16902104) might contribute to the increased risk of cervical cancer in the Chinese Han population, especially in the subjects aged >51 years, population with BMI <24 kg/m2, smokers, and patients with cervical squamous cell carcinoma. Moreover, we also observed the association of rs16902094 with the occurrence of cervical cancer in nondrinkers. These results firstly found that the genetic polymorphism of CASC21 might play an important role in the occurrence of cervical cancer in the Han population from northwest China, which increases the understanding of the role of CASC21 in cervical carcinogenesis.
CASC21 might promote cell proliferation, regulate cell cycle, and enhance tumor metastasis in colon cancer Citation18,Citation19. CASC21 promotes the growth of cancer cells by regulating cyclin-dependent kinase 6(CDK6) Citation19. Downregulation of CDK6 can inhibit the proliferation ability of cervical cancer and promote the apoptosis of cervical cancer cells.Citation20 Little has been reported about the contribution of CASC21 variants to the susceptibility of tumors. This study is the first to show that two SNPs (rs16902094, rs16902104) might contribute to the increased risk of cervical cancer in the Chinese Han population. Rs16902094, located in the intron region was associated with susceptibility to prostate cancer in several European populations.Citation15 Rs16902094 and rs16902104 are adjacent to each other, both of which are on the CASC21 gene. According to our experimental results, our results firstly suggested that rs16902094, and rs16902104 might contribute to the increased risk of cervical cancer in the Chinese Han population. MDR analysis can be speculated that rs16902094 (.49%) and rs16902104 (.52%) were the main influential attribution factor for cervical cancer risk. Bioinformatics analysis suggested that the possible function of rs16902094 and rs16902104 might be related to promoter/enhancer histone marks, DNAse, motifs changed, NHGRI/EBI GWAS hits, and selected eQTL hits. This suggests that these loci may play a part in cervical cancer development by influencing CASC21 gene expression. However, this hypothesis requires to be explored by further functional research.
It is well known that genetic, environmental, and behavioral risk factors may affect cervical cancer development. Katrina V Fox, etc., the study also found that the risk of cervical cancer increases with age.Citation21 In the age stratification of more than 51 years old, the risk of disease of rs16902094 and rs16902104 has been significantly increased, which may partly reflect the age – gene interactions in the occurrence of cervical cancer.
Increased BMI has been considered to increase the risk of many cancers, including cervical cancer.Citation22 Underweight women had significantly lower cervical cancer screening rates compared to other BMI categories.Citation23 Both extremes of weight (underweight and overweight/obesity) were associated with worse survival in patients with cervical cancer.Citation24 Moreover, the lower risk of cervical precancer and higher risk of cervical cancer with increasing body mass index were observed.Citation25 Interestingly, rs16902094 and rs16902104 might confer the risk-increasing effect on the occurrence of cervical cancer among subjects with BMI <24 kg/m2. Based on these results, we speculated the age and BMI for the risk association of CASC21 polymorphisms with cervical cancer susceptibility.
Tobacco smoking is an important risk factor for cervical neoplasia. Smoking status, duration, and intensity are related to a twofold increased risk of high-grade cervical dysplasia and invasive carcinoma.Citation26 Our finding displayed that rs16902094 and rs16902104 were related to the higher risk of cervical cancer in smokers. Alcohol abuse can decrease pelvic control and survival in cervical cancer and increase the risk of cervical cancer.Citation27 Moreover, we also observed the association of rs16902094 with the occurrence of cervical cancer in nondrinkers. Therefore, the role of smoking, drinking, and heredity in the occurrence of cervical cancer needs to be confirmed in further studies.
There are still some limitations in our study, due to the sample size and race. The results only apply to the Han nationality in northwest China. We will continue to study the impact on other ethnic groups. Besides, due to insufficient collection of patients’ HPV information, the correlation between CASC21 and HIV infection in cervical carcinogenesis needs to be further studied.
4. Materials and methods
4.1. Ethics statement
We followed the Helsinki Declaration of the World Medical Association and subsequent amendments. This study has been approved by the Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region (Approval Document No: KY2020041053). All participants in this study were informed and signed informed consent forms.
4.2. Subjects
In order to ensure the accuracy and credibility of the research results, before we plan to conduct this study, we used G*power 3.1.9.7 software (https://stats.idre.ucla.edu/other/gpower/) to estimate the sample size of the case group and the control group through the independent sample T-test. The specific parameters we set are as follows: Tail=two, effect size d = .2; α error probability = .05; power (1-β err prob) = .85, allocation ratio N2/N1 = 1. This calculation yielded a sample consisting of at least 450 cases and 450 controls. In this study, a total of 973 participants within 494 cervical cancer cases and 479 healthy controls were recruited from People’s Hospital of Xinjiang Uygur Autonomous Region from April 2020 to April 2022, which is larger than the total sample size recommended by G*power and statistic power > 85%. In this study, 494 Han nationalities in northwest China unrelated blood samples were randomly collected. According to the diagnostic criteria,Citation28 all the patients were determined to have cervical cancer. The samples were collected from the People’s Hospital of Xinjiang Uygur Autonomous Region. All the patients have no history of radiotherapy or chemotherapy. In addition, 479 control samples were selected from the Han nationality in northwest China. All controls were confirmed by the pathology department to have negative cervical cytology and had no history of cancer, infection, or acute/chronic lesions. Demographic and clinical information were collected from the standardized questionnaires and medical records.
4.3. Variant selection and genotyping
We randomly selected rs16902094, rs16902104, rs13281615, rs1562430, and rs2392780 loci in CASC21 gene for genotyping based on the following: 1) MAF > .05 in the Chinese Han population from 1000 Genomes Chinese Han Beijing population and dbSNP database; 2) HWE > .05, and the call rate for genotyping > 99.5%; 3) the related literature of CASC21 polymorphisms associated with the risk of prostate and breast cancers,Citation15–17 but no reports about cervical cancer. HaploReg v4.1 (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php) were used for the potential function of these polymorphisms.
Peripheral blood samples (5 mL) were collected in EDTA-coated tubes. The GoldMag DNA Purification Kit (GoldMag Co. Ltd., Xi’an, China) was used to extract genomic DNA, which was quantified by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA) and stored at −20°C. The MassARRAY platform is based on MALDI-TOF (matrix-assisted laser desorption/ionization – time of flight) mass spectrometry.Citation29,Citation30 The analytical accuracy of MALDI-TOF MS is quite high, .1–.01% of the determined mass. Agena MassARRAY system (Agena, San Diego, CA, U.S.A.) was used for SNPs genotyping. The specific steps included: PCR amplification of the target sequence, and mass spectrometry to distinguish nucleic acid molecules of different molecular weights. The primer-related information was shown in Table S1. In addition, this study also set up double wells for each sample to ensure the accuracy of the results. About 10% of the samples were randomly selected and re-genotyped for quality control, and the concordance rate was 100%.
4.4. Statistical analyses
PLINK software was used to perform statistical analysis on the original data. The chi-square test compared the differences in SNP genotypes between the case and control groups, and p < .05 indicated that the locus might have a significant correlation with the risk of cervical cancer. HWE test was performed on the control group, and p > .05 indicated that the population was genetically balanced, and the survey data was reliable. OR and 95% CI adjusted by age, BMI, smoking, and drinking were used to evaluate the influence of CASC21 variants on the risk of cervical cancer under the different models (codominant, dominant, recessive, and log‐additive).Citation31 FPRP analysis was used to evaluate the noteworthy associations of the significant findings. The FPRP threshold is .2, and the prior probability is .1, which is used to evaluate the significant association of significant findings. D’ values for pairwise linkage disequilibrium (LD) plots were generated by Haploview software (version 4.2), and the correlation of CASC21 haplotypes with cervical cancer risk was evaluated by logistic regression model. MDR is specifically designed to identify correlations between increased risk and genetic variation for complex diseases in humans. MDR version 3..2 was applied to explore the association between the risk of cervical cancer and multi-SNP interactions. Cross-validation can reduce false positive results caused by random grouping of data to a certain extent, which is usually used to assess the statistical significance of MDR models.
5. Conclusion
In our study, we found that both rs16902094 and rs16902104 polymorphisms increase the risk of cervical cancer in the Han population from northwest China. Our findings add to our knowledge regarding the effect of CASC21 on cervical carcinogenesis.
Authors’ contributions
Lili Han conceived and designed the experiments; Lili Han and Mireayi Shataer performed the experiments; Jing Liu collected samples; Chengyong Wu and Mayinuer Niyazi analyzed the data; Jing Liu contributed reagents/materials/analysis tools; Chengyong Wu drafted the paper, Mayinuer Niyazi revised the paper. All authors read and approved the final version of the manuscript.
Ethics statement
This study has been approved by the Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region (KY2020041053).
supplement table.docx
Download MS Word (15.8 KB)Acknowledgments
Thank all participants for their contributions to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2024.2322207
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–9. doi:10.3322/caac.21660.
- Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6(5):e555–e567. doi:10.1016/S2214-109X(18)30127-X.
- Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8(2):e191–e203. doi:10.1016/S2214-109X(19)30482-6.
- Voltaggio L, McCluggage WG, Iding JS, Martin B, Longacre TA, Ronnett BM. A novel group of HPV-related adenocarcinomas of the lower anogenital tract (vagina, vulva, and anorectum) in women and men resembling HPV-related endocervical adenocarcinomas. Mod Pathol. 2020;33(5):944–952. doi:10.1038/s41379-019-0437-z.
- Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229–232. doi:10.1016/j.jnma.2020.03.002.
- Ramachandran D, Dörk T. Genomic risk factors for cervical cancer. Cancers. 2021;13(20):5137. doi:10.3390/cancers13205137.
- Niu F, Wang T, Li J, Yan M, Li D, Li B, Jin T. The impact of genetic variants in IL1R2 on cervical cancer risk among Uygur females from China: a case-control study. Mol Genet Genomic Med. 2019;7(1):e00516. doi:10.1002/mgg3.516.
- Farbod M, Karimi MZ, Heiranizadeh N, Seifi NS, Akbarian JBM, Jarahzadeh HM, Neamatzadeh H. Association of TNF-α -308G>A polymorphism with susceptibility to cervical cancer and breast cancer - a systematic review and meta-analysis. Klin Onkol. 2019;32(3):170–180. doi:10.14735/amko2019170.
- Liu S, Chen J, Yan Z, Dai S, Li C, Yao Y, Shi L. Polymorphisms in the CCR5 promoter associated with cervical intraepithelial neoplasia in a Chinese han population. BMC Cancer. 2019;19(1):525. doi:10.1186/s12885-019-5738-6.
- Isakova J, Vinnikov D, Bukuev N, Talaibekova E, Aldasheva N: Capital TE, ER C. Cyrillic53 codon 72 polymorphism and human papilloma virus-associated cervical cancer in Kyrgyz women. Asian Pac J Cancer Prev. 2019;20(4):1057–1062. doi:10.31557/APJCP.2019.20.4.1057.
- Li W, Qi Y, Cui X, Huo Q, Zhu L, Zhang A, Tan M, Hong Q, Yang Y, Zhang H, et al. Characteristic of HPV integration in the genome and transcriptome of cervical cancer tissues. Biomed Res Int. 2018;2018:1–7. doi:10.1155/2018/6242173.
- Li N, Cui Z, Huang D, Gao M, Li S, Song M, Wang Y, Tong L, Yin Z. Association of LINC00673 rs11655237 polymorphism with cancer susceptibility: a meta-analysis based on 23,478 subjects. Genomics. 2020;112(6):4148–4154. doi:10.1016/j.ygeno.2020.07.015.
- Wang Y, Luo T. LINC00673 rs11655237 polymorphism is associated with increased risk of cervical cancer in a Chinese population. Cancer Control. 2018;25(1):1073274818803942. doi:10.1177/1073274818803942.
- Zhong Q, Lu M, Yuan W, Cui Y, Ouyang H, Fan Y, Wang Z, Wu C, Qiao J, Hang J. Eight-lncRNA signature of cervical cancer were identified by integrating DNA methylation, copy number variation and transcriptome data. J Transl Med. 2021;19(1):58. doi:10.1186/s12967-021-02705-9.
- Gudmundsson J, Sulem P, Gudbjartsson DF, Blondal T, Gylfason A, Agnarsson BA, Benediktsdottir KR, Magnusdottir DN, Orlygsdottir G, Jakobsdottir M, et al. Genome-wide association and replication studies identify four variants associated with prostate cancer susceptibility. Nat Genet. 2009;41(10):1122–1126. doi:10.1038/ng.448.
- Zhang Y, Zeng X, Lu H, Ji H, Zhao E, Li Y. Association between 8q24 (rs13281615 and rs6983267) polymorphism and breast cancer susceptibility: a meta-analysis involving 117,355 subjects. Oncotarget. 2016;7(42):68002–68011. doi:10.18632/oncotarget.12009.
- Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, Seal S, Ghoussaini M, Hines S, Healey CS, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42(6):504–507. doi:10.1038/ng.586.
- Zhang Q, Bian Y, Zhu Y, Wan L, Kong L, Hu J, Yang M, Li L, Liu B, Qian X. Integrative analysis and validation of dysregulated long non-coding RNAs in colon cancer. J Cell Mol Med. 2020;24(4):2610–2621. doi:10.1111/jcmm.14974.
- Gong T, Li Y, Feng L, Fang M, Dai G, Huang X, Yang Y, Liu S. CASC21, a FOXP1 induced long non-coding RNA, promotes colorectal cancer growth by regulating CDK6. Aging. 2020;12(12):12086–12106. doi:10.18632/aging.103376.
- Hu QL, Xu ZP, Lan YF, Li B. Li B: miR-636 represses cell survival by targeting CDK6/Bcl-2 in cervical cancer. Kaohsiung J Med Sci. 2020;36(5):328–335. doi:10.1002/kjm2.12181.
- Fox KV, Shah CA, Swisher EM, Garcia RL, Mandel LS, Gray HJ, Swensen RE, Goff BA. An evaluation of cervical cancer in women age sixty and over. Gynecol Oncol. 2008;109(1):53–58. doi:10.1016/j.ygyno.2007.12.028.
- Poorolajal J, Jenabi E. The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev. 2016;25(3):232–238. doi:10.1097/CEJ.0000000000000164.
- Charkhchi P, Schabath MB, Carlos RC. Breast, cervical, and colorectal cancer screening adherence: effect of low body mass index in women. J Women Health. 2020;29(7):996–1006. doi:10.1089/jwh.2019.7739.
- Clark LH, Jackson AL, Soo AE, Orrey DC, Gehrig PA, Kim KH. Extremes in body mass index affect overall survival in women with cervical cancer. Gynecol Oncol. 2016;141(3):497–500. doi:10.1016/j.ygyno.2016.03.035.
- Clarke MA, Fetterman B, Cheung LC, Wentzensen N, Gage JC, Katki HA, Befano B, Demarco M, Schussler J, Kinney WK, et al. Epidemiologic evidence that excess body weight increases risk of cervical cancer by decreased detection of precancer. J Clin Oncol. 2018;36(12):1184–1191. doi:10.1200/JCO.2017.75.3442.
- Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, Margall N, Bosch FX, de Sanjosé S, Dillner J, Gram IT, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer. 2014;135(2):453–466. doi:10.1002/ijc.28666.
- Mayadev J, Li CS, Lim J, Valicenti R, Alvarez EA. Alcohol abuse decreases pelvic control and survival in cervical cancer: an opportunity of lifestyle intervention for outcome improvement. Am J Clin Oncol. 2017;40(5):451–457. doi:10.1097/COC.0000000000000187.
- Stumbar SE, Stevens M, Feld Z. Cervical cancer and its precursors: a preventative approach to screening, diagnosis, and management. Prim Care. 2019;46(1):117–134. doi:10.1016/j.pop.2018.10.011.
- Oeth P, Del Mistro G, Marnellos G, Shi T, van den Boom D. Qualitative and quantitative genotyping using single base primer extension coupled with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MassARRAY). Methods Mol Biol. 2009;578:307–343.
- Ellis JA, Ong B. The MassARRAY(®) system for targeted SNP genotyping. Methods Mol Biol (Clifton, NJ). 2017;1492:77–94.
- Liu J, Yang Y, Li H, Liu Y, Sun Y, Wu J, Xiong Z, Jin T. IL1R2 polymorphisms are associated with an increased risk of esophageal cancer. Curr Mol Med. 2020;20(5):379–387. doi:10.2174/1566524019666191025091204.