Stem cells have a high and constitutive endogenous activity of the DNA damage response (DDR) pathwayCitation1 which may differ in some aspects from the function of the DDR pathway in somatic cells. It is likely that such stemness-related functions of the DDR pathway may play a role in the resistance to genotoxic insult seen in stem cells and cancer cells.Citation2 Lately, it has become clear that the context of the DDR pathway is more complex than first anticipated, and as many other signaling pathways it plays roles beyond its first recognized function as guardian of the genome. One example is the finding that the DDR pathway axis ATR-Chk1 plays a critical function in undamaged cells during mitosis.Citation3 An additional function for H2AX in the DDR pathway, was provided by Andäng et al,Citation1 who showed that it mediates proliferation control in stem cells under non-genome damaging conditions. In recent publications by the Hallböök research group, another unexpected function of components of the DDR pathway was reported, again unrelated to direct genome protection. The Hallböök group previously showed that Chk1 in the DDR signaling axis controls the last mitosis in cells undergoing interkinetic nuclear migration (INM) in the developing eye neuroepithelium.Citation4 However, not all cells undergo INM in the neuroepithelium, and the group has now in a carefully executed study, continued to show that Lim1+ horizontal progenitor cells (HPCs), that undergo their last mitosis on the basal side of the retina without INM, have a significantly attenuated dependence on the DDR pathway.Citation5
Figure 1. Schematic of signaling pathways involving the DNA damage response (DDR) pathway in apical and basal cells in the developing neuroepithelium. Only apical cells undergo interkinetic nuclear migration (INM) and are cell cycle arrested by an activated DDR pathway.
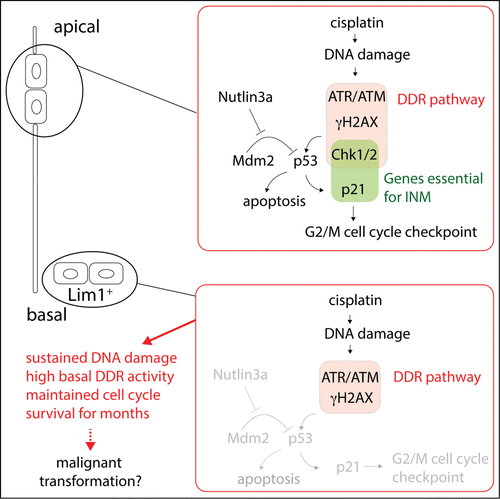
Interestingly, during activation of the ATR-Chk1 (DDR pathway) axis in the developing retinal progenitor cells by Cisplatin, an inducer of ATR/p53 signaling,Citation6 the apical cells were cell cycle arrested as expected, while the basal HPCs cells were not.Citation5 The HPCs nevertheless had a functional p53/p21 pathway regulating cell cycle and apoptosis indicating an inability of the activated DDR pathway to engage the p53 pathway and ultimately apoptosis. However, despite the lack of cell cycle arrest and apoptosis in response to Cisplatin, the DDR pathway was induced and HPCs had elevated γH2AX levels indicating a robust induction of a DDR pathway response. This situation, which has previously been reported in other stem cellsCitation1 and in cancer pre-lesions,Citation7 suggests an ability of the HPCs to withstand genotoxic insult and maintain proliferation. Such ability may be a factor that under for example environmental challenging conditions, allows for accumulation of mutations that later may drive development of cancer initiating cells. On the other hand, critical progenitor cell types may maintain capacity to divide and generate new cells during developmental or repair processes – a last resort for survival when most other more sensitive cells are lost to apoptosis. The attenuated DDR pathway response is then a trade off between immediate survival and risking cancer at a later time.
References
- Andang M, et al. Nature 2008; 451:460-4; PMID:18185516; http://dx.doi.org/10.1038/nature06488
- Squatrito M, Holland EC. Cancer Res 2011; 71:5945-9; PMID:21917735; http://dx.doi.org/10.1158/0008-5472.CAN-11-1245
- Wilsker D, Bunz F. Cell Cycle 2009; 8:1161-3; PMID:19282670; http://dx.doi.org/10.4161/cc.8.8.8148
- Shirazi Fard S, et al. Cell Cycle 2014; 13:408-17; PMID:24247150; http://dx.doi.org/10.4161/cc.27200
- Shirazi Fard S, et al. Cell Cycle 2014; 13:3698-706; PMID:25483080; http://dx.doi.org/10.4161/15384101.2014.964985
- Sangster-Guity N, et al. Oncogene 2011; 30:2526-33; PMID:21258400; http://dx.doi.org/10.1038/onc.2010.624
- Bartkova J, et al. Nature 2005; 434:864-70; PMID:15829956; http://dx.doi.org/10.1038/nature03482
