Abstract
Degeneration or loss of GABAergic neurons frequently may lead to many neuropsychiatric disorders such as epilepsy and autism spectrum disorders. So far no clinically effective therapies can slow and halt the progression of these diseases. Cell-replacement therapy is a promising strategy for treatment of these neuropsychiatric diseases. Although increasing evidence showed that mammalian somatic cells can be directly converted into functional neurons using specific transcription factors or miRNAs via virus delivery, the application of these induced neurons is potentially problematic, due to integration of vectors into the host genome, which results in the disruption or dysfunction of nearby genes. Here, we show that mouse fibroblasts could be efficiently reprogrammed into GABAergic neurons in a combined medium composed of conditioned medium from neurotrophin-3 modified Olfactory Ensheathing Cells (NT3-OECs) plus SB431542, GDNF and RA. Following 3 weeks of induction, these cells derived from fibroblasts acquired the morphological and phenotypical GABAerigic neuronal properties, as demonstrated by the expression of neuronal markers including Tuj1, NeuN, Neurofilament-L, GABA, GABA receptors and GABA transporter 1. More importantly, these converted cells acquired neuronal functional properties such as synapse formation and increasing intracellular free calcium influx when treated with BayK, a specific activator of L-type calcium channel. Therefore, our findings demonstrate for the first time that fibroblasts can be directly converted into GABAergic neurons without ectopic expression of specific transcription factors or miRNA. This study may provide a promising cell source for the application of cell replacement therapy in neuropsychiatric disorders.
Introduction
GABAergic neurons, an important type of inhibitory interneurons, play a crucial role in neural circurity and activity. In contrast to excitatory interneurons, these inhibitory cortical interneurons specifically release the neurotransmitters gamma-aminobutyric acid (GABA) and glycine which actively participate in diverse physiology activities in brain.Citation1 Especially, GABAergic neurons exert pivotal roles in maintaining the balance between the excitatory and inhibitory inputs with regards to cortical circuits. The disruption and alteration of the balance between excitation and inhibition inputs in the cortical circuitry generally result in neuropsychiatric disorders such as epilepsy, autism spectrum disorders and schizophrenia.Citation2 Currently, there is no curative treatment for these neuropsychiatric disorders and existing pharmacotheraphy is extremely erratic and may cause severe side-effects that decrease the quality of life for these patients. Nevertheless, increasing evidence suggest that stem cell implantation might be a promising strategy for the treatment of neurodegenerative disorders. Comparatively, autologous cell therapy is regarded as perspective candidate for cell-based clinical therapies owing to several unique advantages including no immune rejection and no ethical problems. More strikingly, numerous studies also demonstrated that somatic cells can be dedifferentiated into induced pluripotent stem cells (iPSCs) and subsequently re-differentiated into mature specific neurons, which is believed to be a breakthrough strategy for cell-based therapy in restoring the functions of injured nervous system and neurological disorder.Citation3-7 However, iPSCs derived from patient-specific somatic cells are restricted in clinical studies owing to its low differentiation efficiency, time consuming and tumorigenicity.Citation8-11 Thus, people are seeking for alternative strategies such as direct conversion of somatic cells to neurons, by which neurons derived from somatic cells do not go through a pluripotent state. In this way, much lower tumorigenicity and shortened procedure for dedifferentiating and re-differentiation can be achieved. Toward this direction, somatic cells forced to ectopic express of a combination of transcriptional factors or/and neuronal-specific miRNAs (miR9/9* and miR124) have been shown to be directly converted into neural progenitor cells (NPCs),Citation12 neural stem cells (NSCs),Citation13-15 neurons,Citation16,17 or special sub-type of neurons such as glutamatergic neurons, GABAerigic neurons,Citation18,19 motor neurons,Citation20 and dopaminergic (DA) neurons.Citation21-23 In addition, several studies showed that repression of a single RNA binding protein PTB can also directly convert fibroblasts into neurons by up-regulating multiple neuronal-specific transcriptional factors and miR124.Citation24 Unfortunately, these strategies may be problematic because using viral vectors are likely to integrate the genes into the host genome, possibly resulting in the disruption or dysfunction of nearby genes. To overcome these problems, it is of unusual importance to discover new methods for a direct conversion of somatic cells into sub-type of neurons without exogenous gene delivery.
To find out a novel strategy, we attempted to utilize a combination of the conditioned medium of OECs, plus SB431542 and growth factors capable of reprogramming somatic cells to our desired neuronal subtypes based on the following rationales. Firstly, our recent study demonstrated that human OECs and RA cooperate to effectively promote neural differentiation of human bone marrow stromal stem cells (hBMSCs).Citation25 OECs are specialized glial cells from the olfactory bulb and the olfactory mucosa. Numerous studies have revealed that OECs are a promising cell source available for transplantation to improve the functional recovery and axon regeneration following damage to central nervous system (CNS) or peripheral nerve system (PNS) attributing to its unique characteristics.Citation26,27 That is, OEC can produce some neurotrophic factors such as BDNF, NT-3, NT-4/5, NGF, GDNF and VEGF, which effectively promote neuronal cell survival, growth and development.Citation28,29 Additionally, OECs could secrete desert hedgehog (DHH), which promotes cell fate determination of motor neurons and differentiation of interneuron cells in the spinal cordCitation30 and can also regulate the gene expression of FGFs and BMP in CNS.Citation31 Secondly, apart from OECs, we simultaneously supplemented SB431542, an inhibitor of TGF-β pathway, to the induced medium, based on previous reports indicating that the inhibition of TGF-β pathway can enhance the neuronal differentiation of ESCsCitation32 and strengthen the direct conversion process of somatic cells into neural cells.Citation33
We demonstrate in this report that by using a combined medium composed of conditioned medium from NT3-OEC plus SB431542, GDNF and RA, we were able to successfully induce a direct conversion of fibroblasts into GABAergic neurons. Therefore, our findings may provide a novel strategy for the treatment of specific neurological diseases via autologous cell replacement.
Results
Preparation and characterization of MEF cells
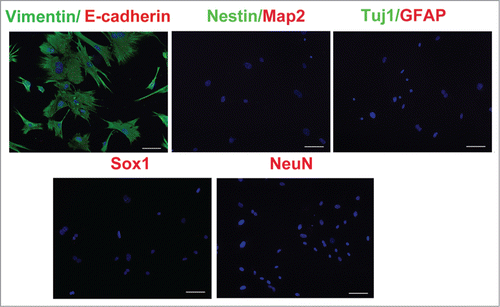
To make sure that the MEF cells that we prepared were pure fibroblasts, we first prepared primary MEFs as described previouslyCitation19 from embryonic day (E) 13.5 wild-type C57BL/6 mice embryos removing spinal cord and cultured the cells in tissue culture flasks. Briefly, MEF were expanded up to passage 2 or 3 for the following conversion experiment in a DMEM medium containing 10% FBS, 1% penicillin/streptomycin. At passage 2, we needed to determine whether the MEFs contained any neuron stem/progenitor cells or neural cells by immunostaining assay with Nestin, Sox1, Tuj-1, Map2, NeuN and GFAP antibodies, respectively. As shown in , neither NSCs/NPCs nor neural cells in the MEFs were found in the MEF culture. All cultured cells were vimentin positive and E-cadherin negative, indicating that the MEFs that we prepared were not contaminated with neural cells or NSCs/NPCs.
Preparation and characterization of the conditioned medium from the NT3-OECs
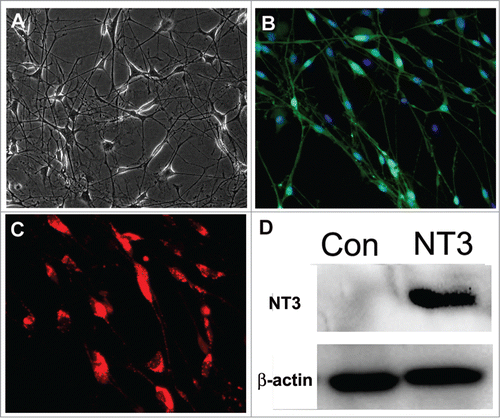
To obtain the conditioned medium, we isolated and cultured OECs in DF12 medium containing 10% FBS. After 2–3 weeks, the cells extended very thin and long processes, and eventually formed a more intricate net (). In addition, immunostaining analysis showed that about 80–90% of the cells immunoreactive to p75 (OEC specific marker) were OECs (). Next, OECs were infected with lentivirus particles containing NT3-RFP. Overexpression of NT3 was indicated in the OECs as they displayed the expression of reporter red fluorescence protein (RFP) (). Consistently, western blotting also confirmed that NT3 level was remarkably higher in the NT3-OECs than that of the control cells ().
Induction of GABAergic neurons from fibroblasts with a combination of the conditioned medium of NT3-OECs plus SB431542, GDNF and RA
To examine the efficiency of our combinational induction system in the trans-differentiation of fibroblasts, we re-plated MEFs onto poly-L-lysine-coated dishes in serum-containing DMEM medium and changed with a combination of 1% B27 neural basal medium supplemented with the conditioned medium of NT3-OECs at a 1:1 ratio plus SB431542, GDNF and RA next day. After 1 week, many MEFs in the cultures exhibited small, compact cell bodies with monopolar or bipolar projections and neuron-like morphology (). 2–3 weeks later, increasing number of cells appeared a typical neuron-like morphology, consisting of multiple neurites with intricate branching (). Moreover, immunostaining analysis showed that, under our induction system, these neuron-like cells were remarkably Tuj-1 positive (). By quantification of positive cells, the percentage of Tuj-1 positive cells over the total cells in the cultures were about 8.2 ± 2.32%, 26.5 ± 1.48%, and 32.3 ± 2.1% at 1, 2 and 3 weeks post-induction, respectively (). These results indicated that MEFs can be directly converted to neurons under this induction system, and the numbers of neuron-like cells were significantly elevated when exposed to the induction medium for 2–3 weeks (). To further verify the GABAergic characteristics of the neuron-like cells derived from MEFs, more neuronal markers were also used to verify their biological phenotypic traits. Quantitative real-time PCR analysis showed that Tuj-1, NeuN and Neurofilament-L (NF-L) were expressed and dramatically increased at 3 weeks post-induction (). Consistently, protein gel blots revealed that protein expression of the 3 molcules were also significantly elevated compared to the control cultures without any treatments (). In addition, immunostaining results also confirmed that the neuron-like cells at 3 weeks post-induction were NeuN positive, Neurofilament-L and MAP2 double positive ().
Figure 3. Direct conversion of MEFs into GABAergic neurons. (A) Phase-contrast microscopic images of the induced cells derived from MEFs and immunostaing with Tuj-1 and GABA antibodies of the induced cells at 1–3 weeks. Scale bar, 50μm. (B) Quantification of Tuj-1 positive cells and GABA positive cells: The percentages of Tuj-1 positive cells over the total cells, and GABA positive cells over Tuj-1 positive cells. Five to 6 representative visual fields for each of the groups were counted. (C) Quantitative real-time PCR analysis of the mRNA levels of Tuj-1, NeuN, Neurofilament L (NF-L), GAT1 and GABA receptors from MEF-derived cells derived from MEFs at 1–3 weeks. MEFs in normal cultures were used as control group, Con. The level of mRNA in MEFs was set as 1. Data were collected from at least 3 separate experiments and are shown as means ± standard deviation (SD). *P < 0.05, **P < 0.01 compared to controls. (D) Western blot analysis of the protein expression of MEF-derived cells with Tuj-1, NeuN, NF-L, GABA, GAT1, synapsin and β-catenin antibodies.W, week. scale bar, 50 μm.
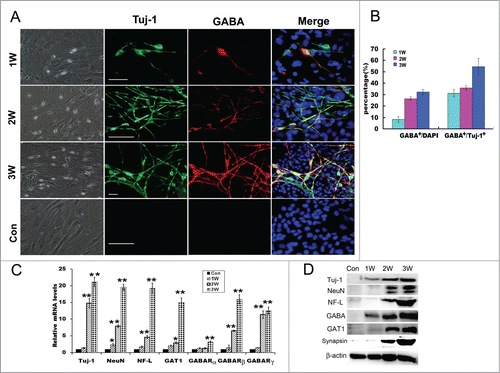
Figure 4. Generation of GABAergic neurons from fibroblasts with the combination of the conditioned medium of NT3-OECs, plus SB431542, GDNF and RA. (A) Immunostaining of the MEF-derived cells at 3 weeks post-induction with Map2, NF-L, NeuN, GATA and GAT1 antibodies. (B) Phase-contrast microscopic of MEF-derived cells and double staining of MEF-derived cells with GATA/Tuj1 or NF-L/GFAP when the MEFs were cultured with the combination of NT3-OECs with SB431542 (SB) or 1% B27 medium supplemented with SB, GDNF and RA for 3 weeks. scale bar, 50 μm.
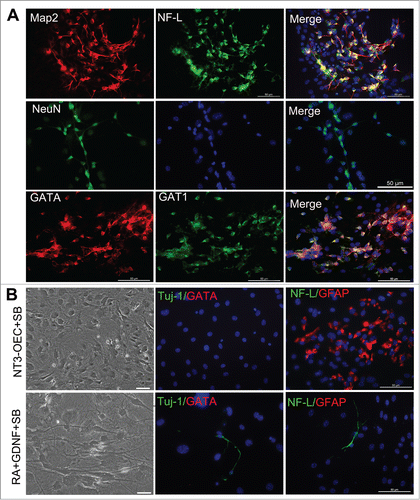
Given that the MEFs are directly converted to neurons, next we are interested in whether these MEFs could be directly converted to a specific type of neurons in the induction culture system, we conducted immunostaining assay with GABA and Tuj-1 antibodies. As shown in , double positive cells at 2 weeks or 3 weeks post-induction were much more in the experimental cultures than that in the control group. Moreover, cell count analysis also showed that a higher percentage of GABAergic neurons was observed in the experimental cultures (31.2 ± 3.1% for 1 week, 35.8 ± 1.9% for 2 weeks, and 54.5 ± 7.2% for 3 weeks) (). To determine the characteristics of GABAergic neurons of the neuron-like cells at 3 weeks post-induction, immnostaining was performed, the results showed that GATA positive cells were also GATA transporter 1 (GAT1) positive (). Consistent with the immunostaining results, quantitative real-time PCR further verified that the mRNA levels of GABA receptors (GABARα, GABARβ and GABARγ) and GAT1 were significantly increased, especially for GABARβ, GABARγ and GAT1 (). In parallel, western blot analysis also confirmed that MEFs-derived cells evidently express GABA and GAT1 after 2–3 weeks of induction (). These results suggest that MEFs were efficiently converted to GABAergic neurons under a combination of the conditioned medium of NT3-OECs plus SB431542, GDNF and RA.
To find out whether MEFs at 3 weeks post-induction can give rise to other subtype of neurons or glial cells with this induction system, we performed double-immunostaining with Tuj-1/VGlut1, Tuj-1/TH and GFAP/NF-L antibodies, respectively. About 10% Tuj-1 positive cells were VGlut1 positive (Fig. S1), indicating that lower proportions of the MEF-derived cells were induced into glutamatergic neurons compared to conversion to GABAergic neurons as 32.3 ± 2.1% GABA/Tuj-1 double positive cells were seen (). Strikingly, no dopaminergic neurons were found when induced with the same system (Fig. S1). Nevertheless, a few of MEFs were also converted into glial cells (Fig. S1). These results suggested the induction system could efficiently promote conversion of MEFs to GABAergic neurons.
To clarify which components of the combined medium of NT3-OECs plus SB431542, GDNF and RA cause the conversion from mEFs into GABAergic neurons, different induction conditions were used as follows: (1) The conditioned medium of NT3-OECs; (2) The conditioned medium of NT3-OECs with 1% B27 medium and SB431542; (3) The conditioned medium of NT3-OEC with 1% B27 medium, GDNF and RA; (4) 1% B27 medium with SB431542, GDNF and RA. We found that while virtually all MEFs were apoptotic or dead under the conditioned medium of NT3-OEC with or without GDNF and RA at one week (Data not shown), a number of flat cells appeared in the conditioned medium of NT3-OEC with SB431542, and some of the flat cells became GFAP positive, but Tuj-1 and GATA negative (). In the cultures treated with SB431542, GDNF and RA, only a very small number of cells appeared neuron-like morphology and were Tuj-1 positive but GABA negative, which was much less than what was seen in the cultures treated with NT3-OEC, SB431542, GDNF and RA (). Taken together, these results suggest that the components of NT3-OEC, SB431542, GDNF and RA in our induction medium might exert a synergistic effect in the conversion of MEFs to GABAergic neurons.
Generation of functional neuron-like cells from fibroblasts under the combination of NT3-OECs, SB431542, GDNF and RA
To detect whether the neurons derived from fibroblasts are likely functional, we firstly performed the immunostaining and protein gel blotting experiments to determine the expression of synapsin in the induced cells. As shown in , immunostaining results showed that synapsin positive cells overlapped with Tuj-1 positive cells, displaying punctate distribution in the cell bodies and neurites after 2–3 weeks of induction. The data above indicated that the synapse has formed and these MEF-derived neurons are likely functional. Consistent with the above immunostaining results, the protein levels of synapsin could also be detected after 2–3 weeks of induction, exhibiting a much stronger blot band of synapsin at 3 weeks compared to that of the control cultures by western blotting ().
Figure 5. Characterization of the neuron-like cells derived from MEFs. (A) Immunostaining of the neuron-like cells at 1–3 weeks post-induction with synapsin and Tuj-1 antibodies. scale bar, 50 μm. (B) Functional characterization of the L-type Calcium channel. The panels show typical calcium imagines response observed in neuron-like cells at 2–3 weeks post-induction. 1F/F0 represents the ratio of fluorescence intensity of cells for 0s and an indicated time. Bay-K (10 μM) in the presence or absence of nifedipine (5 μM) was added at the marked time point. (C) The represent figures of calcium imaging of the induced neurons or MEFs with or without nifedipine following in the treatment with BayK. The total number of cells analyzed in each experiment was 40–50 cells, represent results were present. scale bar, 20 μm.
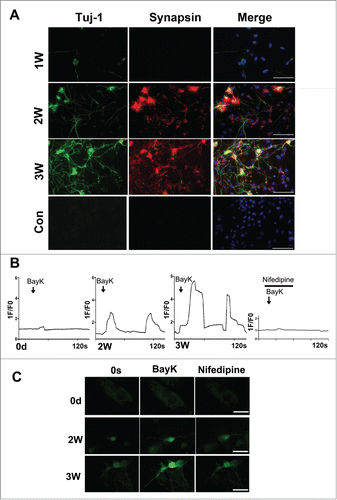
Since a neuron-specific membrane-related electrical activity is the most important indicator of functional neuronsCitation34-36, we assessed this function of the neuron-like cells at different time points using calcium imaging. As shown in the , the neuron-like cells display the increasing neuron-specific calcium influx when treated with BayK, as demonstrated by about 3-fold and 6-fold increase of the fluorescence intensity at 2 and 3 weeks post-induction, respectively. More importantly, these converted cells could display 2–3 times stronger fluorescence intensity after withdrawal of BayK and were blocked by nifedipine, a specific L type calcium channel blocker. In contrast, there was not a significant increase of fluorescence intensity when treated with BayK in cultured MEFs. Based on the above data, the converted neuron-like cells indeed acquired the functional properties of neurons.
Discussion
Although recent studies demonstrated that induced neurons can be directly converted from somatic cells using a combination of transcription factors such as Ascl1, Brn2 and Mytl1,Citation19 and that specific neurons subtypes can be generated by various combination of transcription factors,Citation20,22 the strategy is likely problematic in future clinic application because of a potential risk of the integration of viral vectors into the host genome. In this study, we demonstrated a new protocol that a combination of the conditioned medium of NT3-OEC with SB431542, GDNF and RA can directly convert fibroblasts into GABAergic neurons, avoiding the risk of delivery mediated by viral vectors containing exogenous reprogramming factors.
Previous studies reveal that OEC can differentiate MSCs into desired type of neurons.Citation25,37 The possible underlying mechanism of OECs participation in inducing MSCs to neuronal cells is associated with secretion of specific growth factors and cell adhesion molecules including laminin, fibronectin and tenasin, some of which play an important role in neural cell differentiation, development and survival.Citation38 Accordingly, we used the conditioned medium of OECs as an important component in our induction system. In addition, we utilized NT3-OECs for the following 2 reasons: First, NT3 promotes neural cell proliferation, differentiation, survival and maturation;Citation39 Second, NT3 can further promote cell fate specification and the growth factor secretion.Citation40 Furthermore, we supplemented GDNF and RA in our induction system based on previous findings that GDNF promotes neuron survival,Citation41 differentiation, neurite outgrowth and dopamine release.Citation42 Finally, RA was also added into the induction system as it is well-known that RA, an widely used inducer in neural differentiation of stem cells,Citation43 can bind to specific RA receptors and therefore regulates the expression of some transcription factors responsible for cell patterning and differentiation, such as Nolz1, which reduces the self-renewal of neural stem/progenitor cells and promotes neuronal differentiation and growth.Citation44 To clarify which component of the combined medium play a crucial role in the conversion of MEFs to GABAergic neurons, MEFs were induced with various combinations of NT3-OEC, SB431542, GDNF and RA for 3 weeks. Our results revealed that the combination of RA, SB431542 and GDNF could only induce a small number of MEFs into pan-neurons without GABAergic neurons (). Surprisingly, when the 3 components were supplemented to the conditioned medium of NT3-OEC, this induction system could significantly enhance the conversion efficiency, displaying 32.3 ± 2.1% Tuj-1 positive cells and 54.5 ± 7.2% GABA positive cells among Tuj-1 positive cells () These results suggested that the components of NT3-OEC, SB431542, GDNF and RA in our induction system might exert a synergetic effect in direct conversion of MEFs to GABAergic neurons.
It should be pointed out that there are several unique advantages of the direct conversion of MEFs into specific subtype of neurons by our combined conditioned medium plus additional supplements. First, our induction culture system can directly convert fibroblasts into neurons with a high reprogramming efficiency of 32.3 ± 2.1% (). Secondly, the high proportion of GABAergic neurons is generated using this culture system, and the percentage of GABA positive cells over Tuj-1 positive cells reaches 54.5 ± 7.2% (), suggesting that the present reprogramming approach is relatively excellent, and particularly useful as a cell resource for cell-replacement therapy for GABAergic neuronal loss-mediated neurologic disorders. Thirdly, our induction approach remarkably shortens the process of conversion of fibroblasts, as demonstrated by the expression of neuron marker Tuj-1 and GABAergic neuron marker GABA after only 1–3 week of induction and the increase of numbers of Tuj-1 positive cells and GABA positive cells when exposed to the combinational medium for 2–3 weeks (). Fourthly, these neuron-like cells derived from MEFs also possess functional neuronal characteristics including synapse formation and significant calcium influx into the neuron-like cells when treated with BayK. As shown in the , the neuron-like cells display the neuron-specific calcium influx in a time-dependent manner as demonstrated by about 3-fold and 6-fold increases in the fluorescence intensity at 2 or 3 weeks post-induction, respectively following addition of BayK. Moreover, the intracellular free calcium influx has gradually exhibited a relatively stable tendency, indicating the converted neuron became mature as the induction time proceeds.
Taken together, we have presented a novel protocol for the first time to induce somatic cells to be directly converted into GABAregic neurons without involving exogenous gene transfer. This method can be useful for cell-replacement therapy for many neuropsychiatric disorders.
Materials and Methods
Cell culture
Mouse embryonic fibroblasts (MEFs) were isolated by removing spinal cord from C57BL/6 mouse fetus on embryonic day 13.5 (E13.5) as previously describedCitation19 and cultured in DMEM with 10% FBS, 1% penicillin/streptomycin (all from life technology). MEFs were used at passage 2 or 3 (P2 or P3) for the following reprogramming experiments.
Mouse OECs were isolated from the olfactory bulb and cultured as previously describedCitation45 in DF12 medium containing 10% FBS, 2 mM glutamine, and 1% penicillin/streptomycin.
Lentiviral vector construction, viral particles production and transduction
Mouse NT3 cDNA was amplified using high-fidelity DNA polymerase, cloned into the lentiviral vector pHIV-H2BmRFP (from addgene) with HapI and Xba1 sites and constructed a plasmid named as NT3-RFP. Viral particles were generated by transient transfection of lentiviral plasmids using the X-tremeGENE 9 DNA Transfection Reagent (Roche) into the 293T cells. The supernatant was collected after 48 and 72 hours of transfection, and further concentrated by ultracentrifugation to produce virus stocks, which were stored at −80°C for the subsequent use. OECs grown in the DF12 medium with 10% FBS were infected with the viral particles. Twenty-four hours later, the culture medium was replaced with DF12 medium supplemented 1% G5 (all from life technology). Subsequently, the supernatant of the OECs was harvested once a day until the day 5. Finally these supernatant was mixed, centrifuged as conditioned medium, and stored −20°C for the next experiments.
Direct conversion of MEFs to neurons
For induction of MEFs with the combined medium, we re-plated MEFs at P2 or P3 on poly-L-lysine (Sigma) coated dishes or coverslips in DMEM medium with 10% FBS. After 24 hours, the cultures were switched to 1% B27 neural basal medium (life technology) supplemented with 50% (v/v) conditioned medium of NT3-OECs, 10 μM SB431542 (Sigma), 20 ng/ml GDNF (R&D) and 5 uM RA (Sigma). Then the cultures were kept for additional 2–3 weeks for the following experiments.
Immunofluorescence staining
Cells grown on coverslips were fixed with 4% paraformaldehyde (PFA, Sigma), blocked with 3% BSA, incubated overnight at 4°C with the primary antibodies as follows: rabbit anti-Vimentin (1:200, BD), mouse anti-E-cadherin (1:200, BD), mouse anti-Nestin (1:100, Cell Signaling Technology), Rabbit anti-Pax6 (1:50, Abcam), rabbit anti-GFAP (1:500, DAKO), mouse anti-Tuj1 (1:200, Sigma), rabbit anti-synapsin (1:200, Abcam), rabbit anti-GΑΒΑ (1:100, Abcam), mouse anti-p75 (1:200, Abcam), rabbit NeuN (1:100, Cell Signaling Technology), rabbit Map2 (1:100, Cell Signaling Technology), mouse Neurofilament-L (NF-L, 1:100, Cell Signaling Technology), mouse VGlut1 (1:100, millipore), chicken TH (1:200, GeneTex) and guinea pig GATA transporter 1 (GAT1,1:100, Synaptic System). After washing with PBS 3 times, cells were incubated with the corresponding secondary antibodies conjugated with Alexa Fluor 488 and Alexa Fluor 594-conjugaged at 400-fold dilution for 2 hrs in 3% BSA in PBS. Followed by DAPI (Sigma) counterstaining, the coverslips were mounted and immunostaining images were visualized by using an inverted fluorescence microscope.
Quantitative real-time PCR
RNA was extracted by using the Direct-Zol™ RNA Mini kit (ZYMO RESEARCH) and reversely transcribed to cDNA using PrimedScript™ RT Master Mix (Takara). The mRNA levels were quantified by SYBR Green-based quantitative real-time PCR (Takara) using an ABI Prism 7900 HT (Applied Biosystems). Results were confirmed in at least 3 separate analyses. The sequences for gene primers are shown in supplementary information, Table S1.
Western blot assays
Western blot analyses were performed as previously reported.Citation46 The following antibodies were used to detect specific proteins: mouse anti-Tuj-1 (Sigma), rabbit anti-GABA (Abcam), rabbit anti-NeuN (Cell Signaling Technology), mouse anti-Neurofilament-L (Cell Signaling Technology), guinea pig anti-GAT1 (Synaptic System) and mouse anti-β-Actin (Sigma).
Calcium imaging
To substantiate the neuron-like cell neuronal properties, we performed assessment of calcium imaging of single cells as previously describedCitation47,48 Briefly, MEFs were plated onto autoclaved coverglass chambers coated with 0.01% poly-L-lysine for 4 hours and then the cultures were changed with above induction system and were maintained for additional 1–3 weeks. To measure Ca2+ influx and efflux, the cultures were incubated with 2 μM Ca2+-sensitive dye Fluo4-AM (Life technology, dissolved in DMSO) in 1% B27 neural basal medium for 30 min at 37°C, and then were washed twice with extracellular medium (EM) (140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 10 mM HEPES, pH 7.4, 10 mM glucose), 5 min each time. Measurements of calcium imaging were performed at 37°C in EM with confocal Zeiss microscopy with perfusion system, using image sizes of 256 × 256 pixels and acquisition rates of one frame per 2 second. The fluorescence intensity for a single cell was measured and set as 1 F at indicated time points, and the fluorescence intensitys at 0s was set as F0. To better observe Ca2+ influx, 10 μm BayK (BayK8644, Stemgent), a specific L-type calcium channel activator was applied for 10 s on the cells and then repeatedly wash with EM for 10 min. Next, 5 μm nifedipine (Abcam), a specifi L-type calcium channel blocker, was used to block Ca2+ influx before the addition of Bay-K. For calcium imaging in Ca2+-free extracellular medium, cells were treated for 1 min with Ca2+-free medium containing 1 mM EGTA without CaCl2 before the Calcium imaging experiments. Control measurements in the presence of extracellular Ca2+-containing medium were performed before and after experiments in Ca2+-free medium.
Statistical analysis
Data derived from at least 3 independent experiments were presented and analyzed with the SPSS 13.0 software. The relative mRNA levels were quantified by normalization to GAPDH expression. Statistical significances were tested by Student's t-test. P-values < 0.05 (*) were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1060382_supplemental_files.doc
Download MS Word (31 KB)Funding
The study was supported by funds to H. Xu from the Chinese Ministry of Science and Technology (2012CB967903), to H. Yang from the National Nature Science Foundation of China (81371411) and to W.-Q.G. from the Chinese Ministry of Science and Technology (2012CB966800 and 2013CB945600), the National Natural Science Foundation of China (81130038 and 81372189), Science and Technology Commission of Shanghai Municipality (Pujiang Program), Shanghai Education Committee Key Discipline and Specialty Foundation (J50208), Key Discipline and Specialty Foundation of Shanghai Health Bureau and KC Wong foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Reference
- Dreifuss JJ, Kelly JS, Krnjevic K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res 1969; 9:137-54; PMID:5346460; http://dx.doi.org/10.1007/BF00238327
- Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13:107-20; PMID:22251963
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009; 136:964-77; PMID:19269371; http://dx.doi.org/10.1016/j.cell.2009.02.013
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917-20; PMID:18029452; http://dx.doi.org/10.1126/science.1151526
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663-76; PMID:16904174; http://dx.doi.org/10.1016/j.cell.2006.07.024
- Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, Shim JW, Jo AY, Kim BW, Lee H, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest 2011; 121:2326-35; PMID:21576821; http://dx.doi.org/10.1172/JCI45794
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A 2008; 105:5856-61; PMID:18391196; http://dx.doi.org/10.1073/pnas.0801677105
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods 2010; 7:197-9; PMID:20139967; http://dx.doi.org/10.1038/nmeth.1426
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009; 4:472-6; PMID:19481515; http://dx.doi.org/10.1016/j.stem.2009.05.005
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 2007; 27:3069-77; PMID:17376968; http://dx.doi.org/10.1523/JNEUROSCI.4562-06.2007
- Soldner F, Jaenisch R. Medicine. iPSC disease modeling. Science 2012; 338:1155-6; PMID:23197518; http://dx.doi.org/10.1126/science.1227682
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A 2011; 108:7838-43; PMID:21521790; http://dx.doi.org/10.1073/pnas.1103113108
- Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012; 10:465-72; PMID:22445517; http://dx.doi.org/10.1016/j.stem.2012.02.021
- Kim SM, Flasskamp H, Hermann A, Arauzo-Bravo MJ, Lee SC, Lee SH, Seo EH, Storch A, Lee HT, Scholer HR, et al. Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc 2014; 9:871-81; PMID:24651499; http://dx.doi.org/10.1038/nprot.2014.056
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012; 11:100-9; PMID:22683203; http://dx.doi.org/10.1016/j.stem.2012.05.018
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 2011; 9:113-8; PMID:21802386; http://dx.doi.org/10.1016/j.stem.2011.07.002
- Ang CE, Wernig M. Induced neuronal reprogramming. J Comp Neurol 2014; 522:2877-86; PMID:24771471; http://dx.doi.org/10.1002/cne.23620
- Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A 2005; 102:18183-8; PMID:16330768; http://dx.doi.org/10.1073/pnas.0506535102
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463:1035-41; PMID:20107439; http://dx.doi.org/10.1038/nature08797
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011; 9:205-18; PMID:21852222; http://dx.doi.org/10.1016/j.stem.2011.07.014
- Oh SI, Park HS, Hwang I, Park HK, Choi KA, Jeong H, Kim SW, Hong S. Efficient reprogramming of mouse fibroblasts to neuronal cells including dopaminergic neurons. ScientificWorldJournal 2014; 2014:957548; PMID:24991651
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011; 476:224-7; PMID:21725324; http://dx.doi.org/10.1038/nature10284
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 2011; 108:10343-8; PMID:21646515; http://dx.doi.org/10.1073/pnas.1105135108
- Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 2013; 152:82-96; PMID:23313552; http://dx.doi.org/10.1016/j.cell.2012.11.045
- Xie ST, Lu F, Zhang XJ, Shen Q, He Z, Gao WQ, Hu DH, Yang H. Retinoic acid and human olfactory ensheathing cells cooperate to promote neural induction from human bone marrow stromal stem cells. Neuromolecular Med 2013; 15:252-64; PMID:23288654; http://dx.doi.org/10.1007/s12017-012-8215-9
- Lu J, Ashwell K. Olfactory ensheathing cells: their potential use for repairing the injured spinal cord. Spine (Phila Pa 1976) 2002; 27:887-92; PMID:11935115; http://dx.doi.org/10.1097/00007632-200204150-00021
- Roloff F, Ziege S, Baumgartner W, Wewetzer K, Bicker G. Schwann cell-free adult canine olfactory ensheathing cell preparations from olfactory bulb and mucosa display differential migratory and neurite growth-promoting properties in vitro. BMC Neurosci 2013; 14:141; PMID:24219805; http://dx.doi.org/10.1186/1471-2202-14-141
- Ramon-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull 1998; 46:175-87; PMID:9667810; http://dx.doi.org/10.1016/S0361-9230(97)00463-2
- Wewetzer K, Grothe C, Claus P. In vitro expression and regulation of ciliary neurotrophic factor and its alpha receptor subunit in neonatal rat olfactory ensheathing cells. Neurosci Lett 2001; 306:165-8; PMID:11406321; http://dx.doi.org/10.1016/S0304-3940(01)01891-2
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999; 23:713-24; PMID:10482238; http://dx.doi.org/10.1016/S0896-6273(01)80030-1
- Liem KF, Jr., Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development 2000; 127:4855-66; PMID:11044400
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009; 27:275-80; PMID:19252484; http://dx.doi.org/10.1038/nbt.1529
- Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kogler G, Muller FJ, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods 2012; 9:575-8; PMID:22484851; http://dx.doi.org/10.1038/nmeth.1972
- Yuste R, MacLean J, Vogelstein J, Paninski L. Imaging action potentials with calcium indicators. Cold Spring Harbor Protocols 2011; 2011:985-9; PMID:21807854; http://dx.doi.org/10.1101/pdb.prot5650
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron 2012; 73:862-85; PMID:22405199; http://dx.doi.org/10.1016/j.neuron.2012.02.011
- Zheng M, Cao P, Yang J, Xu XZ, Feng Z. Calcium imaging of multiple neurons in freely behaving C. elegans. J Neurosci Methods 2012; 206:78-82; PMID:22260981; http://dx.doi.org/10.1016/j.jneumeth.2012.01.002
- Tatard VM, D'Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, Bouckenooghe T, Menei P, Montero-Menei CN, Schiller PC. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone 2007; 40:360-73; PMID:17085092; http://dx.doi.org/10.1016/j.bone.2006.09.013
- Yang H, He BR, Hao DJ. Biological roles of olfactory ensheathing cells in facilitating neural regeneration: a systematic review. Mol Neurobiol 2015; 51(1):168-79; PMID:24615159
- Zhou XF, Rush RA. Functional roles of neurotrophin 3 in the developing and mature sympathetic nervous system. Mol Neurobiol 1996; 13:185-97; PMID:8989769; http://dx.doi.org/10.1007/BF02740622
- Ohtsuka M, Soumiya H, Hanai M, Furukawa S, Fukumitsu H. Neurotrophin-3 influences the number and the laminar fate of cortical progenitors in the developing cerebral cortex of mice through the MEK/ERK1/2 signaling pathway. Biomed Res 2013; 34:231-9; PMID:24190235; http://dx.doi.org/10.2220/biomedres.34.231
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996; 380:252-5; PMID:8637574; http://dx.doi.org/10.1038/380252a0
- Johansson M, Friedemann M, Hoffer B, Stromberg I. Effects of glial cell line-derived neurotrophic factor on developing and mature ventral mesencephalic grafts in oculo. Exp Neurol 1995; 134:25-34; PMID:7672036; http://dx.doi.org/10.1006/exnr.1995.1033
- Zhou JM, Chu JX, Chen XJ. An improved protocol that induces human embryonic stem cells to differentiate into neural cells in vitro. Cell Biol Int 2008; 32:80-5; PMID:17945517; http://dx.doi.org/10.1016/j.cellbi.2007.08.015
- Urban N, Martin-Ibanez R, Herranz C, Esgleas M, Crespo E, Pardo M, Crespo-Enriquez I, Mendez-Gomez HR, Waclaw R, Chatzi C, et al. Nolz1 promotes striatal neurogenesis through the regulation of retinoic acid signaling. Neural Dev 2010; 5:21; PMID:20735826; http://dx.doi.org/10.1186/1749-8104-5-21
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 2000; 25:425-35; PMID:10719896; http://dx.doi.org/10.1016/S0896-6273(00)80905-8
- Xu HM, Liao B, Zhang QJ, Wang BB, Li H, Zhong XM, Sheng HZ, Zhao YX, Zhao YM, Jin Y. Wwp2, an E3 ubiquitin ligase that targets transcription factor Oct-4 for ubiquitination. J Biol Chem 2004; 279:23495-503; PMID:15047715; http://dx.doi.org/10.1074/jbc.M400516200
- Resende RR, da Costa JL, Kihara AH, Adhikari A, Lorencon E. Intracellular Ca2+ regulation during neuronal differentiation of murine embryonal carcinoma and mesenchymal stem cells. Stem Cell Dev 2010; 19:379-94; PMID:19032055; http://dx.doi.org/10.1089/scd.2008.0289
- Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-D-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience 2007; 146:1169-81; PMID:17418494; http://dx.doi.org/10.1016/j.neuroscience.2007.02.041