Bui et al. report in Cell Cycle 2017; 16:879–893 that androgen can induce a quiescent dormant state that is self-sustained in a cell-autonomous manner through a “hit and run” mechanism in androgen receptor-expressing prostate cancer cells. Thus, these authors suggest that repeated cycles of androgen deprivation and supplementation [i.e. bipolar androgen therapy (BAT)] could be effective for inhibiting the very early phase of metastasis development by inducing and/or reinforcing a self-sustained quiescent state in disseminated solitary cancer cells.
The results of Bui et al. thus complement our own rationale for on-going clinical trials of such BAT therapy in which castration resistant metastatic prostate cancer [mCRPC] patients continuously maintained on androgen ablation via luteinizing hormone releasing hormone agonist therapy are given intermittent cycles of pharmacologic levels of testosterone (T) via intramuscularly injection with 400mg of testosterone cypionate every 28 d to raises the serum T to > 1500 ng/dl by 2 d post-injection, with a decline to near castrate level (100 ng/dl) by 28 d post-injection.Citation1
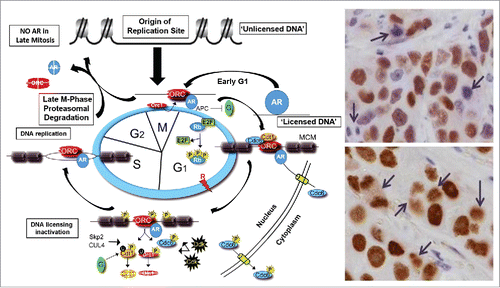
Our approach of bipolar cycling between pharmacological high followed by a rapid decline to castrate levels of T is based upon the coupled facts that during prostatic carcinogenesis, Androgen Receptor (AR) acquires an oncogene gain of function with regard to DNA replication licensing required for malignant cell proliferation and that during the subsequent progression during androgen ablation therapy to mCRPC, AR protein expression is greatly (> 50–100 fold) increased.Citation2-6 During early G1 of the cell cycle, nuclear AR in mCRPC cells binds to DNA at origins of replication sites (ORS) as part of the origin of replication complex (ORC) complex that is needed for licensing DNA replication during the S-phase, left panel in . AR remains associated with the ORC during cell cycle progression until late mitosis when, as a DNA licensing factor, it must be removed via its degradation so that re-licensing can occur in the next cell cycle, upper right panel in Fig. 1.Citation2-6 In the presence of pharmacologic serum testosterone, increased ligand over-stabilizes ORC bound AR prevents its sufficient degradation, lower right panel in . This lack of sufficient mitotic AR degradation due to ligand dependent over stabilization inhibits DNA relicensing, resulting in cell death in the subsequent cycle.Citation3,5
Figure 1. Overview of role and timing of Androgen Receptor (AR) in licensing DNA Replication in metastatic Castration Resistant Prostate Cancer Cells. For full details see reference 4. When androgen is low during androgen ablation, AR is degraded appropriately during late mitosis such that mitotic figures (denoted by arrows in upper right panel) do not contain detectable AR protein as determined via immunohistochemical staining. When androgen is too high one day following injection with a high pharmacological level of testosterone, AR is now detectable in mitotic figures (denoted by arrows in lower right panel) and in the next cell cycle, this cell will die due to its inability to relicense its DNA for S-phase replication.
An additional rationale for BAT is the observation that there are differences in the AR transcriptome when high-AR cell lines are exposed to either high or low androgen concentrations.Citation6 Under high-androgen conditions, AR represses several genes, including AR and those involved in androgen synthesis, DNA synthesis, and proliferation. Therefore, high-dose T may lead castration-resistant cells to transition from a more oncogenic transcriptome associated with castrate T levels to a high-androgen transcriptome that does not support cancer proliferation. We hypothesized that such continuous rapidly cycling between the polar extremes from near-castrate to supraphysiologic (i.e., pharmacologic) serum T levels prevents adaptive changes in AR expression, prolonging the length of time during which patients respond to this therapy. Furthermore, because recent studies have shown that the double- strand DNA breaks and apoptosis induced by high doses of androgens are transient, rapid cycling of T could result in repeated rounds of DNA damage, enhancing antitumor effects.Citation7
At present, BAT is being tested in a large (n = 180) randomized trial (NCT02286921; TRANSFORMER) in asymptomatic mCRPC patients who have failed on abiraterone. In this study, BAT is being compared with enzalutamide with a primary end point of progression-free survival (PFS). However, this trial will not allow to determine whether BAT is effective in inducing or reinforcing dormancy in disseminated solitary cancer cells and thus to decrease the frequency of metastatic recurrence. As suggested by Bui et al, this would require to test the effect of short duration BAT when started just after radical prostatectomy with a primary end point of metastasis-free survival.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Schweizer MT, Antonarakis ES, Wang H, Ajiboye AS, Spitz A, Cao H, Luo J, Haffner MC, Yegnasubramanian S, Carducci MA, Eisenberger MA, Isaacs JT, Denmeade SR. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2. doi:10.1126/scitranslmed. PMID:25568070
- Litvinov IV, Vander Griend DJ, Antony L, Dalrymple S, De Marzo AM, Drake CG, Isaacs JT. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci. USA. 2006;103:15085−90. doi:10.1073/pnas.0603057103. PMID:17015840
- Vander Griend DJ, Litvinov IV, Isaacs JT. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle. 2007;6:647−51. doi:10.4161/cc.6.6.4028. PMID: 17387277
- D'Antonio JM, Vander Griend DJ, Isaacs JT. DNA licensing as a novel androgen receptor mediated therapeutic target for prostate cancer. Endocr Relat Cancer. 2009;16:325−32. doi:10.1677/ERC-08-0205. PMID:19240183
- Isaacs, JT, D'Antonio JM, Chen S, Antony L, Dalrymple SP, Ndikuyeze GH, Luo J, Denmeade SR. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491−505. doi:10.1002/pros.22504. PMID:22396319
- Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, Chen S, Nelson PS, Liu XS, Brown M, Balk SP. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457−71. doi:10.1016/j.ccr.2011.09.001. PMID:22014572
- Hedayati M, Haffner MC, Coulter JB, Raval RR, Zhang Y, Zhou H, Mian O, Knight EJ, Razavi N, Dalrymple S, Isaacs JT, Santos A, Hales R, Nelson WG, Yegnasubramanian S, DeWeese TL. Androgen deprivation followed by acute androgen stimulation selectively sensitizes AR-positive prostate cancer cells to ionizing radiation. Clin Cancer Res. 2016;22:3310−9. doi:10.1158/1078-0432.CCR-15-1147. PMID:26831716
