ABSTRACT
The minichromosome maintenance (MCM) complex, consisting of six subunits, Mcm2-7, is loaded onto replication origins through loading factors (origin recognition complex [ORC], Cdc6, and Cdt1) and forms an MCM double hexamer that licenses the initiation of DNA replication. Previous studies with Xenopus egg extracts showed that loading factors, especially Cdc6, dissociate from chromatin on MCM loading, but the molecular mechanism and physiological significance remain largely unknown. Using a cell-free system for MCM loading onto plasmid DNA in Xenopus egg extracts, we found that MCM loaded onto DNA prevents DNA binding of the loading factors ORC, Cdc6, and Cdt1. We further report that a peptide of the C-terminal region of MCM3 (MCM3-C), previously implicated in the initial association with ORC/Cdc6 in budding yeast, prevents ORC/Cdc6/Cdt1 binding to DNA in the absence of MCM loading. ATP-γ-S suppresses inhibitory activities of both the MCM loaded onto DNA and the MCM3-C peptide. Other soluble factors in the extract, but neither MCM nor Cdt1, are required for the activity. Conservation of the amino acid sequences of MCM3-C and its activity in vertebrates implies a novel negative autoregulatory mechanism that interferes with MCM loading in the vicinity of licensed origins to ensure proper origin licensing.
Introduction
In eukaryotes, DNA replication is initiated from multiple origins that are fired only once within one round of the cell cycle in order to duplicate the genome exactly (once-per-cell-cycle DNA replication). The minichromosome maintenance (MCM) complex, consisting of six subunits, Mcm2-7, is the catalytic unit of replicative helicase, and the elaborate regulation of MCM helicase during the cell cycle, both in its loading and activation step, is the central molecular mechanism to ensure once-per-cell-cycle DNA replication [Citation1–3]. MCM is loaded onto replication origins in late M to early G1 phase through the action of the loading factors origin recognition complex (ORC), Cdc6, and Cdt1. ORC, which consists of six subunits, Orc1-6, binds to DNA and marks the replication origins. Although budding yeast ORC recognizes short specific DNA sequences of the replication origins, metazoan ORC does not show strict DNA sequence specificity. A recent study clarified that even budding yeast ORC can bind and load MCM onto any DNA sequence if competitor DNA is absent [Citation4]. Two other loading factors, Cdc6 and Cdt1, are loaded onto replication origins depending on ORC pre-bound to the origins. In budding yeast, MCM complexed with Cdt1 is recruited by Cdc6/ORC bound to DNA [Citation4,Citation5], whereas in metazoans, Cdt1 binds to Cdc6/ORC independent of MCM [Citation6], and the Cdc6/ORC/Cdt1 complex recruits MCM to the origins [Citation7]. After the completion of MCM loading, MCM forms a double hexamer, in which N-terminal tiers of two single hexamers are attached head to head [Citation4]. In metazoans, biochemical analysis of MCM bound to chromatin also identified double hexameric MCM formation on origin licensing [Citation8].
The MCM double hexamer loaded onto DNA is inactive as a helicase. When cells enter S phase, cyclin-dependent kinase, Dbf4-dependent kinase, and a number of activation factors (e.g., Sld2/RecQ4, Sld3/Teslin, Dpb11/TopBP1) convert the double hexamer into two CMG (Cdc45-MCM-GINS) complexes that act as a replicative helicase [Citation9–11]. During the S-G2-M phase, loading factors are suppressed in multiple ways to prevent MCM reloading, which causes re-replication in a single cell cycle. For example, in metazoans, Cdt1 is inactivated by its inhibitor geminin, which is a target of APC/C; thus, Cdt1 is active only from late M to G1 phase [Citation12].
The MCM loading step has been extensively studied by using a reconstituted budding yeast system. The C-terminal region of Mcm3 has been reported to directly interact with Cdc6/ORC bound to DNA in order to initiate MCM loading [Citation13]. This interaction requires the extreme C-terminal region of Mcm3, and mutations in four amino acids at the C-terminal end diminish the interaction and subsequent MCM loading. The interaction can be detected only in the presence of ATP-γ-S, which stabilizes the Cdc6/ORC-MCM/Cdt1 complex [Citation14]. In the presence of ATP, the C-terminal region of Mcm3 dissociates Cdc6 from ORC bound to DNA. Taking into account that the C-terminal region of Mcm3 activates ATPase activity of Cdc6/ORC, authors have proposed a quality control model in which the C-terminal region of Mcm3 resets the loading reaction by dissociating Cdc6 from ORC when the normal loading step is compromised [Citation13]. The importance of the C-terminal region of Mcm3 has not been tested in other organisms.
Recent single-molecule analysis with a reconstituted yeast system clearly demonstrates that two MCM single hexamers are recruited, one by one, to origin DNA bound to one ORC and that Cdc6 and Cdt1 bind to and dissociate from the origin DNA for each loading step of the single hexameric MCM [Citation15]. On assembly of the double hexameric MCM, ORC is finally dissociated from DNA, that was an unexpected finding that may be difficult to reconcile with previous chromatin immunoprecipitation (ChIP) data showing the simultaneous presence of MCM and ORC on the licensed origins [Citation16–18].
In metazoans, the detailed molecular mechanism of MCM loading has not yet been clarified. However, the conserved structural features of Orc1 and 5, Cdc6, and Mcm2-7, as AAA+ ATPases, provide us with information on the fundamental role of ATP in MCM loading reactions. ATP binding to ORC is essential for binding to DNA [Citation19]. ATP hydrolysis by budding yeast ORC is not essential for MCM loading, but it has been reported that it is required for reiterative loading of MCM [Citation20]. A Cdc6 mutant defective in ATP binding cannot support MCM loading, whereas another Cdc6 mutant defective in ATP hydrolysis can support MCM loading in vitro [Citation21,Citation22]. A recent report suggests that the latter mutant Cdc6 could not support cell viability because of the failure to initiate DNA replication [Citation23]. Interestingly, induced degradation of such a Cdc6 mutant after MCM loading is sufficient to initiate DNA replication; thus ATP hydrolysis-mediated Cdc6 clearance after MCM loading is thought to be an important process [Citation23]. Unlike that of ORC and Cdc6, the ATPase activity of MCM has been reported to be essential for MCM loading in vitro [Citation21,Citation22]. Perhaps open-closed conformational changes induced by ATP hydrolysis in the ring-shaped MCM hexamer is important for loading [Citation24]; a recent study shows the dynamic changes in the MCM hexameric ring during its loading reactions [Citation25]. The requirement for ATP binding to Cdc6 and ORC is believed to be conserved in metazoans. In fact, in Xenopus egg extract, ATP, but not ADP, supports Cdc6 loading, and ATP hydrolysis by Cdc6 appears to be important for MCM loading [Citation26,Citation27]. In addition, with the egg extract, the concomitant release of Cdc6 on loading of MCM onto chromatin has been reported and is implicated in inducing the distribution of MCM to each origin [Citation28]. However, the molecular mechanism is not fully understood.
From numerous studies on the number of MCM loading components bound to chromatin arose a long-standing issue called the “MCM paradox.” The number of ORCs (and Cdc6 molecules) bound to chromatin is comparable to the number of origins, whereas the number of MCM complexes bound to chromatin is 10–40 times greater than that in all organisms studied so far [Citation29–32]. In addition, ChIP experiments with mammalian cells suggest that the distance between chromatin-bound MCM and chromatin-bound ORC is at least 500–1000 bp [Citation33]. Previous studies suggest that these excess MCM complexes are used as dormant origins, which are activated only when normal S-phase progression is compromised [Citation34–36]. It is, however, still not clear how small amounts of ORC can load a large amount of MCM onto chromatin.
Using Xenopus egg extracts, it has been reported that the amount of MCM loaded onto linear DNA is proportional to the length of the DNA, but that a constant amount of ORC binds to DNA regardless of its length [Citation37]. Considering that a previous study showed that MCM can move at least 1 kb away from the ORC binding site [Citation38], it has been widely believed that repeated loading of MCM by a single ORC bound to DNA results in the distributive loading of MCM along DNA [Citation32,Citation39]. On the other hand, with plasmid DNA, an alternative view has been presented. Inhibition of MCM loading, by addition of geminin, lowering of the reaction temperature, or MCM depletion, is known to result in stabilization of ORC, Cdc6, and Cdt1 binding to DNA in Xenopus egg extracts. Under these conditions, the number of ORC/Cdc6/Cdt1 molecules bound to DNA is comparable to the number of MCM complexes loaded onto DNA under normal loading conditions, and it is proposed that one ORC/Cdc6/Cdt1 molecule loads one MCM complex, possibly one double hexameric MCM, and the loading factors are dissociated from DNA on MCM loading, thus ensuring the recycling of loading factors and multiple MCM loading [Citation40]. Although this model offers intriguing possibilities about the MCM paradox, the molecular mechanism underlying the dissociation of the loading factors is not yet known.
Here we report that MCM loaded onto DNA interferes with further binding of the loading factors, especially Cdc6. Addition of a peptide consisting of the C-terminal region of Mcm3 recapitulated the inhibition of ORC/Cdc6/Cdt1 binding on MCM loading. Our results support the novel inhibitory autoregulation of MCM loaded onto DNA through the C-terminal region of Mcm3, shedding new light on a control mechanism of origin licensing reactions that permit the licensing of numerous origins throughout the chromosome precisely and efficiently.
Results
Reciprocal DNA binding of MCM and its loading factors in egg extracts
Chromatin loading of metazoan MCM has been extensively studied with Xenopus egg extracts, and previous studies show that MCM loading is accompanied by the dissociation of its loading factors, ORC, Cdc6, and Cdt1, in particular Cdc6, from chromatin and DNA [Citation28,Citation40,Citation41]. We have reexamined the time course of DNA binding of MCM and the loading factors by using plasmid DNA coupled with magnetic beads (DNA beads). The DNA beads were incubated in egg extracts at 4˚C to prevent MCM loading and to maximally bind ORC/Cdc6/Cdt1 to the beads (A). When the temperature rapidly shifted to 23˚C, MCM started to bind to DNA within 1 min, and the amount bound increased with incubation time. In an almost reciprocal manner, ORC/Cdc6/Cdt1 which was bound to DNA, dissociated from it. We noticed that among ORC/Cdc6/Cdt1, Cdc6 always dissociated from DNA upon MCM loading almost completely, but Orc1 and Cdt1 did modestly, and Orc2 did slightly. These differences might reflect that these factors behave slightly differently during the MCM loading. Nevertheless, these results confirm the previous observations that ORC/Cdc6/Cdt1 dissociates from DNA on MCM loading. It is equally important to note that rebinding of ORC/Cdc6/Cdt1 is apparently prevented during the incubation.
Figure 1. Inhibition of ORC/Cdc6/Cdt1 binding by MCM loaded on chromatin. (A) Plasmid DNA bound to magnetic beads was incubated in 10 µL of egg extract at 23˚C or at 4˚C for 16 min. For shifting the temperature from 4˚C to 23˚C, DNA beads were incubated at 4˚C for 16 min, and then shifted to 23˚C and incubated for the indicated time. DNA beads were isolated and washed with EB (50 mM HEPES-KOH at pH 7.5, 100 mM KCl, 2.5 mM MgCl2) containing 0.25% NP40 and bound proteins were analyzed by immunoblotting. (B) Scheme to examine the effect of MCM bound to DNA on the DNA binding of ORC/Cdc6/Cdt1 in the presence of geminin. (C) For the first incubation, DNA beads were incubated in 10 µL of egg extract in the presence or absence of geminin for the indicated time at 23˚C. DNA beads were then washed by either EB + 0.25% NP40 (low salt, L) or EB + 0.25% NP40 + 0.4 M NaCl (high salt, H) and transferred to the egg extracts (10 μL) containing geminin for the second incubation. After incubation at 23˚C for 20 min, the beads were washed with EB containing 0.25% NP40 (low salt) and bound proteins were analyzed by immunoblotting.
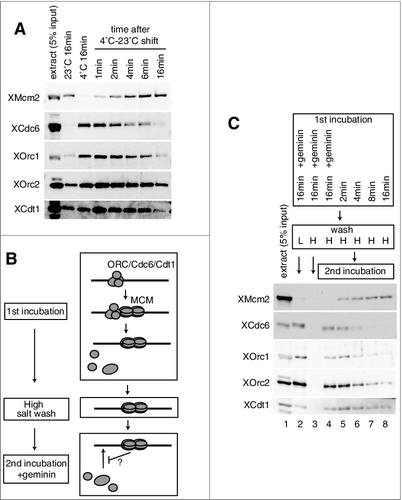
We hypothesized that the MCM double hexamer assembled on DNA might prevent ORC/Cdc6/Cdt1 rebinding to DNA. To test this idea directly, we incubated DNA beads in the extracts with or without geminin (first incubation), and then washed them with buffer containing high salt (high salt wash, H), which removed most of the ORC/Cdc6/Cdt1 bound to DNA; tightly bound MCM, however, remained on the DNA beads. The washed DNA beads were then further incubated in fresh extracts in the presence of geminin to allow maximal binding of ORC/Cdc6/Cdt1 to DNA (second incubation) (B). During the first incubation in the extracts in the absence of geminin, the amount of MCM bound to DNA that was resistant to the high salt wash increased with incubation time and reached a maximum at 16 min of incubation (C). After the second incubation, the amount of loading factors bound to DNA was detected by washing with buffer containing low salt (L). When MCM loading was inhibited by geminin in the first incubation, the loading factors were maximally bound to DNA (lane 2), but they were removed by the high salt wash (lane 3). However, similar amounts of loading factors were bound to the high-salt washed beads after the second incubation (lane 4). In the absence of geminin in the first incubation, the amount of loading factors, in particular Cdc6, bound to DNA in the second incubation was inversely proportional to the amount of MCM tightly bound to DNA (C, lanes 5–8). These results suggest that a high-salt-wash-resistant complex, presumably double-hexameric MCM bound to DNA prevents the rebinding of the loading factors, in particular Cdc6, during the second incubation. Notably, the estimated amount of high-salt-wash-resistant MCM is less than 2% of the MCM single hexamer in the extract (compare lanes 1 and 8). These results therefore suggest that MCM that is tightly bound to DNA could prevent the binding of ORC/Cdc6/Cdt1 to DNA.
We further found that neither MCM nor Cdt1 depletion from the 2nd extracts affected the inhibitory activity of MCM tightly bound to DNA for ORC/Cdc6 rebinding, and Cdc6 depletion did not affect the inhibition of ORC rebinding to the DNA (A, lanes 5–9). Both Cdc6 and some ORC subunits are AAA+ ATPases and their DNA binding requires ATP. Therefore we next tested if addition of ATP-γ-S affects the inhibition. We found that addition of ATP-γ-S during the 2nd incubation abolished the inhibition of ORC/Cdc6/Cdt1 rebinding, although lowering the 2nd incubation temperature to 4˚C did not affect the inhibition of ORC/Cdc6/Cdt1 rebinding by the MCM bound to DNA (B, lanes 2–5). These results therefore suggest that the inhibition of ORC/Cdc6 rebinding by MCM tightly bound to DNA is not simply caused by an occupation of the binding sites by MCM, but rather by an active process which is prevented by ATP-γ-S.
Figure 2. Inhibition of Cdc6, ORC, and Cdt1 binding by MCM on the plasmid. (A) The first incubation was performed, as in the legend for C, for 16 min in the presence or absence of geminin. After incubation, the beads were washed with the high-salt buffer, and further incubated in the second extract using either mock-, Mcm2-, Cdc6-, or Cdt1-depleted extracts in the presence of geminin. Immuno-depletion of each factor was performed by treating the egg extracts with protein A beads bound with either control, αXMcm2, αXCdt1, or αXCdc6 antisera. Various depleted extracts (0.5 uL) and proteins bound to the beads were analyzed by immunoblotting. (B) The first incubation was performed, as in the legend for C, for 16 min. The second incubation was performed in the extracts with or without 5 mM ATP-γ-S at either 23˚C or 4˚C in the presence of geminin.
Inhibition of MCM loading onto DNA by a C-terminal region of Mcm3
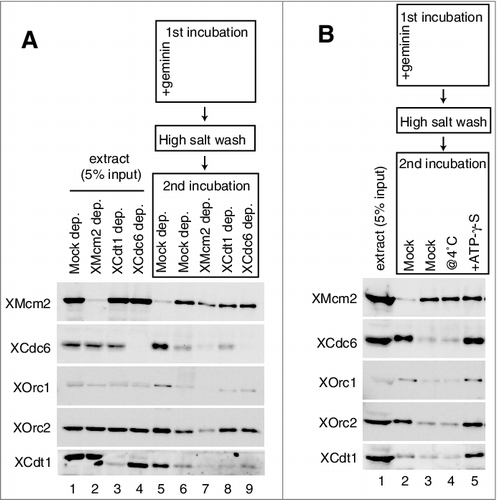
How could MCM assembled onto DNA prevent ORC/Cdc6/Cdt1 binding? One candidate for prevention is the C-terminal region of Mcm3, which could interact with ORC/Cdc6 on DNA and could also dissociate Cdc6 by activating its ATPase activity in the budding yeast [Citation13]. To investigate the function of a C-terminal region of Xenopus Mcm3 in the extracts, we first examined whether the addition of a peptide derived from the Mcm3 C-terminal region (MCM3-C, recombinant Xenopus maternal Mcm3 consisting of aa 702–807) could interfere with MCM loading in the extracts. To this end, DNA beads were incubated in egg extracts, either in the presence or absence of MCM3-C, and the proteins bound to DNA were analyzed by immunoblotting (A). In the absence of MCM3-C, Mcm2 was gradually loaded onto DNA and reached a plateau at about 8–16 min of incubation. Cdc6 rapidly bound to DNA within 2 min, and binding gradually decreased during incubation. ORC and Cdt1 showed similar behavior to that of Cdc6, even though their decreases in binding were modest in comparison to that of Cdc6. In the presence of MCM3-C, however, binding of Mcm2 was inhibited and most MCM binding was abolished in the presence of the 3.2 µM peptide, which is about fivefold of the endogenous concentration of MCM3 in the extract, assuming as about 0.6 µM [Citation42]. Rapid binding of Cdc6 also decreased depending on the amount of MCM3-C added to the extracts. Cdt1 behaved like Cdc6, but relatively higher amounts of MCM3-C were required to inhibit binding (A and Supplemental Fig. 1A). In contrast, the time course of ORC binding was not apparently affected by adding MCM3-C and, in some cases, the addition of MCM3-C stabilized ORC binding (A, compare mock and 3.2µM; see also C, compare lanes 3 and 4). This tendency was especially apparent at an early time point (Supplemental Fig. 1A). Because 1.6 µM MCM3-C only modestly inhibits MCM loading but strongly inhibits Cdc6 binding (A, 1.6µM), we tested whether the MCM bound to DNA in the presence of MCM3-C was different from that obtained in its absence (B). By varying the concentration of peptides in the extracts, we found that 0.8 µM MCM3-C did not apparently affect DNA binding of MCM after the low-salt wash, but the high-salt-wash-resistant MCM was decreased to 40% of that observed in the absence of the peptide (B). These results showed that the effect of MCM3-C on MCM loading reactions is not simply due to the initial inhibition of the loading factors, such as Cdc6 (see Discussion).
Figure 3. Inhibition of MCM and ORC/Cdc6/Cdt1 binding to DNA by the C-terminal fragment of MCM3. (A) Plasmid DNA bound to magnetic beads was incubated in 10 µL of egg extract at 23˚C in the absence (mock) and presence of 1.6 or 3.2 µM (∼ 5 × endogenous Mcm3) MCM3-C (His6-3Strep-XMcm3m(702-807)). After incubating for the indicated time, DNA beads were isolated and washed with EB + 0.25% NP40 and bound proteins were analyzed by immunoblotting. (B) DNA beads were incubated in 10 µL of egg extract for 16 min in the presence of various concentrations of MCM3-C as indicated. Beads were isolated and washed with either the low-salt or the high-salt buffer and bound Mcm2 was analyzed by immunoblotting. The graph shows quantified data of Mcm2 bound to DNA obtained by the low-salt wash (diamond) or the high-salt-wash (square). The vertical axis indicates the percentage of signal intensity relative to the maximum value obtained in the absence of MCM3-C. (C) DNA beads were incubated in 10 µL of egg extract in the presence or absence of geminin and MCM3-C (1.6 µM) for 20 min. Beads were isolated and washed with the low-salt EB buffer and the bound proteins were analyzed by immunoblotting. (D) DNA beads were incubated in 10 µL of egg extract in the presence of geminin for 15 min in the presence or absence of 1.6 µM MCM3-C (@0 min). Alternatively, DNA beads were incubated in the presence of geminin but the absence of MCM3-C for 15 min, and then MCM3-C (final concentration 1.6 μM) or the dialysis buffer was added and further incubated for 15 min (@15min). Beads were isolated and washed with the low-salt buffer and the bound proteins were analyzed by immunoblotting.
To investigate the effect of MCM3-C on the binding of ORC and Cdc6 more directly, we next examined DNA binding of these proteins in the presence of geminin, which maximizes Cdc6/ORC binding to DNA. MCM3-C efficiently inhibits Cdc6 and ORC binding to DNA in the presence of geminin (C, compare lanes 5 and 6). Cdt1 binding was also inhibited by MCM3-C in a concentration-dependent manner (Supplemental Fig. 1D). Two mechanisms may be involved in the inhibition of protein binding to DNA at a steady state. One is the direct inhibition of the binding step and the other is the promotion of the dissociation step of bound proteins. To distinguish between these possibilities, we incubated DNA beads in the extracts in the presence of geminin for 15 min to allow maximal Cdc6/ORC binding to DNA, and then we incubated them for another 15 min without (lane 5) or with (lane 6) addition of MCM3-C (D). We found that the addition of MCM3-C after Cdc6/ORC binding efficiently inhibited Cdc6/ORC binding to DNA, and that the amount of these proteins bound to DNA rapidly decreased to the basal level within 2 min after the addition of MCM3-C (Supplemental Fig. 1E). These results suggest that MCM3-C inhibits the occupancy of these proteins on DNA by promoting their dissociation from DNA (see more details in Discussion). We also observed that MCM3-C could promote the dissociation of Cdc6/ORC bound to sperm chromatin instead of the DNA beads (Supplemental Fig. 1B and C). In contrast, MCM3-C could not promote the dissociation of MCM bound to DNA in the extracts (Supplemental Fig. 1F).
MCM3-C inhibits Cdc6/ORC binding independent of MCM/Cdt1 but is sensitive to ATP-γ-S in the extract
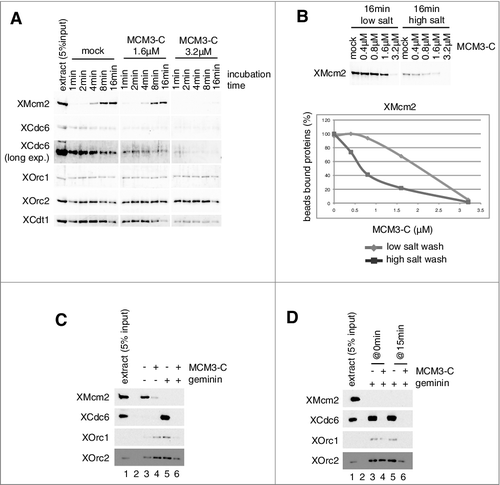
Geminin in the extracts maximizes the binding of ORC, Cdc6, and Cdt1 to DNA, and it has also been reported that MCM binds to DNA loosely in the presence of geminin, so that it is removed from DNA after a low-salt wash [Citation40]. To understand the contribution of these factors in the inhibition by MCM3-C, we immunodepleted each of these factors from the egg extracts and examined the inhibition of Cdc6/ORC binding by MCM3-C (A and B). On Mcm2 depletion, we observed that only a small amount of MCM was bound to DNA (A, lane 6), possibly due to residual MCM in the depleted extracts, and that the binding of ORC and Cdc6 was increased compared with the mock-depleted extracts that allowed maximal MCM loading onto DNA (A, compare lanes 4 and 6). The increased binding of Cdc6 in the absence of geminin was inhibited by adding MCM3-C to the extracts (A, compare lanes 6 and 7). The inhibition of Cdc6 binding by MCM3-C was essentially similar both in the mock- and the Mcm2-depleted extracts in the presence of geminin (lanes 8–11).
Figure 4. Inhibition of Cdc6 and ORC binding to DNA by MCM3-C under various conditions. (A and B) Experiments were performed with mock-, Mcm2-, Cdt1- and Cdc6 depleted extracts as described in the legend for C. (C) Experiments were performed as in the legend for C, except that DNA beads were incubated at either 23˚C or 4˚C. (D) Egg extracts were dialyzed with EB, and then supplemented with either 5 mM ATP or ATP-γ-S. The experiments that followed were performed as in the legend for C.
We next examined the effect of Cdt1 or Cdc6 depletion. By depleting Cdt1 or Cdc6 from the extract, MCM loading was suppressed to a background level that was observed with the mock depleted extract in the presence of geminin but similar ORC binding was observed with both depleted extracts (B, compare lanes 6, 8 and 10), suggesting that Cdc6 and Cdt1 were effectively immunodepleted from the extract without affecting ORC binding. With Cdt1-depleted extracts, we found similar inhibition of Cdc6 binding by the addition of MCM3-C in the presence and absence of geminin (B, lanes 6, 7, 12, and 13). These results therefore show that Cdc6 binding is inhibited by MCM3-C in the absence of MCM and Cdt1 binding to DNA. We further investigated whether Cdc6 is involved in the inhibition of ORC binding. Addition of MCM3-C to the Cdc6-depleted extracts resulted in inhibition of ORC binding to DNA, both in the presence and absence of geminin (B, compare lanes 8 and 9 and 14 and 15). These results indicate that inhibition of Cdc6/ORC binding requires neither MCM nor Cdt1 and that MCM3-C inhibits ORC binding independent of Cdc6.
A previous study suggested the involvement of ATP hydrolysis in controlling the binding of ORC/Cdc6 to DNA [Citation26]. Therefore, we conducted the experiments at 4˚C, which should slow the hydrolysis reactions. Compared with the reaction at 23˚C, MCM loading was inhibited at 4˚C (C, compare lanes 2 and 6) and binding of ORC/Cdc6 was increased to a similar level as that observed in the presence of geminin (C, lanes 6 and 8). MCM3-C effectively inhibits the binding of ORC/Cdc6, both in the presence and absence of geminin at 4˚C, showing that inhibition by MCM3-C was not affected by lowering the reaction temperature (C, lanes 6–9).
We next tested the effect of ATP-γ-S on inhibition by MCM3-C, as in the case of MCM tightly bound to DNA (B). The extracts were first dialyzed against buffer (EB) to reduce endogenous nucleotide, and the dialyzed extract was supplemented with either ATP or ATP-γ-S. By dialyzing the extracts against more than 1,000 volumes of EB, we assume that ATP concentrations could be reduced to nearly 1/1,000 fold of the endogenous one. But we observed the robust binding of ORC and Cdc6 to DNA in the presence of geminin with and without the addition of 5 mM ATP to the dialyzed extract (D, lanes 3 and 5), suggesting that residual ATP in the dialyzed extract could support DNA binding of ORC/Cdc6. Addition of MCM3-C to the dialyzed extracts similarly inhibited the binding of ORC/Cdc6 both in the presence and absence of 5 mM ATP (D, compare lanes 3, 4, 5, and 6), showing that MCM3-C effectively inhibits ORC/Cdc6 binding in the dialyzed extracts. In contrast to ATP, the addition of ATP-γ-S to the extracts stabilized Cdc6/ORC binding (D), and we reproducibly observed that a small amount of MCM was bound to DNA in the presence of geminin, presumably because of the stabilization of some intermediates of MCM loading (D, lane 7). Interestingly, ATP-γ-S, but not ATP, suppressed the inhibition of the Cdc6/ORC binding by MCM3-C (D, compare lanes 6 and 8). Similar results were also obtained when the dialysis was omitted, but the effect of ATP-γ-S was modest (Supplemental Fig. 1G).
Determination of amino acids responsible for inhibition of Cdc6 binding by MCM3-C
MCM3-C used in the present study consisted of aa 702–807 of maternal Xenopus MCM3. This region is less conserved between maternal and zygotic Xenopus MCM3 (cf. A) [Citation43]. Therefore, we first tested whether maternal and zygotic Xenopus MCM3-C showed different activities for the inhibition of Cdc6/ORC binding. A titration experiment demonstrated that these fragments retained similar inhibitory activity (B). In addition, we found that human MCM3-C (aa 706–808) retained similar inhibitory activity (Supplemental Fig. 2C). These results therefore suggest that conserved amino acids may be involved in the activity.
Figure 5. Functional region and amino acids of MCM3-C involved in the inhibition of Cdc6 binding to DNA. (A) Amino acid sequences of C-terminal regions of various metazoan Mcm3; Ciona intestinalis (Ci), Xenopus laevis maternal (Xlm), Danio rerio maternal (Drm), Xenopus laevis zygotic (Xlz), Danio rerio zygotic (Drz), Homo sapience (Hs), Gallus gallus (Gg). MCM3-C conserved motifs are further aligned with corresponding sequences of budding yeast (Sc) and C. elegans (Ce) Mcm3. (B) Experiments were performed as in the legend for C except that either maternal (XMCM3m-C aa 702–807 ( = MCM3-C)) or zygotic (XMCM3z-C aa 700–806) MCM3-C was used. (C and D) Experiments were performed as in the legend for C except that either 0.8 µM of various truncation mutants (C) or various point mutants (D) were used.
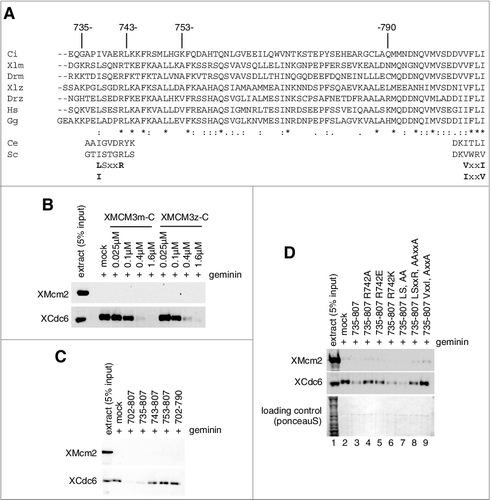
To identify the conserved amino acids, we first tried to identify minimum peptides needed for inhibitory activity. Using different truncated forms of MCM3-C, we found that the truncation of aa 702–734 (peptide 735–807) did not affect the inhibitory activity, indicating the truncated region is dispensable (C). While, the truncation of either aa 735–742 (743-807) or aa 791–807 (702-790) led to lose the inhibitory activity, showing that these regions are indispensable for inhibition (C). Essentially the same results were obtained using GST fusion proteins instead of His6-strep3 tag proteins used in , excluding the influence of the tag on the activity (Supplemental Fig. 2A). Since the simultaneous addition of recombinant MCM3-C proteins lacking aa 735–742 and those lacking aa 791–807 did not reconstitute the inhibitory activity, these two deleted regions should be present in the same molecule for this activity (Supplemental Fig. 2B).
By comparing the corresponding amino acid sequences of 735–742 in metazoans, we found the evolutionally conserved L/ISxxR motif (A). When arginine 742 was mutated to either alanine or glutamate, the binding of Cdc6 was modestly inhibited (D, lanes 4 and 5). The mutation into lysine, however, hardly affected the activity (D, lane 6). The LS mutant showed similar inhibitory activity to that of the wild type, whereas the LSxxR mutant showed similar inhibitory activity to that of the R mutant (D, compare lanes 3, 7 and 8), suggesting the importance of arginine 742 in the inhibition.
It has been reported that the extreme C-terminal region of MCM3 is essential for in vitro association with Cdc6/ORC in the yeast reconstituted system, as well as for in vivo viability [Citation13]. The alanine mutation of the yeast VxxV motif, corresponding to metazoan V/IxxI motif, diminishes the in vitro and in vivo activity of MCM [Citation13]. Consistent with these findings, and the present result with the truncated peptide (702-790), the VxxI mutant of MCM3-C was defective for inhibitory activity (D, lane 9).
Requirement of soluble factors in the extract for Cdc6/ORC dissociation from DNA by MCM3-C
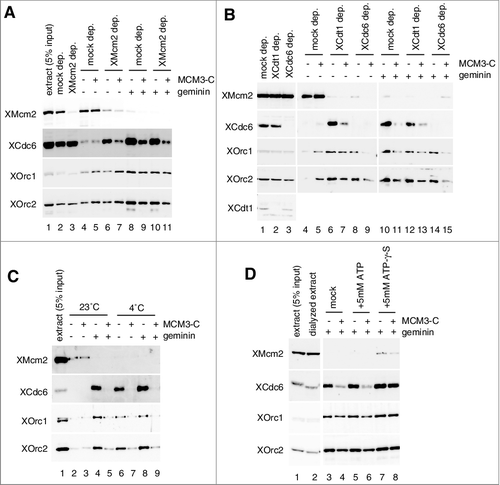
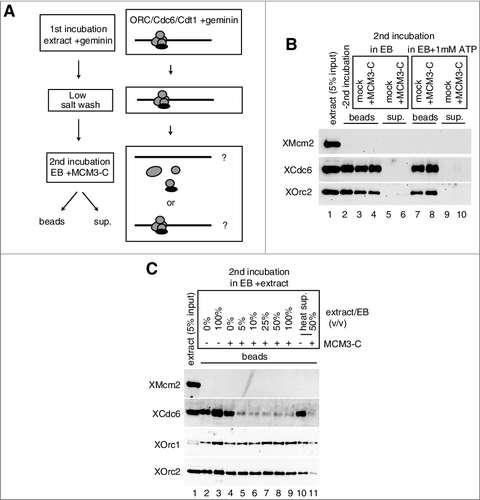
We showed that MCM3-C could promote the dissociation of Cdc6/ORC that was bound to DNA in the extracts (D). In addition, we found that neither MCM nor Cdt1 was required for dissociation (), but whether one or more additional factors, besides Cdc6/ORC/Cdt1 and MCM3-C, are required in the extracts for dissociation remains unknown. To address this issue, we isolated and washed DNA beads, which had been incubated in extracts containing geminin (first incubation), and then incubated them in the extraction buffer (EB) in the presence or absence of MCM3-C (second incubation) (A). Under these conditions, MCM3-C could not dissociate Cdc6 and ORC bound to the beads and they were not found in the supernatant after the addition of MCM3-C (B, lanes 3–6). Inclusion of 1 mM ATP to the buffer in the second incubation did not affect the dissociation of Cdc6/ORC (B, lanes 7–10). While, small amounts of extract (5%) included in the buffer for the second incubation, could efficiently promote the dissociation of Cdc6/ORC from DNA beads as that observed with the extracts in an MCM3-C-dependent manner (C, lanes 4 and 5). These results demonstrate that some soluble factors but not ATP in the extracts are involved in the MCM3-C-dependent dissociation of ORC/Cdc6. Interestingly, we found that the heat-treated supernatant (heat sup) of the extracts, where most of the heat-denatured proteins, including ORC and Cdc6 in the extract had been removed, supported Cdc6/ORC dissociation by MCM3-C (C, compare lanes 10 and 11). This suggests that some heat stable factor(s) may be involved in the MCM3-C dependent dissociation of Cdc6/ORC from DNA.
Figure 6. Requirement of soluble factor(s) in the extract for the dissociation of Cdc6/ORC by MCM3-C. (A) Experimental scheme for examining the requirement of soluble factor(s) for the dissociation of Cdc6 and ORC by MCM3-C. (B) DNA beads were incubated in 10 µL of egg extract containing geminin at 23˚C for 20 min (first incubation). Beads were then isolated and washed with the low-salt buffer and resuspended with EB or EB + 1mM ATP with or without 1.6 µM MCM3-C. After 20 min incubation at 23˚C (second incubation), supernatant and beads were separated and analyzed by immunoblotting following SDS-PAGE. (C) Experiments were performed as in (B) except that EB was supplemented with either egg extract or heat-treated supernatant (heat sup) at various ratios (%) for the second incubation. The heat sup was obtained by incubating egg extract at 98˚C for 2 min and centrifuging at 15,000 g for 15 min.
Discussion
In this paper, we describe a novel mechanism, which we call “MCM interference”, that regulates the licensing of replication origins by inhibitory autoregulation possibly through a C-terminal region of Mcm3 on DNA. It provides a new layer of control of the origin licensing reaction, which may contribute to the precise and efficient loading of MCM onto chromatin.
Experimental evidence for inhibitory autoregulation of origin licensing
Previous studies with Xenopus egg extracts showed that loading of MCM onto chromatin is accompanied by the dissociation of loading factors, in particular Cdc6, from chromatin (see Introduction). Similarly, a more persuasive experiment has been done with plasmid DNA in which the loading reaction was initiated after the assembly of ORC/Cdc6/Cdt1 [Citation40] (A). We have performed a similar experiment in which we paid more attention to the initial stages of the reaction, confirming the mirror image time course of MCM binding and of Cdc6 dissociation from DNA. Although such a coincidence could be interpreted as a simple dissociation of loading factors once they are used for MCM loading, our study provides the first evidence for inhibition of binding of the loading factors by preassembled MCM on DNA (C). Since the assembled MCM is resistant to a high-salt wash, it is reasonable to assume that bound MCM forms a double hexamer on DNA. Assuming the concentration of MCM in the extracts was as previously reported [Citation42], we could estimate that about one to two double MCM hexamers maximally bound to one plasmid DNA in the absence of geminin could inhibit the further binding of ORC/Cdc6/Cdt1 in the presence of geminin almost completely. It is not known whether the bound MCM act on Cdc6/ORC in cis or trans configuration, we speculate that the bound MCM could preferentially act in cis, considering the efficient inhibition of Cdc6 binding (C). If a single hexamer in the extracts retained such activity, the excess presence of these hexamers in the extracts should inhibit the loading reaction. In fact, we found that MCM in the extracts did not affect the inhibitory activity of the MCM loaded onto the plasmid (A). Thus, it is reasonable to assume that only the MCM on DNA has specific inhibitory activity for the binding of loading factors. Considering the size of the plasmid (3-5 kb) used in the present study, one or two MCM double hexamer bound to the plasmid could inhibit the binding of Cdc6/ORC not only in the vicinity of, but at a distance from, its loaded site.
Possible Role of MCM3-C in mediating the autoinhibitory activity of the double hexameric MCM
A previous study with a yeast in vitro system showed that MCM3-C interacts with ORC/Cdc6 on the plasmid DNA and activates the ATPase activity of Cdc6 to release it and resets inappropriate intermediates during MCM loading [Citation13]. In the present study, we showed that MCM3-C inhibited the binding of the loading factors Cdc6, Cdt1, and ORC to DNA in a similar manner to that observed for the MCM-loaded plasmids. The inhibition of Cdc6 binding was not affected by the presence of MCM or Cdt1 in the extracts, and the inhibition of ORC binding was observed in the presence and absence of Cdc6, either by MCM3-C or by the MCM-loaded plasmid (A and B, A, B). Notably, both inhibitory activities were suppressed by ATP-γ-S in the extracts, but not by lowering the reaction temperature to 4˚C, which also stabilizes ORC, Cdc6, and Cdt1 binding to DNA (B, C and D). These data support the view that the autoinhibitory activity of MCM on DNA is mediated by MCM3-C. However, the effective concentration of the MCM3-C in the extract is as much as 1,000-fold higher than that of the MCM bound to DNA. The estimated concentration of the MCM on DNA is nM range, while that of the MCM3-C peptide is µM. Apparent difference in the effective concentrations could be reconciled by considering the local concentration of the MCM bound to DNA. Because the MCM is tethered on DNA which is immobilized on the magnetic beads, the actual concentration of the MCM should be much more than the value calculated based on the total reaction volume. In particular, it has been shown that the effective concentration of the tethered ligand is much higher than free ligand in a solution, and could be calculated using the radius of gyration of the tether [Citation44]. Assuming that MCM is tethered on 1kb DNA and its radius of gyration is less than 0.1 µm, the local concentration of the MCM bound to DNA is estimated to be as much as µM range. It is therefore reasonable to assume that the MCM3 C-terminal region of the MCM loaded onto DNA, presumably as a double hexamer, has autoinhibitory activity against further binding of the loading factors, in particular Cdc6.
According to recent structural analysis of the MCM double hexamer, the C-terminal region of MCM3 is highly disordered [Citation45]. Perhaps the MCM3 C-terminal region adopts two different conformations: one the noninhibitory conformation of the single MCM hexamer that allows the association with Cdc6/ORC, and the other the inhibitory conformation in the double hexamer that promotes the dissociation of Cdc6/ORC (). It is worth noting that good candidate regions for the structural differences between the single and double hexamer might be mapped near R742, which is dispensable for initial loading in yeast [Citation13], but plays an important role in the inhibition of Cdc6/ORC binding by the MCM3-C in the extracts.
Figure 7. Model for the inhibitory autoregulation of MCM loading in the extract. Single hexameric MCM is recruited to DNA by prebound ORC/Cdc6/Cdt1 via interaction between MCM3 C-terminal-Cdc6 and between MCM6-Cdt1. Bound Cdc6 and Cdt1 are released on MCM loading, and the second MCM loading is promoted by rebinding of Cdc6 and Cdt1. After double hexamer formation, the conformation of a C-terminal region of MCM3 has changed and this inhibitory conformation could dissociate ORC/Cdc6/Cdt1 from DNA or inhibit their rebinding. The MCM3-C mimics the inhibitory conformation and could inhibit the first binding of ORC/Cdc6/Cdt1, or the second binding step of Cdc6/Cdt1. The inhibitory effect of MCM3-C is suppressed by ATP-γ-S and requires soluble factor(s) in the extract.
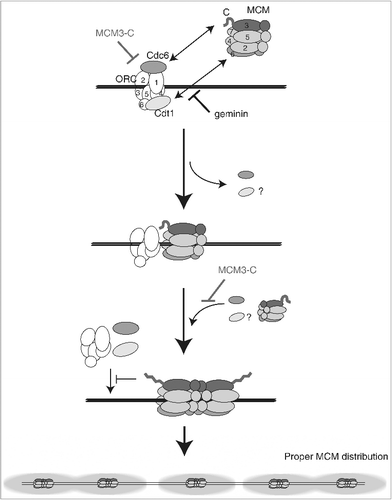
Considering the mechanistic aspects of MCM3-C for the inhibition of Cdc6/ORC binding, it is important to stress that the MCM3-C could promote the dissociation of Cdc6/ORC bound to DNA (D). Even though we do not know the rate constants of Cdc6 binding to and dissociation from DNA, it is possible to assume that this process is in a rather rapid equilibrium than that of ORC, which is stably bound to DNA [Citation15]. Therefore, the MCM3-C could promote the dissociation of Cdc6 by just inhibiting the re-binding of Cdc6 or actually accelerate its dissociation. At present, we could not distinguish these two possibilities. In contrast to the inhibition of Cdc6 binding, we found that the MCM3-C apparently stabilized the ORC binding in the absence of geminin. One explanation for the stabilization of ORC binding during the MCM loading reaction, is that MCM3-C affects two different reaction steps, the first Cdc6 binding and the second Cdc6 binding (). In the presence of geminin, MCM loading is prevented before the second Cdc6 binding, allowing stabilization of ORC-Cdc6-Cdt1, and MCM3-C could promote the dissociation of ORC, Cdc6, and Cdt1. This effect of MCM3-C may be related to previous reports that ORC binding becomes salt-sensitive after the licensing reaction [Citation46,Citation47]. On the other hand, in the absence of geminin, MCM loading reaction could proceed to the second Cdc6 loading step. In such a situation, there would be an intermediate form of the MCM double hexamer, such as an ORC-MCM complex, formed after the dissociation of Cdc6 and Cdt1, which could stabilize ORC binding in the presence of MCM3-C. In accordance with this idea, we found that in the presence of a modest amount of MCM3-C, a considerable amount of MCM was bound to DNA but was most of it was sensitive to a high-salt wash, presumably not forming double hexamers (B). Future analysis using a reconstituted MCM loading system and single-molecule analysis using Xenopus proteins will enable us to address this issue.
Previous studies showed that ATP-γ-S inhibited the dissociation of Cdc6 and Cdt1 from the first intermediate containing a single MCM hexamer and stabilized the ORC/Cdc6/Cdt1/MCM complex [Citation14]. Because Cdc6 and some ORC subunits (Orc1 and 5) are AAA+ ATPase family proteins, ATP-γ-S stabilizes Cdc6/ORC binding because of its poor hydrolysis by Cdc6/ORC. In fact, in yeast, a Cdc6 mutant in which ATP hydrolysis is impaired results in a defect in Cdc6 dissociation during MCM loading [Citation21–23]. In the present study, however, lowering the temperature to 4˚C, which should slow the hydrolysis activity of ORC/Cdc6, did not affect the inhibition of Cdc6/ORC binding by MCM3-C. This result may suggest that ATP-γ-S induces a conformational change in ORC/Cdc6, thus preventing the action of MCM3-C.
Physiological significance of the inhibitory autoregulation of the licensing system
Negative autoregulation of the binding of ORC/Cdc6 by the MCM loaded on DNA suggests that once the MCM double hexamer is assembled on chromatin, further loading of MCM would be prohibited through the promotion of Cdc6/Cdt1/ORC dissociation from DNA (). Previous and present observations about the coincidental release of Cdc6 and other loading factors on loading of MCM [Citation40] (A) could be reinterpreted as the auto-inhibition of the loading factors by the MCM on DNA. More important, the autoregulation appears not to be restricted to the vicinity of the loaded site, but to spread at a distance, possibly a few kilobases from the MCM-loaded site. This feature could explain previous findings that there is a distinct localization of ORC and MCM in the interphase nuclei and that the distance between the ORC and MCM is more than 500 bp apart in mammalian cells [Citation33]. The present findings are also consistent with the previous observation in a yeast in vitro system, showing that ORC is released after the formation of the double hexameric MCM on the origin [Citation15], and the apparent absence of the formation of more than one MCM double hexamer per 1 kbp plasmid [Citation4].
Negative autoregulation would also facilitate the binding of ORC and other loading factors to unlicensed regions of chromosomal DNA, which may contribute to appropriate and efficient loading of MCM double hexamers onto the entire genome (). In most eukaryotes, the amounts of Cdc6/Cdt1/ORC are limited compared with those of MCM; thus, it would be important to ensure licensing of all origins with limited amounts of Cdc6/Cdt1/ORC. In addition, the present findings would provide a mechanism for the “MCM paradox”, involving reiterative binding of ORC/Cdc6/Cdt1 dissociated from DNA by the “MCM interference”, in contrast with the previously assumed reiterative MCM loading by ORC stably bound to origins [Citation39]. Interestingly, a yeast strain containing a mutation corresponding to R742 of Xenopus MCM3 can load an MCM double hexamer efficiently in vitro, but shows some growth defects in vivo [Citation13]. It would be of interest to address the importance of autoregulation in vivo using such mutant cells.
“MCM interference” and “quality control”
The “MCM interference” shows some features similar to previously reported “quality control of the MCM loading” proposed with a reconstituted licensing system derived from budding yeast [Citation13]. Both of them are triggered by a C-terminal region of MCM3, dissociate Cdc6, and are inhibited by ATP-γ-S. Therefore, it is assumed that their fundamental molecular mechanisms are largely overlapping. But there are different features between them, 1. not only Cdc6 but also Cdt1, ORC are dissociated by the MCM interference. 2. not single hexamer before MCM loading but MCM double hexamer formed onto DNA appears to be responsible for the interference. 3. The interference could inhibit the loading of ORC/Cdc6/Cdt1 not only in the vicinity of, but at a distant from its loaded site. 4. Soluble factor(s) other than MCM and its loading factors is required for the interference. The most apparent difference is that the “quality control” is visible when normal MCM loading is compromised before the first MCM single hexamer loading, but the interference occurs upon normal MCM loading. The “MCM interference” proposed in the present study would extend the function of C-terminal region of MCM3 to the dissociation of loading factors that occurs upon and after normal MCM loading.
To summarize, we first showed that MCM loaded onto DNA could interfere with the binding of loading factors to DNA. Inhibition of binding was observed only when DNA was prepared under conditions that allow MCM loading. Therefore, it is reasonable to assume that the observed inhibition of Cdc6/ORC binding was mediated by MCM loaded onto the DNA. From this finding, we propose a novel autoinhibitory regulation mechanism, “MCM interference”, in origin licensing. Second, we found that MCM3-C shows a similar inhibition of Cdc6/ORC binding to DNA as that observed with MCM loaded onto DNA, thus suggesting that “MCM interference” is mediated by the C-terminal region of MCM3. Some complexities are, however, associated with the autoinhibitory mechanism observed in the present study. The dissociation of Cdc6/ORC from DNA apparently requires additional factors in the extracts, and identification of the factor(s) in the extract will help clarify the physiological role of “MCM interference” in origin licensing.
Materials and methods
Preparation of Xenopus egg extracts and DNA beads
Xenopus egg extracts and sperm chromatin were prepared as described, immunodepletion was performed as described [Citation41,Citation48]. To prepare heat sup, we incubated egg extracts at 98˚C for 2 min, and then centrifuged them at 15000 rpm for 15 min at 4˚C. Typically, less than 10% of the proteins were recovered in the supernatant.
DNA-coupled beads were prepared as described [Citation48]. Briefly, biotinylated plasmids obtained by UV cross-linking were incubated with streptavidin-coupled Dynabeads (Thermo Fisher Scientific). pBluescript-derived (3 kb), pFastBac-derived (5 kb), and pGEX-derived (5 kb) plasmids were used for the study. All plasmids exhibited similar results (data not shown). A typical amount of DNA coupled to streptavidin beads was about 30 ng/µL.
Preparation of recombinant MCM3-C
MCM3-C fragments were amplified by PCR using KOD+ DNA polymerase (Toyobo), and cloned into a pET28c (Novagen)-derived vector, in which N-terminal 8xHis, 3xStrep tag was inserted between NcoI and NheI sites, using NheI and XhoI sites. Site-directed mutagenesis was performed with two-step PCR using overlapping primers. Sequences of primers and plasmids will be provided on request. BL21 codon+ (Agilent) cells, transformed with plasmids, were cultured in 500 mL of LB containing kanamycin at 30˚C until the OD600 reached 1.0, and then expression was induced by adding 0.2 mM IPTG for 4 h. Cells were harvested and lysed in 15 mL of wash buffer (50 mM Tris HCl at pH 7.5, 150 mM NaCl, 20 mM imidazole, 0.2% Triton X-100) containing 1/500 protease inhibitor cocktail (Nacalai Tesque) and 0.1 mg/mL lysozyme for 15 min at room temperature, followed by sonication. The lysate was clarified by centrifugation at 24,000g for 30 min at 4˚C, and the supernatant was incubated with COSMOGEL His-Accept (Nacalai Tesque) for 3 h at 4˚C. Beads were washed with wash buffer, and then bound proteins were eluted with elution buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 300 mM imidazole, 0.2% Triton X-100, 5% glycerol). Eluate was dialyzed against EB before being frozen in liquid nitrogen. GST fusion proteins were similarly prepared except that imidazole was omitted from the buffer, and GSH-sepharose4B Fast Flow (GE Healthcare Life Sciences) and glutathione were used instead of Ni beads and imidazole for elution. Recombinant GST-geminin was also prepared as above and used at about 400 nM.
Cdc6/ORC dissociation assay
In typical experiments, 5 µg of DNA beads were incubated in 10 µL of egg extracts in the presence or absence of 1 µL of MCM3-C (final 1.6 or 3.2 µM) and 0.4 µL of geminin (final 1 µM) for 20 min at 23˚C with gentle agitation. The reaction mixture was diluted with ice-cold EB (50 mM HEPES-KOH, 100 mM KCl, 2.5 mM MgCl2) containing 0.25% NP40, and the beads were recovered by a magnetic apparatus (Thermo Fisher Scientific). The beads were further washed twice with 100 µL of ice-cold EB containing 0.25% NP40. The high-salt wash was supplemented with 0.4 M NaCl. When the beads were further incubated in the second extracts, the last wash was done with EB. Bead-bound proteins were solubilized with 20 µL of Laemmli sample buffer, 10 µL of which was analyzed by SDS-PAGE followed by immunoblotting. Proteins of interest were detected with rabbit antisera as described previously [Citation41]. anti-Cdt1 antiserum was used at 1/250 dilution and the others were used at 1/1000 dilution. Protein A-HRP (GE Life Sciences) was used to detect the primary antibodies on the membrane at 1/500 dilution for anti-Cdt1 and at 1/2000 dilution for the others. Either ImmunoStar Zeta or ImmunoStar LD (Wako) was used as the HRP substrate and signals were detected by LAS1000 (Fujifilm). Images obtained from the same gel were arranged by using Photoshop to prepare the figures. Quantification of signals was performed with ImageJ. The protein signals in egg extracts and in bead-bound fractions were compared. The amount of protein in egg extracts was obtained from a previous report [Citation42].
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
downloadFromZipFile.pdf
Download PDF (1.5 MB)Acknowledgments
We thank Ms. Hana Nakanishi for her help at the early stage of this project. We are grateful to Dr. Hisao Masukata for discussion and critical reading.
References
- Deegan TD, Diffley JF. MCM: one ring to rule them all. Curr Opin Struct Biol. 2016;37:145–151. doi:10.1016/j.sbi.2016.01.014. PMID:26866665
- Abid Ali F, Costa A. The MCM Helicase Motor of the Eukaryotic Replisome. J Mol Biol. 2016;428:1822–1832. doi:10.1016/j.jmb.2016.01.024. PMID:26829220
- Li Y, Araki H. Loading and activation of DNA replicative helicases: the key step of initiation of DNA replication. Genes Cells. 2013;18:266–277. doi:10.1111/gtc.12040. PMID:23461534
- Remus D, Beuron F, Tolun G, et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi:10.1016/j.cell.2009.10.015. PMID:19896182
- Tanaka S, Diffley JF. Interdependent nuclear accumulation of budding yeast Cdt1 and Mcm2-7 during G1 phase. Nat Cell Biol. 2002;4:198–207. doi:10.1038/ncb757. PMID:11836525
- Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi:10.1038/35007104. PMID:10766247
- DePamphilis ML, Blow JJ, Ghosh S, et al. Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol. 2006;18:231–239. doi:10.1016/j.ceb.2006.04.001. PMID:16650748
- Gambus A, Khoudoli GA, Jones RC, et al. MCM2-7 form double hexamers at licensed origins in Xenopus egg extract. J Biol Chem. 2011;286:11855–11864. doi:10.1074/jbc.M110.199521. PMID:21282109
- Yeeles JT, Deegan TD, Janska A, et al. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature. 2015;519:431–435. doi:10.1038/nature14285. PMID:25739503
- Ilves I, Petojevic T, Pesavento JJ, et al. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol Cell. 2010;37:247–258. doi:10.1016/j.molcel.2009.12.030. PMID:20122406
- Tanaka S, Araki H. Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb Perspect Biol. 2013;5:a010371. doi:10.1101/cshperspect.a010371. PMID:23881938
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi:10.1016/S0092-8674(00)81209-X. PMID:9635433
- Frigola J, Remus D, Mehanna A, et al. ATPase-dependent quality control of DNA replication origin licensing. Nature. 2013;495:339–343. doi:10.1038/nature11920. PMID:23474987
- Sun J, Evrin C, Samel SA, et al. Cryo-EM structure of a helicase loading intermediate containing ORC-Cdc6-Cdt1-MCM2-7 bound to DNA. Nat Struct Mol Biol. 2013;20:944–51. doi:10.1038/nsmb.2629. PMID:23851460
- Ticau S, Friedman LJ, Ivica NA, et al. Single-molecule studies of origin licensing reveal mechanisms ensuring bidirectional helicase loading. Cell. 2015;161:513–525. doi:10.1016/j.cell.2015.03.012. PMID:25892223
- Wyrick JJ, Aparicio JG, Chen T, et al. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science. 2001;294:2357–2360. doi:10.1126/science.1066101. PMID:11743203
- Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi:10.1016/S0092-8674(01)80009-X. PMID:9335335
- Tanaka T, Knapp D, Nasmyth K. Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell. 1997;90:649–660. doi:10.1016/S0092-8674(00)80526-7. PMID:9288745
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi:10.1146/annurev.biochem.71.110601.135425. PMID:12045100
- Bowers JL, Randell JC, Chen S, et al. ATP hydrolysis by ORC catalyzes reiterative Mcm2-7 assembly at a defined origin of replication. Mol Cell. 2004;16:967–978. doi:10.1016/j.molcel.2004.11.038. PMID:15610739
- Coster G, Frigola J, Beuron F, et al. Origin licensing requires ATP binding and hydrolysis by the MCM replicative helicase. Mol Cell. 2014;55:666–677. doi:10.1016/j.molcel.2014.06.034. PMID:25087873
- Kang S, Warner MD, Bell SP. Multiple functions for Mcm2-7 ATPase motifs during replication initiation. Mol Cell. 2014;55:655–665. doi:10.1016/j.molcel.2014.06.033. PMID:25087876
- Chang F, Riera A, Evrin C, et al. Cdc6 ATPase activity disengages Cdc6 from the pre-replicative complex to promote DNA replication. Elife. 2015;4. doi:10.7554/eLife.05795.
- Samel SA, Fernandez-Cid A, Sun J, et al. A unique DNA entry gate serves for regulated loading of the eukaryotic replicative helicase MCM2-7 onto DNA. Genes Dev. 2014;28:1653–1666. doi:10.1101/gad.242404.114. PMID:25085418
- Ticau S, Friedman LJ, Champasa K, et al. Mechanism and timing of Mcm2-7 ring closure during DNA replication origin licensing. Nat Struct Mol Biol. 2017;24:309–315. doi:10.1038/nsmb.3375. PMID:28191892
- Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BMC Biochem. 2001;2:15. doi:10.1186/1471-2091-2-15. PMID:11737877
- Frolova NS, Schek N, Tikhmyanova N, et al. Xenopus Cdc6 performs separate functions in initiating DNA replication. Mol Biol Cell. 2002;13:1298–1312. doi:10.1091/mbc.01-08-0382. PMID:11950940
- Oehlmann M, Score AJ, Blow JJ. The role of Cdc6 in ensuring complete genome licensing and S phase checkpoint activation. J Cell Biol. 2004;165:181–190. doi:10.1083/jcb.200311044. PMID:15096526
- Donovan S, Harwood J, Drury LS, et al. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci U S A. 1997;94:5611–5616. doi:10.1073/pnas.94.11.5611. PMID:9159120
- Mahbubani HM, Chong JP, Chevalier S, et al. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol. 1997;136:125–35. doi:10.1083/jcb.136.1.125. PMID:9008708
- Burkhart R, Schulte D, Hu D, et al. Interactions of human nuclear proteins P1Mcm3 and P1Cdc46. Eur J Biochem. 1995;228:431–438. doi:10.1111/j.1432-1033.1995.tb20281.x. PMID:7705359
- Powell SK, MacAlpine HK, Prinz JA, et al. Dynamic loading and redistribution of the Mcm2-7 helicase complex through the cell cycle. EMBO J. 2015;34:531–543. doi:10.15252/embj.201488307. PMID:25555795
- Ritzi M, Baack M, Musahl C, et al. Human minichromosome maintenance proteins and human origin recognition complex 2 protein on chromatin. J Biol Chem. 1998;273:24543–24549. doi:10.1074/jbc.273.38.24543. PMID:9733749
- Woodward AM, Gohler T, Luciani MG, et al. Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. J Cell Biol. 2006;173:673–683. doi:10.1083/jcb.200602108. PMID:16754955
- Blow JJ, Ge XQ, Jackson DA. How dormant origins promote complete genome replication. Trends Biochem Sci. 2011;36:405–414. doi:10.1016/j.tibs.2011.05.002. PMID:21641805
- Ge XQ, Jackson DA, Blow JJ. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 2007;21:3331–3341. doi:10.1101/gad.457807. PMID:18079179
- Edwards MC, Tutter AV, Cvetic C, et al. MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in Xenopus egg extracts. J Biol Chem. 2002;277:33049–33057. doi:10.1074/jbc.M204438200. PMID:12087101
- Harvey KJ, Newport J. CpG methylation of DNA restricts prereplication complex assembly in Xenopus egg extracts. Mol Cell Biol. 2003;23:6769–6779. doi:10.1128/MCB.23.19.6769-6779.2003. PMID:12972597
- Hyrien O. How MCM loading and spreading specify eukaryotic DNA replication initiation sites. F1000Res. 2016;5. doi:10.12688/f1000research.9008.1. PMID:27635237
- Waga S, Zembutsu A. Dynamics of DNA binding of replication initiation proteins during de novo formation of pre-replicative complexes in Xenopus egg extracts. J Biol Chem. 2006;281:10926–10934. doi:10.1074/jbc.M600299200. PMID:16497662
- Ode KL, Fujimoto K, Kubota Y, et al. Inter-origin cooperativity of geminin action establishes an all-or-none switch for replication origin licensing. Genes Cells. 2011;16:380–396. doi:10.1111/j.1365-2443.2011.01501.x. PMID:21426446
- Wuhr M, Freeman RM, Jr, Presler M, et al. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol. 2014;24:1467–1475. doi:10.1016/j.cub.2014.05.044. PMID:24954049
- Shinya M, Machiki D, Henrich T, et al. Evolutionary diversification of MCM3 genes in Xenopus laevis and Danio rerio. Cell Cycle. 2014;13:3271–3281. doi:10.4161/15384101.2014.954445. PMID:25485507
- Phillips RKJ, Theriot J, Garcia HG. Physical Biology of the Cell. Garland Sci. 2013.
- Li N, Zhai Y, Zhang Y, et al. Structure of the eukaryotic MCM complex at 3.8 A. Nature. 2015;524:186–191. doi:10.1038/nature14685. PMID:26222030
- Rowles A, Tada S, Blow JJ. Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J Cell Sci. 1999;112(Pt 12):2011–2018. PMID:10341218
- Gardner NJ, Gillespie PJ, Carrington JT, et al. The High-Affinity Interaction between ORC and DNA that Is Required for Replication Licensing Is Inhibited by 2-Arylquinolin-4-Amines. Cell Chem Biol. 24:981–992 e4. PMID:28781123
- Sanuki Y, Kubota Y, Kanemaki MT, et al. RecQ4 promotes the conversion of the pre-initiation complex at a site-specific origin for DNA unwinding in Xenopus egg extracts. Cell Cycle. 2015;14:1010–1023. doi:10.1080/15384101.2015.1007003. PMID:25602506
