ABSTRACT
Obesity hypoventilation syndrome (OHS) is a sleep disorder that has acquired great importance worldwide because of its prevalence and association with obesity leading to increased morbidity and mortality with reduced quality of life. The primary feature is insufficient sleep-related ventilation, resulting in abnormally elevated arterial carbon dioxide pressure (PaCO2) during sleep and demonstration of daytime hypoventilation. There are three main mechanisms that can generate diurnal hypoventilation in obese patients: alteration of the respiratory mechanics secondary to obesity; central hypoventilation secondary to leptin resistance and sleep disorder with sleep hypoventilation and obstructive apnoeas, which can be potentially solved with the use of positive airway pressure: non-invasive ventilation (NIV) and continuous positive airway pressure (CPAP). There are no established guidelines for the treatment of OHS, and only a few randomised controlled trials have been published. In this review, we have gone over the role of positive airway pressure, in particular the mechanisms that produce improvement, ventilatory modes available, clinical applications, technical considerations and future research. In addition, we added a review on NIV efficacy in chronic obstructive pulmonary disease (COPD), both in acute respiratory failure due to exacerbation and mainly in stable setting where more controversy and scientific contributions are coming.
Introduction
Sleep disorders have been grouped into eight categories according to the Third Classification of Sleep Disorders (ICSD3) Citation(1). One of the most studied groups and the most common are respiratory sleep disorders, in which obstructive sleep apnoea syndrome (OSA) and obesity hypoventilation syndrome (OHS) have acquired great importance worldwide because of its prevalence and association with obesity leading to increased morbidity and mortality with reduced quality of life (Citation1,Citation2).
The primary feature of sleep-related hypoventilation disorders is insufficient sleep-related ventilation, resulting in abnormally elevated PaCO2 during sleep; in addition, demonstration of daytime hypoventilation is required for a diagnosis of OHS. The diagnostic criteria are summarised in Citation(1).
Table 1. Diagnostic criteria for OHS.
Currently there are no established guidelines for the treatment of OHS, and only a few randomised controlled trials have been published. Available treatments can be divided into treatment of sleep-disordered breathing (including positive airway pressure; PAP), medical treatment and surgical treatment. In this review, we have gone over the role of positive airway pressure, considering two modalities: non-invasive ventilation (NIV) and CPAP in OHS, in particular the mechanisms that produce improvement, ventilatory modes available, clinical applications, technical considerations and future research.
Pathophysiology (potential mechanism of improvement with PAP)
Obesity compromises respiratory function; primarily in the supine position it induces hypoventilation by increasing the mechanical load on the respiratory system, resulting in fatigue and weakness of the respiratory muscles (Citation3,Citation4).
This difficulty and the tendency for decreased ventilation in the normal sleep physiology, hypotonia of the intercostal muscles and lower lung volume can lead to nocturnal oxyhaemoglobin desaturation and hypercapnia. Moreover, the accumulation of fat in the lateral parts of the pharynx intensifies extraluminal pressure and can modify the geometry of the upper airway, facilitating total (apnoeas) or partial (hypopnoeas) collapses Citation(5).
There are three main mechanisms that can generate diurnal hypoventilation in obese patients, which include: alteration of the respiratory mechanics secondary to obesity; central hypoventilation secondary to leptin resistance; and an altered compensatory response to chronic sleep hypoventilation and acute hypercapnia due to obstructive apnoeas and hypopnoeas (Citation6,Citation7).
The mechanisms by which diurnal hypercapnia improves with PAP are complex and not fully understood. (See for the potential mechanism of improvement with CPAP and NIV in OSH). PAP can influence in the following mechanisms: abnormal respiratory mechanics, including respiratory muscle dysfunction; central responses to hypercapnia and/or neurohormonal dysfunction (leptin resistance) and sleep-disordered breathing.
Figure 1. Potential mechanism of improvement with CPAP and NIV in OHS. Both CPAP and NIV can influence the following mechanisms to improve daytime hypercapnia: decrease the work breathing with the subsequent muscular rest, decrease the central resistance to leptin and decrease nocturnal obstructive events with the subsequent improvement in the threshold arousal and sleep hypercapnia. OHS, obesity hypoventilation syndrome; CPAP, continuous positive airway pressure; and NIV, non-invasive ventilation.
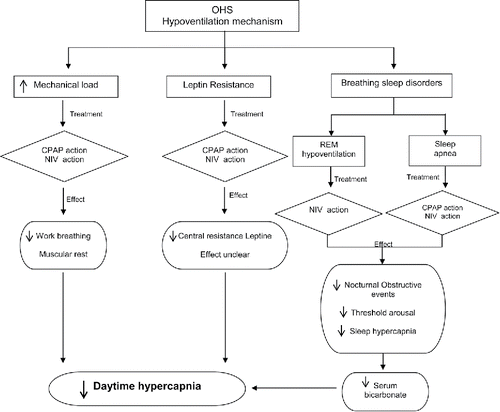
Respiratory mechanics: NIV can reduce inspiratory muscular activity Citation(8) and, consequently, it could decrease mechanical load, favouring muscular rest and greater muscular efficacy during the day after nocturnal NIV treatment. CPAP may decrease the mechanical load avoiding upper airway repetitive obstructions during sleep.
The improvement in muscular efficacy and the resolution of micro-atelectasis can result in greater lung volume. However, evidence of improvement in vital capacity and lung volume with chronic NIV treatment is contradictory. Several studies (Citation9–11) have shown no change in these parameters after an effective NIV treatment. In contrast, two other studies (Citation12,Citation13) have reported significant improvements in vital capacity and expiratory reserve volume after NIV therapy. In a recent study NIV treatment demonstrated to induce more functional benefits than CPAP with increases in FVC, forced expiratory volume in one second (FEV1) and distance in the 6 minutes walking test Citation(14).
Breathing control: Leptin is a protein released by adipose tissue whose functions are to reduce appetite and increase energy expenditure (Citation15–17). This protein is a potent respiratory stimulant Citation(18). Obesity can result in leptin resistance; obese subjects have significantly higher levels of this protein than lean subjects, but this does not reduce appetite, providing indirect evidence of resistance to its effects in the central nervous system Citation(19). Studies in animals have shown that this protein has a potent ventilation stimulus and its absence (or lack of effect) could produce hypoventilation Citation(20).
Patients with OHS might be resistant to leptin or may have a central leptin resistance (Citation18,Citation21,Citation22). The level of serum leptin decreases to normal limits in patients with OSA treated with CPAP (Citation23,Citation24), but it is assumed that apnoeas and hypopnoeas are the cause of the elevated leptin levels rather than being the result of them (Citation20,Citation25). Leptinaemia also decreases with NIV treatment (Citation26,Citation27) as does daytime hypercapnia, and some studies have shown a correlation between leptinaemia and a reduction in the hypercapnic ventilatory response Citation(28). Nevertheless, a study Citation(27) reported contradictory results—leptin increased with NIV. Therefore, the role of leptin in how NIV treatment achieves improvement is still unclear.
Sleep period: Nocturnal hypoventilation is the confluent factor of all sleep-breathing disorders. Although OHS can exist without sleep apnoea, approximately 90% of OHS patients have sleep apnoea (Citation10,Citation29). Repetitive obstructive events produce increasing hypercapnia that is not resolved with the hyperventilations that happen at the end of obstructive events. Despite correction of these nocturnal obstructive events with CPAP, daytime PaCO2 does not return to normal in all cases. Several studies have emphasised that the CPAP response may depend on the predominance of nocturnal obstructive events (Citation30,Citation31). Non-invasive ventilation can prevent obstructive events and reduce hypoventilation during sleep (including REM sleep). Both NIV and CPAP should decrease nocturnal hypercapnia, leading to lower daytime serum bicarbonate and consequently less blunting of the central carbon dioxide response Citation(32).
Evidence base
General treatment
Weight loss is the ideal treatment for OHS. It can improve respiratory failure, pulmonary hypertension and sleep disorders Citation(33). However, it is difficult to achieve and maintain significant weight loss in these patients. Limited long-term data are available about the efficacy of bariatric surgery in OHS Citation(34) and it is not an alternative for most of the patients because of significant morbidity and mortality Citation(35). Moderate weight loss can also improve PaCO2, although this outcome has not been confirmed over the long term Citation(33).
Positive airway pressure (CPAP and NIV)
For the first time, CPAP was used in a small series of patients in 1982 Citation(36) and NIV was used in 1992 Citation(37); currently, NIV and CPAP are used extensively worldwide. Clinical series have reported improvements in symptoms, arterial blood gases and sleep disorders with these treatments (Citation9,Citation38).
The short-term benefits of CPAP include improvement in gas exchange and sleep breathing disorders Citation(2) with a response between 50% and 80% of cases; it can improve the gas exchange in a period of 30–90 days. This improvement is directly proportional to the hours of CPAP use, as each hour of use of CPAP decreased 1.8 mmHg PaCO2 and PaO2 increases by 3 mmHg. It should be considered as initial therapy in these patients (Citation39,Citation40) especially for patients with severe OSA. Because not all OHS patients respond to CPAP treatment, it is suspected that responders may have predominance of apnoeic episodes over the alveolar hypoventilation Citation(30).
The long-term benefits of CPAP include improvement in lung function and respiratory drive to CO2. CPAP therapy for 24–48 weeks significantly increased FEV1 and forced vital capacity (FVC) (Citation12,Citation41).
As we have mentioned, CPAP treatment is able to prevent nocturnal obstructive events in patients with OHS, but nocturnal and daytime PaCO2 do not revert to normal values in all cases. A controlled study Citation(39) compared the impact of overnight CPAP titration in 23 patients with OHS and 23 patients with eucapnic OSA who were matched for body mass index (BMI), apnoea hypopnoea index (AHI), and lung function. Forty-three percentage of patients had refractory hypoxaemia during CPAP titration. From this and other studies (Citation39,Citation42–45), patients unresponsive to CPAP treatment appeared to have more obesity, higher PaCO2, and lower PaO2, and nocturnal oxygen saturation (SaO2), than responsive patients.
In a study in 2013, in 29 newly diagnosed, clinically stable OHS patients, CPAP treatment was commenced if the AHI was >15. Lung function, night-time oximetry, blood adipokine and C-reactive protein levels were assessed prospectively on enrolment and after 3 months. Treatment failure at 3 months was defined as daytime PaCO2 >45 mm Hg and/or oxygen saturation (SpO2) <90% for >30% of the night-time oximetry study. The percentage of time spent below 90% saturation improved from 8.4% (0.0–39.0%) to 0.3% (0.4–4.0%). Awake PaCO2 decreased from 50 (Citation47–53) mm Hg to 43 (Citation40–45) mm Hg. Seven patients failed CPAP treatment after 3 months. Therefore, this study concluded that CPAP treatment improves night-time oxygenation and daytime hypoventilation in selected clinically stable OHS patients who also have OSA. Patients with worse night-time saturation while on CPAP and higher daytime PaCO2 at 1 month were more likely to fail CPAP treatment Citation(46).
A Spanish group was one of the pioneers in demonstrating, in a series of cases, that NIV is able to improve clinical and arterial blood gas parameters Citation(47) and longitudinal studies have found a decrease in days of hospital admission when introducing this treatment (Citation10,Citation48). At the present time, there are three randomised controlled studies that compare different treatments in OHS, one of them compares CPAP to NIV Citation(49), other NIV to conservative measures Citation(14) and more recently the first results of the largest clinical trial (Pickwick study) done in this topic that include the three most extended treatment modalities: lifestyle modification, CPAP and NIV have been published Citation(14).
The first of the randomised trials compared the short-term efficacy of NIV and CPAP treatments in 36 OHS patients, selected for their favourable response to an initial night of CPAP treatment Citation(49). From 45 eligible patients, nine (20%) did not achieve acceptable improvement with CPAP based in the following criteria: SaO2 remaining below 80% continuously (>10 minutes) in the absence of apnoeas; acute increase in transcutaneous PaCO2 during REM sleep (>10 mm Hg); or increase in afternoon to morning PaCO2 of >10 mm Hg in patients with an awake PaCO2> 55 mm Hg. After 3 months, the results were that the daytime sleepiness, clinical, gasometric and polysomnographic improvement at 3 months was similar between the two types of treatments (CPAP and NIV groups).
The second trial was a small randomised study of 38 patients with mild hypercapnia compared NIV versus a control group with conservative measures; in this study, as well as arterial blood gases and polysomnographic variables, the glucidic and lepidic metabolism and the inflammatory profile were analysed. The NIV group had a significant reduction in diurnal PaCO2, bicarbonate and an increase in pH. The treatment with NIV, as could be expected, was associated with a huge improvement in all sleep variables analysed, sleep architecture, mean oxygen SaO2, time spent with oxygen SaO2 under 90%, AHI, with a positive and significant correlation between mean SaO2 during sleep and daytime arterial blood gases. In contrast no change was observed in any of the metabolic and inflammatory parameters studied; but follow-up was only 1 month that no other conclusions could be drawn Citation(50). In this work, the patients had a lower BMI, and were less hypercapnic than subjects included in other trials (Citation14,Citation49).
The Pickwick study, still under development, is the largest clinical trial performed in patients with OHS to date; this is a very ambitious project that intends to provide the necessary evidence to answer which is the best choice in OHS treatment Citation(20). This compares the results obtained through treatment with NIV, CPAP or lifestyle change measures with a goal of including more than 300 patients. The first results from the Pickwick study to determine the efficacy of NIV, CPAP and lifestyle modification in OHS are available Citation(14). The first publication includes 221 OHS patients with severe OSA randomised in three groups: NIV, CPAP and lifestyle counselling. Polysomnographic data, arterial blood gases, spirometry, 6 minutes walking distance test and quality of life questionnaires were performed at baseline and after 2 months. PaCO2 improved with each of the three treatments but the improvement was greater with NIV with a significant difference only relative to the conservative measures group. In the CPAP group the PaCO2 reduction was dependent on treatment compliance. NIV and CPAP decreased bicarbonate blood levels, but after adjusting by baseline values only the NIV achieves statistical significance compared to the control group. Sleep variables markedly improved with NIV and CPAP without differences between these treatments. Only in the NIV group, increments in FVC, FEV1 and 6 minutes walking test values were found. In another publication from this Pickwick study, 86 OHS patients without severe OSA were randomised and treated for 2 months either with NIV or lifestyle modifications Citation(51). After 2 months of treatment PaCO2 and serum bicarbonate improved significantly in the NIV group compared to the control group. The impact of these treatments in the long term and its influence in hospital resource utilisation, cardiovascular and all-cause morbidity and mortality as well as the cost effectiveness is still unknown.
AVAPS
Recent ventilators offer the possibility of estimating the expiratory tidal volume and responding by adjusting the inspiratory positive airway pressure (IPAP) to maintain ventilation. This new mode of treatment for patients with OHS is called average volume-assured pressure support (AVAPS) or target volume. This mode of ventilation ensures the delivery of a current volume during a double pressure or bilevel ventilation. Expiratory flow volume and leakage are estimated based on inspiratory and expiratory pneumotacrographic flows. The tidal volume is typically set at 8–10 ml/kg of ideal weight. The expiratory pressure is adjusted to resolve upper airway obstruction and the inspiratory pressure is automatically adjusted to reach the target tidal volume. This mode also provides a backup frequency for control of central apnoeas that may be emergent during positive pressure treatment (Citation50,Citation52).
In a small group of patients already treated with NIV, this ventilation modality has demonstrated a similar efficacy to the standard Citation(53). Storre et al. Citation(54) compared bi-level pressure support with AVAPS in a randomised crossover trial. Ten patients with OHS who did not respond to CPAP treatment were included. Six weeks of therapy with AVAPS achieved greater improvement in nocturnal and daytime PaCO2 than bi-level pressure support. However, changes in sleep quality and quality of life were similar between the two ventilation modes. Janssen Citation(55) compared bilevel with usual settings with AVAPS in a group of 12 patients in two consecutive nights and they found that although volume targeting improved control of nocturnal hypoventilation, the sleep fragmentation and impaired subjective sleep quality increase. Finally, Murphy et al. Citation(56) compared, in a single-blind randomised study, this ventilation mode during 3 months in 50 super-obese patients, studying daytime PCO2 as a main outcome and they concluded that fixed bilevel pressure support is as effective as AVAPS. Therefore, until more data is available, AVAPS may be beneficial in OHS patients without super-obesity.
Clinical applications
CPAP or NIV
NIV and CPAP have a role in OHS treatment. Both therapies improve gas exchange, sleep breathing disorders (SBD) and probably lung function and central respiratory impulse to CO2. Nocturnal hypoventilation can be effectively improved but not in all (Citation57–60) and diurnal PaCO2 reduced or restored to normal values Citation(59).
In clinical practice, the selection of patients for one or the other treatment is not clearly established, but conceptually CPAP should be the initial modality of treatment if severe OSA is present, because of its relative simplicity, low cost and effectiveness Citation(58). In OHS patients without significant OSA NIV should be preferred.
Therefore, a more refined selection of patients for NIV or CPAP treatment could be used until more long-term information becomes available. Patients with a large number of apnoeas and, consequently, high sleep time in apnoea may very well respond to CPAP Citation(33). On the other hand, in patients without a significant number of apnoeas, their nocturnal hypoventilation could depend on other mechanisms (i.e. obesity), or if previously CPAP failed then NIV should be preferable.
If CPAP prevents obstructive events and maintains adequate oxygenation and ventilation, CPAP could be a good choice for long-term treatment, and NIV should be used if this is not the case. However, some non-controlled studies and one randomised controlled trial have shown that NIV may be more strongly indicated than CPAP in patients with a predominance of hypoventilation over obstructive events during sleep, as well as with a higher obesity, higher PaCO2 and lower PaO2 while awake (Citation14,Citation32,Citation34,Citation39,Citation42–44,Citation60,Citation61) (see for the NIV and CPAP treatment in OHS).
Figure 2. Non-invasive ventilation and CPAP treatment role in OHS. CPAP should be the initial modality of treatment if severe OSA is present (predominance of obstructive events); if in subsequent evaluations the patient responds with adequate oxygenation and ventilation CPAP should be continued; if the patient does not have adequate response with CPAP treatment, or if initially the OHS patient has no significant OSA or has a hypoventilation predominance, NIV should be preferred. OHS, obesity hypoventilation syndrome; OSA, obstructive sleep apnoea; CPAP, continuous positive airway pressure; and NIV, non-invasive ventilation.
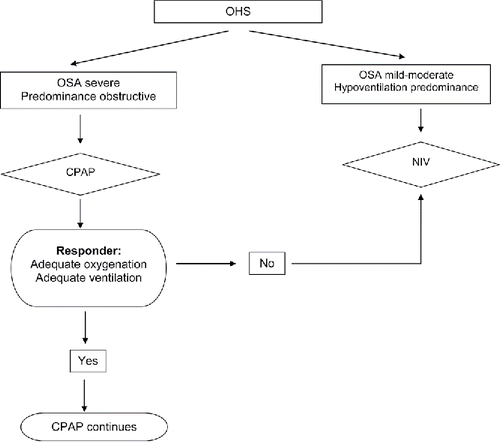
In patients with acute hypercapnic respiratory failure, NIV should be the first choice, due to an apparently higher efficacy, the severity of respiratory failure, and because probably OSA is not the only cause of the acute respiratory failure of these patients.
Length of treatment
The duration of treatment with NIV or CPAP is not well established, although it seems to be indefinite.
In a prospective study Citation(62), 12 OHS patients, successfully treated with NIV for 1 year, were required to cease the treatment for 3 months. They had diurnal and nocturnal PaO2 ≥ 60 mm Hg and PaCO2 ≤ 45 mm Hg as well as nocturnal SaO2 ≥ 90% for more than 70% of the night. After the withdrawal period, daytime and nocturnal pH, PaO2 and PaCO2 were similar to the previous period. Although these patients would probably show worse blood gas measurements with greater withdrawal time, this study suggests the possibility of discontinuing NIV for short (weekend) or intermediate (vacation) periods in some patients who desire it.
When to treat
The standard indication of NIV initiation is in patients who meet the definition and diagnostic criteria of OHS: those with obesity (BMI > 30 kg/m2) with daytime hypercapnia (PaCO2 > 45 mm Hg) and without other potential causes of hypercapnia such as severe obstructive or restrictive pulmonary disease (significant kyphoscoliosis or neuromuscular diseases), severe hypothyroidism or other central hypoventilation syndromes Citation(33).
However, there is a group of patients that can be considered as an early phase of OHS, being those patients with obesity with relevant nocturnal hypoventilation without diurnal hypercapnia and that at least in some of these individuals will result in their evolution in daytime hypercapnia following a progressive increase of bicarbonate. A non-randomised study Citation(59) compared the efficacy of sequential treatments with oxygen and NIV in a group of 11 patients with obesity, who had nocturnal hypoventilation without daytime respiratory failure or relevant apnoeic episodes during sleep. Only NIV improved all clinical symptoms and nocturnal PCO2, maintaining a level of oxygenation similar to oxygen therapy; early initiation of NIV treatment can improve clinical symptoms and possibly prevent the development of daytime hypercapnia.
Obese patients with daytime PaCO2 close to the upper normal limit (45 mmHg) and bicarbonate ≥27 mmol/L could also be considered mild OHS patients and treated with PAP (Citation40,Citation49,Citation63).
Additional oxygen therapy
In one study, obese patients with nocturnal hypoventilation but without daytime hypercapnia were treated first with oxygen and then with NIV; oxygen increased transcutaneous PaCO2 during sleep compared with NIV Citation(59).
While oxygen therapy is commonly supplemented to NIV in patients with persistent hypoxaemia, there are no available data about the long-term benefits or clear deleterious effect of this procedure.
Recently in a post hoc analysis in the Pickwick study, 302 sequentially screened OHS patients who were randomly assigned to non-invasive ventilation, CPAP or lifestyle modification were reanalysed. The objective was to assess the medium-term treatment efficacy of adding supplemental oxygen therapy to commonly prescribed treatment modalities in OHS. Outcomes at 2 months included arterial blood gases, symptoms, quality of life, blood pressure, polysomnography, spirometry, 6-minute walk distance and hospital resource utilisation. In the non-invasive ventilation group, supplemental oxygen reduced systolic blood pressure although this could be also explained by a reduction in body weight experienced in this group. In the CPAP group, supplemental oxygen increased the frequency of morning confusion. In the lifestyle modification group, supplemental oxygen increased compensatory metabolic alkalosis and decreased the AHI during sleep. Chronic oxygen therapy produced marginal changes that were insufficient to consider it, globally, as beneficial or deleterious. Long-term studies examining outcomes such as incident cardiovascular morbidity and mortality are necessary Citation(64).
Follow-up
There are currently no standard recommendations for follow-up of patients with OHS treated with NIV. As previously mentioned the improvement is proportional to the hours of PAP use, as each hour of use decreased 1.8 mmHg PaCO2 and PaO2 increased by 3 mmHg, and this improvement in gas exchange will be seen in a period of 30–90 days (Citation39,Citation40). So we could consider a first visit after a week of the beginning of the NIV, at the first month and then each 3 or 6 months, depending always on the good compliance and tolerance and clinical evolution of each patient. However we cannot assure the correct follow-up time since there are no clinical trials that corroborate it. A long-term efficacy and treatment compliance monitoring (or tele-monitoring) is necessary.
Technical considerations
Setting and titration
Continuous positive airway pressure
CPAP can be titrated in a similar way as that for OSA but there is not uniform NIV titration consensus Citation(65). The pressure used for OHS treatment is usually higher than that used for patients with OSA (without daytime hypercapnia). The mean pressure required in some studies was about 14 cm H2O (Citation39,Citation49).
Determining optimal CPAP in OSA patients is well understood Citation(66). For OHS, in some studies (Citation39,Citation49) pressure was increased once obstructive events were eliminated to improve nocturnal oxygen desaturation. However, it is not clear if this process has benefits for nocturnal or daytime hypercapnic respiratory failure.
Today, auto-CPAP titration is a common procedure for achieving optimal CPAP in OSA patients Citation(67), because most studies of the efficacy of auto-titration excluded patients with OHS, the efficacy of these devices is unknown in these patients.
Non-invasive ventilation
Bilevel pressure support is the most frequently employed mode. IPAP can be high, and the average is frequently around 18–20 cm H2O (Citation48,Citation49). The level of expiratory positive air pressure (EPAP) or CPAP can vary depending on the presence and severity of OSA. Although a fixed respiratory rate is not required in this ventilation mode (pressure support), some studies used a security rate of 12–15 breaths/minute instead of spontaneous bilevel ventilation to assure a respiratory rate during sleep Citation(14). Although this setting could introduce asynchronies, Contal et al. Citation(68) observed that low or high levels of backup rates reduce the number of central and mixed respiratory events. The starting backup rate should be equal to or slightly less than the patient's spontaneous frequency during sleep (at least 10 breaths per minute), if the respiratory rate during sleep is unknown, a frequency of 2 rpm below the respiratory rate at rest of the patient Citation(65).
Classically, treatment with NIV in OHS has not been nocturnally titrated. However, most patients with OHS have concurrent sleep apnoea, requiring at least EPAP adjustment. Furthermore, IPAP can prevent hypopnoeas and hypoventilation, depending on the level of pressure used. Therefore, arguments for performing titration during sleep in OHS are similar to those for OSA. Two approaches using PSG have been recommended: conventional titration of EPAP (CPAP) as it is used in OSA, and IPAP used to improve nocturnal desaturation (SaO2 > 90%) (Citation49,Citation65,Citation69); in the second approach EPAP could be used to prevent apnoeas (static obstruction) and IPAP could be used to prevent hypopnoeas (dynamic obstruction) and improve nocturnal desaturation (hypoventilation) (Citation14,Citation70). The last method can result in lower EPAP and similar IPAP, which would be more comfortable for the patient, potentially improving adherence and compliance.
At the present time there is not wide agreement if PSG, respiratory polygraphy or simpler tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation should be used for PAP adjustment and titration Citation(71).
NIV has been effective in the management of chronic respiratory failure, modern ventilators, controlled by complex algorithms, and with integrated monitoring allows for sophisticated customisation of ventilatory support to an individual. Experience of monitoring during sleep from patients predominantly with sleep apnoea can be transferred and extended to patients receiving NIV. To understand how assisted ventilation helps patients, an understanding of the pathophysiology of ventilatory failure and the way in which ventilators work is required. For unassisted breathing to be effective the respiratory muscle pump must have the capacity to sustain ventilation against a given load. It also requires neural drive from the central nervous system. In other words, there needs to be a balance between load and capacity, with the system receiving adequate drive Citation(72).
The effectiveness of NIV might therefore be more correctly assessed by sleep studies than by daytime assessment. The most available and simple monitoring can be done from flow and pressure curves from the mask or the ventilator circuit. Examination of these tracings can give useful information to evaluate if the settings chosen by the operator were the right ones for that patient. Ventilatory modality, mode of triggering, pressurisation slope, use or not of positive end expiratory pressure and type of exhalation as well as ventilator performances may all have physiological consequences. Leaks and upper airway resistance variations may, in turn, modify these patterns Citation(73).
Complex respiratory events, which may have a detrimental effect on both quality of sleep and control of nocturnal hypoventilation, occur during sleep in patients treated with NIV. Among these events are patient–ventilator asynchrony, increases in upper airway resistance (with or without increased respiratory drive) and leaks. Detection of these events is important in order to select the most appropriate ventilator settings and interface. Simple tools are available for monitoring the efficacy of long-term NIV. Pulse oximetry is the simplest approach. It is a rough indicator of major problems related to NIV but loses sensitivity in subjects with oxygen supplementation. Abnormalities on pulse oximetry tracings are non-specific and do not allow a distinction to be made between, for instance, V/Q mismatch versus alveolar hypoventilation. However, pulse oximetry is a very useful screening tool in stable patients receiving home ventilation. By using pulse wave amplitude analysis, oximetric recordings can also provide indirect assessment of sleep fragmentation when using NIV. Transcutaneous capnography helps discriminate between hypoxaemia related to ventilation/perfusion mismatch and hypoventilation, documents correction of nocturnal hypoventilation and may detect ventilator-induced hyperventilation, a possible cause for central apnoea/hypopnoea and glottic closure Citation(71).
Treatment time
OHS patients need nocturnal NIV to obtain dramatic improvement. However in patients with extreme obesity and residual daytime hypercapnia, one option is to extend treatment to the daytime, although there is no formal evidence for the effectiveness of this regimen in OHS.
Interfaces
The most extensive experience in long-term treatment for chronic failure is with the nasal mask. Nevertheless, as with other diseases treated using PAP, switching to another kind of mask or nasal prongs could be beneficial if there is a justifiable cause (e.g. important oral leakage might indicate a switch to an oronasal mask) Citation(74).
Future research
CPAP and NIV have been and nowadays are extensively used for the treatment of OHS even when solid long-term scientific evidence is lacking. There is still a need of controlled studies or at least big long observational series to answer important clinical questions about long-term benefits with PAP treatment, if bicarbonate is a useful marker of early disease, how must this treatment should be titrated, the relevance of the adherence, when oxygen should be added, the impact of the new ventilatory modes, how can the data recordings from the ventilator be used or the role and new tools for tele-monitoring.
We are waiting for the long-term data results from the Spanish Pickwick study, and two important trials are ongoing comparing manual and automatic titration of NIV in OHS. A huge increase in the knowledge in OHS is coming in the next years and some relevant questions in this topic will be clarified.
Non-invasive ventilation in COPD exacerbation and stable situations
NIV is a recognised therapy for several chronic diseases that result in nocturnal and daytime hypoventilation, as we saw previously, especially in conditions that cause restriction of the chest wall like OHS and neuromuscular diseases. It is also approved for treatment of acute or chronic situation of respiratory diseases like chronic obstructive pulmonary disease (COPD) but there is not enough evidence on the efficacy of NIV in chronic setting.
NIV in COPD exacerbation
Role of NIV in the management of COPD exacerbation
NIV plays a critical role in the management of acute COPD exacerbations with hypercapnic respiratory failure. NIV is a very effective treatment for hypercapnic respiratory failure in COPD exacerbations, reducing endotracheal intubation rate, treatment failure and short-term mortality, as well as improving acidosis in the first few hours of treatment Citation(75). Despite this, NIV is not an appropriate option for all patients; the indications and relative and absolute contraindications shown in should be considered. Recently, a large multicentric observational study has observed similar outcomes in COPD patients treated with NIV in intermediate care units with and without a severe acidosis (pH < 7.25), suggesting that severe may not be a limiting factor Citation(76).
Table 2. Indications and contraindications for non-invasive ventilation.
Once NIV is initiated, arterial blood gases monitoring is required to ensure resolution of acidosis and improvement of gas exchange Citation(77).
Potential mechanisms to explain the improvement with NIV
Limited information exists about the efficacy and mechanisms by which NIV produces improvement in an acute setting. They are probably not very different from those mentioned previously. However to reduce the load on respiratory muscles and to avoid micro-atelectasis, as well as to improve central chemosensitivity, compliance of the chest wall and lungs and sleep quality can be the most important factors Citation(78).
Evidence base
Most of the systematic reviews included hospitalised patients with respiratory failure due to a COPD exacerbation, confirmed acute or acute-on-chronic hypercapnic respiratory failure. Most the studies compared usual care plus NIV to usual care alone, although a few randomised patients to habitual care plus NIV or usual care plus sham NIV. When the trials were considered collectively by means of a meta-analysis, patients who received NIV had a lower mortality rate, less rate of intubation, shorter duration of hospital stay, and days in intensive care unit (ICU), and less complications of treatment Citation(79).
Guidelines base
The 2017 GOLD strategy document recommends the use of NIV mainly during respiratory failure due to COPD exacerbation if the patient has severe dyspnoea, muscular fatigue, use of accessory muscles or persistent hypoxaemia despite supplemental oxygen. Also the NIV can improve the survival of patients with COPD after hospitalisation, especially in those with daytime hypercapnia PaCO2 greater than 57 mmHG Citation(80).
The European Respiratory Society and the American Thoracic Society (ERS/ATS) recommendations are for hospitalised patients with acute or acute-on-chronic hypercapnic respiratory failure due to a COPD exacerbation Citation(79).
Treatment time
British Thoracic Society (BTS) guidelines advise that the time on NIV should be maximised in the first 24 hours, depending on patient tolerance and/or complications. Thereafter, NIV use during the day can be narrowed over the following 2–3 days, depending on the PaCO2 levels measured while self-ventilating, before being discontinued overnight. NIV can be discontinued when there has been normalisation of pH and PCO2 and a general improvement in the patient's condition Citation(81).
NIV in stable COPD
Once the COPD patients develop chronic respiratory failure (CRF) (when PaCO2 is persistently > 45 mmHg with compensated respiratory acidosis), their prognosis worsens and they are more likely to develop exacerbations, be admitted to hospital and/or ICU and experience a deterioration of their disease. However, to date, few interventions have been shown to reduce the frequency of exacerbations in patients with advanced COPD and CRF.
Home long-term oxygen therapy (LTOT) has been shown to improve survival in patients with severe COPD and chronic hypoxaemia. Long-term NIV has been proposed, in addition to LTOT, to treat chronic hypercapnia, with the theoretical rationale of improving gas exchange, unloading the respiratory muscles and resetting the central respiratory drive Citation(82).
NIV has been shown to improve outcomes in stable COPD patients however, the evidence for the long-term benefits of NIV remains rather scarce and a matter of controversy (Citation83,Citation84).
Role of NIV in the management of stable COPD
One of the uses of NIV could be for COPD patients with chronic respiratory failure and severe COPD. Acute-on-chronic respiratory failure occurs when there is an acute deterioration of the pre-existing state of chronic respiratory failure. One of the goals of long-term NIV at home is to persistently reduce hypercapnia and improvement in mortality in these patients (Citation84,Citation85).
Evidence base
In 2012 a meta-analysis searched the effectiveness of NIV, compared with no ventilation while receiving usual care for stable COPD patients. The database included 10 eligible studies published between 2004 and 2010. The results of this analysis show short-term beneficial effects of NIV on oxygen and carbon dioxide levels and on exercise tolerance. There were no long-term beneficial effects of NIV on mortality, FEV1, oxygen levels, carbon dioxide levels and exercise tolerance. The qualitative assessment found a beneficial effect of NIV on breathlessness but no effect on hospitalisations Citation(85).
Later, a new meta-analysis in 2013 evaluated the effect of NIV on severe, stable COPD comparing with sham ventilation or no ventilation. Eight parallel and three crossover randomised controlled trials met the inclusion criteria. Analysis from parallel randomised controlled trials showed NIV did not change the mortality for 12 or 24 months (OR 0.82, 95% CI: 0.48–1.41). Although NIV improved the PaCO2 did not improve FEV1, maximal inspiratory pressure or 6-minute walk distance. The conclusions of this meta-analysis were similar to those previously mentioned; therefore, NIV should be cautiously used in severe stable chronic obstructive pulmonary disease Citation(86).
The effectiveness of NIV in COPD patients with prolonged hypercapnia after ventilator support for acute respiratory failure remains unclear, so in 2014 a randomised, controlled, parallel-group study investigated if nocturnal NIV prolongs the time to remission for respiratory causes or death in the following 12 months. The study included 201 patients admitted to hospital with acute respiratory failure and prolonged hypercapnia >48 hours after termination of ventilator support. They were randomised to NIV or standard treatment; the results for 1 year after discharge shown that 65% versus 64% of patients (NIV vs standard treatment) were readmitted to hospital for respiratory causes. Both daytime PaCO2 and transcutaneous PCO2 during the night were significantly improved with NIV in comparison with standard treatment. The number of exacerbations, lung function, mood state, daily activity levels or dyspnoea was not significantly different Citation(87).
In 2014 a prospective, multicentre, randomised and controlled clinical trial was conducted, with the aim of markedly reducing hypercapnia with NIV in survival patients with advanced, stable hypercapnic COPD. A total of 195 patients with stable GOLD stage IV COPD and PaCO2 of 51.9 mmHg or higher and pH higher than 7.35 were recruited from 36 respiratory units in Germany and Austria. Patients were randomly allocated to continue optimised standard treatment (control group) or to receive additional NIV for at least 12 month (intervention group). One-year mortality was 12% in the intervention group and 33% in the control group with a hazard ratio of 0.24 (95% CI 0.11–0.49; p = 0.0004). The conclusion of this study was that the addition of long-term NIV to standard treatment improves survival of patients with hypercapnic, stable COPD when NIV is targeted to greatly reduce hypercapnia Citation(88).
A new meta-analysis was published in 2016 in the UK. The review was undertaken in order to compare the relative effectiveness of different types of domiciliary NIV and usual care on hospital admissions, mortality, and health-related quality of life. Thirty-one studies were included. The results showed that for stable patients, there was no survival benefit from NIV, but there was a possible trend toward less hospitalisations and an improvement in health-related quality of life. For post-hospital patients, survival benefit could not be demonstrated within the three randomised controlled trials, although there was evidence of benefit from four non-randomised controlled trials. Post hoc analyses suggested that improvements in hypercapnia with NIV were associated with reduced hospital admissions in both populations. The conclusion was the effectiveness of domiciliary NIV remains uncertain; however, some patients may benefit Citation(89).
In summary, NIV in stable COPD patients can improve gas exchange, and maybe quality of life and sleep quality. Long-term NIV may be an effective treatment to reduce readmissions in stable COPD patients with episodes of acute hypercapnic respiratory failure. However the benefit of domiciliary NIV to prolong survival is unclear yet.
Future research
The efficacy of home-long term NIV treatment after an in-hospital acute-on-chronic respiratory failure treated with NIV is an area that requires additional study, including the optimal NIV technique and interface type selection Citation(79).
Further research is required to identify patients who may have more benefits with domiciliary NIV as well as to explore the relevance of improvements in hypercapnia in influencing clinical outcomes. Optimal time points for beginning home NIV and equipment settings need to be established Citation(89).
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd edition. Darien, IL: American Academy of Sleep Medicine, 2014.
- Pérez de Llano LA, Golpe R, Piquer MO, Racamonde AV, Caruncho MV, López MJ, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Aldrich T, Arora NS, Rochester DF. The influence of airway obstruction and respiratory muscle strength on maximal voluntary ventilation. Am Rev Respir Dis 1982; 126:195–199.
- Lavietes M, Clifford E, Silverstein D, Stier F, Reichman LB. Relationship of static respiratory muscle pressure and maximum ventilatory ventilation. Respiration 1979; 38:121–126.
- Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax 2008; 63:738–746.
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010; 55:1347–1362.
- Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med 2011; 183:292–298.
- Pankow W, Hijjeh N, Schüttler F, Penzel T, Becker HF, Peter JH, et al. Influence of noninvasive positive pressure ventilation on inspiratory muscle activity in obese subjects. Eur Respir J 1997; 10:2847–2852.
- Masa JF, Celli BR, Riesco JA, Hernández M, Sánchez de Cos J, Disdier C. The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest 2001; 119:1102–1107.
- Pérez de Llano LA, Golpe R, Ortiz Piquer M, Veres Racamonde A, Vázquez Caruncho M, Caballero Muinelos O, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation síndrome. Chest 2005; 128:587–594.
- Chouri-Pontarollo N, Borel JC, Tamisier R, Wuyam B, Levy P, Pépin JL. Impaired objective daytime vigilance in obesity-hypoventilation syndrome: impact of noninvasive ventilation. Chest 2007; 131:148–155.
- Heinemann F, Budweiser S, Dobroschke J, Pfeifer M. Non-invasive positive pressure ventilationimproves lung volumes in the obesity hypoventilation syndrome. Respir Med 2007; 101:1229–1235.
- De Lucas-Ramos P, de Miguel-Díez J, Santacruz-Siminiani A, González-Moro JM, Buendía-García MJ, Izquierdo-Alonso JL. Benefits at 1 year of nocturnal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Respir Med 2004; 98:961–967.
- Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, González M, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick Study. Am J Respir Crit Care Med 2015; 192:86–95.
- O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 1999; 159:1477–1484.
- Considine R, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292–295.
- Tankersley C, O'Donnell C, Daood MJ, Watchko JF, Mitzner W, Shwartz A, et al. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol 1998; 85:2261–2269.
- Atwood CW. Sleep-related hypoventilation: the envolving of role of leptin. Chest 2005; 128:1079–1081.
- Pierce AM and Brown, LK. Obesity hypoventilation syndrome: current theories of pathogenesis. Curr Opin Pulm Med 2015, 21:557–562.
- López-Jiménez MJ, Masa JF, Corral J, Terán J, Ordaz E, Troncoso MF, et al. Mid- and long-term efficacy of non-invasive ventilation in obesity hypoventilation syndrome: The Pickwick's study. Arch Bronconeumol 2016; 52:158–165.
- Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology 2012; 17:402–411.
- Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of the leptin in the respiratory system: an overview. Respir Res 2010; 11:1–16.
- Ip MS, Lam KS, Ho C, Tsang KW, Law W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 2000; 118(3):580–586.
- Phipps PR, Starrit E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax 2002; 57:75–76.
- Fitzpatrick M. Leptin and the obesity hypoventilation syndrome: a leap of faith? Thorax 2002; 57:1–2.
- Yee BJ, Cheung J, Phipps P, Banerjee D, Piper AJ, Grunstein RR. Treatment of obesity hypoventilation syndrome and serum leptin. Respiration 2006; 73:209–212.
- Redolfi S, Corda L, La Piana G, Spandrio S, Prometti P, Tantucci C. Long-term noninvasive ventilation increases chemosensitivity and leptin in obesity-hypoventilation syndrome. Respir Med 2007; 101:1191–1195.
- Campo A, Freihbeck G, Zueta JJ, Iriarte J, Seijo LM, Alcaide AB, et al. Hypercapnic response in obese patients. Eur Respir J 2007; 30:223–231.
- Kessler R, Chaovat A, Schinkewitch P, Faller M, Casel S, Krieger J, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001; 120:369–376.
- Berger KI, Ayappa I, Chatr-amontri B, Marfatia A, Sorkin IB, Rapoport DM, et al. Obesity hypoventilation syndrome as a Spectrum of respiratory disturbances during sleep. Chest 2001; 120:1231–1238.
- Pérez de Llano LA, Golpe R, Ortiz Piquer M, Racamonde AV, Caruncho MV, López MJ, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008; 75:34–39.
- Mokhlesi B, Tulaimat A, Faibursowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118:948–956.
- Martí-Valeri C, Sabaté A, Masdevall C, Dalmau A. Improvement of associated respiratory problems in morbidly obese patients after open roux-en-y gastric bypass. Obes Surg 2007; 17:1102–1110.
- Surgeman HJ, Fairman RP, Sood RK, Engle K, Wolfe L, Kellum JM. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr 1992; 55:5975–6015.
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983; 128:177–181.
- Waldhorn RE. Nocturnal nasal intermittent positive pressure ventilation with bi-level positive airway pressure (BiPAP) in respiratory failure. Chest 1992; 101:516–521.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grustein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest 2007; 131:1678–1684.
- Mokhlesi B, Tulaimat A, Evans AT, Wang, Itani AA, Hassaballa HA, et al. Impact of adherence with positive airway pressure therapy on hypercapnia in obstructive sleep apnea. J Clin Sleep Med 2006; 2:57–62.
- Budweiser S, Riedl SG, Jorres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesityhypoventilationsyndrome undergoing noninvasive ventilation. J Intern Med 2007; 261:375–383.
- Resta O, Guido P, Picca V, Sabato R, Rizzi M, Scarpelli F, et al. Prescription of nCPAP and nBIPAP in obstructive sleep apnoea syndrome: Italian experience in 105 subjects. A prospective two centre study. Respir Med 1998; 92:820–827.
- Rabec C, Merati M, Baudouin N, Foucher P, Ulukavac T, Reybet-Degat O. Management of obesity and respiratory insufficiency. The value of dual-level pressure nasal ventilation. Rev Mal Respir 1998; 15:269–278.
- Schäfer H, Ewig S, Hasper E, Lüderitz B. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med 1998; 92:208–215.
- Piper AJ, Sullivan CE. Effects of short-term NIPPV in the treatment of patients with severe obstructive sleep apnea and hypercapnia. Chest 1994; 105:434–440.
- Salord N, Mayos M, Miralda RM, Farré A, Carreras M, Sust R, et al. Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea. Respirology 2013; 18(7):1135–1142.
- Masa JF, Celli BR, Riesco JA, Hernández M, Sánchez de Cos J, Disdier C. The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest 2001; 119:1102–1107.
- Janssens JP, Derivaz S, Breitenstein E, Muralt B, Fitting JW, Chevrolet JC, et al. Changing patterns in long-term noninvasive ventilation: a 7 year prospective study in the Geneva lake area. Chest 2003; 123:67–79.
- Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63:395–401.
- Borel JC, Tamisier R, Gonzalez-Bermejo J, Baquet JP, Monneret D, Arnold N, et al. Noninvasive ventilation in mild obesity hypoventilation syndrome. A randomized controlled trial. Chest 2012; 141:692–702.
- Masa JF, Corral J, Caballero C, Barrot E, Terán-Santos J, Alonso-Álvarez ML, et al. Noninvasive ventilation in obesity hypoventilation syndrome without severe sleep apnea. Thorax 2016; 71(10):899–906.
- Johnson KG, Johnson DK. Treatment of sleep-disordered breathing with positive airway pressure devices: technology update. Med Devices 2015:8 425–437.
- Jaye J, Chatwin M, Dayer M, Morrell MJ, Simonds AK. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. Eur Respir J 2009;33:566–571.
- Storre JH, Seuthe B, Fiechter R, Milioglou S, Dreher M, Sorichters S, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest 2006; 130:815–821.
- Janssens JP, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity-hypoventilation. Respir Med 2009; 103:165–172.
- Murphy PB, Davidson C, Hind MD, Simonds A, Williams AJ, Hopkinson NS, et al. Volumen targered versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomized controlled trial. Thorax 2012; 67:727–734.
- Lee WY, Mokhlesi B. Diagnosis and management of obesity hypoventilation syndrome in the ICU. Crit Care Clin 2008; 24:533–549.
- Chau EH, Babak Mokhlesi, Chung F. Obesity hypoventilation syndrome and anesthesia. Sleep Med Clin 2013; 8(1):135–147.
- Masa JF, Celli BR, Riesco JA, Sánchez de Cos J, Disdier C, Sojo A. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall diseases. Chest 1997; 112(1):207–213.
- Hida W, Okabe S, Tatsumi K, Kimura H, Akasiba T, Chin K, et al. Nasal continuous positive airway pressure improves quality of life in obesity hypoventilation syndrome. Sleep Breath 2003; 7(1): 3–12.
- Smith IE, King MA, Siklos PW, Shnnerson JM. Treatment of ventilatory failure in the Prader-Willi syndrome. Eur Respir J 1998; 11(5):1150–1152.
- De Miguel Díez J, De Lucas Ramos P, Pérez Parra JJ, Buendía García MJ, Cubillo Marcos JM, González-Moro JM. Analysis of withdrawal from noninvasive mechanical ventilation in patients with obesity-hypoventilation syndrome. Medium term results. Arch Bronconeumol 2003; 39(7):292–297.
- Pepin JL, Borel JC, Janssens JP. Obesity Hypoventilation Syndrome: An underdiagnosed and untreated condition. Am J Respir Crit Care Med 2012; 186(12):1205–1207.
- Masa JF, Corral J, Romero A, Caballero C, Terán-Santos J, Alonso-Álvarez ML, et al. The effect of supplemental oxygen in obesity hypoventilation syndrome. J Clin Sleep Med 2016; 12(10):1379–1388.
- Berry RB, Chediak A, Brown LK, Finder J, Gozal D, Iber C, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010; 6(5):491–509.
- Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 2008; 4(2): 157–171.
- Masa JF, Jiménez A, Durán J, Capote F, Monasterio C, Mayos M, et al. Alternative methods of titrating continuous positive airway pressure: a large multicentre study. Am J Respir Crit Care Med 2004; 170(11):1218–1224.
- Contal O, Adler D, Borel JC, Espa F, Perring S, Rodenstein D, et al. Impact of different backup respiratory rates on the efficacy of noninvasive positive pressure ventilation in obesity hypoventilation syndrome: a randomized trial. Chest 2013,143(1):37–46.
- Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest 2007; 132(4):1322–1336.
- Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest 1990; 98(2): 317–324.
- Janssens J-P, Borel J-C, Pepin J-L, SomnoNIV Group. Nocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Thorax 2011; 66(5):438–445.
- Elliott MW. Non-invasive ventilation during sleep: time to define new tools in the systematic evaluation of the technique. Thorax 2011; 66(1):82–84.
- Rabec C, Rodenstein D, Leger P, Rouault S, Perrin C, Gonzalez-Bermejo J. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax 2011;66(2):170–178.
- Elliott MW. The interface: crucial for successful noninvasive ventilation. Eur Respir J 2004; 23(1):7–8.
- Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; 1: CD004104.
- Masa JF, Utrabo I, Gomez de Terreros J, Aburto M, Esteban C, Prats E, et al. Noninvasive ventilation for severely acidotic patients in respiratory intermediate care units: Precision medicine in intermediate care units. BMC Pulm Med 2016;16(1):97.
- Alison Patricia Butler, Laura-Jane E. Smith, Alexander John Mackay. Pulmonary Emergencies: ERS Chapter 4. Acute exacerbations of COPD. Edited by Leo Heunks, Alexandre Demoule and Wolfram Windisch. ERS Monogr 2016; 74:48–65. DOI: 10.1183/2312508X.10001416.
- Diaz-Lobato S, Alises SM, Rodriguez EP. Current status of noninvasive ventilation in stable COPD patients. Int J Chron Obstruct Pulmon Dis 2006; 1(2):129–135.
- Wedzicha JA, Ers Co-Chair, Miravitlless M, Hurst JR, Calverley PM, Albert RK, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 49(3): 1600791.
- From the Global Strategy for the Diagnosis. Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from: http://goldcopd.org
- Davidson AC, Banham S, Elliott M, Kennedy D, Gelcer C, Glossop A, et al. BTS/ICS guidelines for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax 2016; 71(Suppl. 2):ii1–35.
- Clini E, Sturani C, Rossi A, Viaggi S, Corrado A, Donner CF, et al. The Italian multicentre syudy on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20(3): 529–538. DOI: 10.1183/09031936.02.02162001
- Cuvelier A, Muir JF. Noninvasive ventilation and obstructive lung diseases. Eur Respir J 2001; 17(6):1271–1281.
- Budweiser S, Jorres RA, Pfeifer M. Treatment of respiratory failure in COPD. Int J Chron Obstruct Pulmon Dis 2008; 3(4):605–618.
- McCurdy BR. Noninvasive positive pressure ventilation for acute respiratory failure in patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012;12(8):1–102.
- Shi JX, Xu J, Sun WK, Su X, Zhang Y, Shi Y. Effect of noninvasive positive pressure ventilation on patients with severe, stable, chronic obstructive pulmonary disease: a meta-analysis. Chin Med J(Engl) 2013; 126(1):140–146.
- Struik FM, Sprooten RT, Kerstjens HA, Bladder G, Zijnen M, Asin J, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilator support for acute respiratory failure: a randomized, controlled, parallel-group study. Thorax 2014;69(9):826–834.
- Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicenter, randomised, controlled clinical trial. Lancet Respir Med 2014;2(9):698–705.
- Dretzke J, Moore D, Dave C, Mukherjee R, Price MJ, Bayliss S, et al. The effect of domiciliary noninvasive ventilation on clinical outcomes in stable and recently hospitalized patients with COPD: a systematic review and meta-analysis. Int J COPD 2016; 11:2269–2286.
