Abstract
Rural population-based estimates of airflow obstruction based on spirometry are unavailable from southern India. This study assessed the prevalence of spirometry-defined airflow obstruction in Vellore, Tamil Nadu.
A cross sectional survey was done in nine villages, among adults aged ≥30 years, where previous cardiovascular surveys had been conducted (1994, 2011). Population proportional to size sampling was used to select 20 clusters, with sampling from all streets proportional to the number of households. One person randomly selected per household was interviewed for symptoms and risk factors. A respiratory therapist performed pre and post bronchodilator spirometry on all, following American Thoracic Society criteria. Airflow obstruction was defined as pre-bronchodilator Forced Expiratory Volume 1 s/Forced Vital Capacity (FEV1/FVC) < Lower Limit of Normal (LLN, derived from local prediction equations) and compared to other criteria.
Of 1015 participants, 787 completed technically acceptable spirometry. The prevalence of airflow obstruction was 9.0% (95% CI: 5.8%–9.6%, 71). Fixed obstruction (post bronchodilator FEV1/FVC < LLN) was 4.6% (95% CI: 3.1%–6.1%, 36), and 4.1% (95% CI: 2.7%–5.5%, 32) using post bronchodilator FEV1/FVC < 70%. The GOLD criteria missed 56% (40) of those with airflow obstruction, of which 87.5% were females. Although 63.4% with airflow obstruction had moderate to severe disease, 82.2% were not on treatment and only 48.9% reported symptoms in the previous year.
This study estimates prevalence of airflow obstruction based on spirometry in rural southern India. Despite significant impairment on spirometry, majority were undiagnosed, and half did not report symptoms.
Keywords:
Introduction
According to the Global Burden of Disease (GBD) study, age standardised death rates of COPD have decreased by 14% between 1990-2016, with only a 5% decrease in India [Citation1]. The overall prevalence of Chronic Respiratory Diseases (CRDs) however, is increasing, due to population growth and ageing in low and middle-income countries. Disability Adjusted Life Years (DALYs) lost due to CRDs increased from 4.5% in 1990 to 6.4% in 2016, with COPD contributing to three-fourths of these DALYs. The prevalence of COPD increased from 3.3% to 4.2% between 1990 and 2016 [Citation2]. The Burden of Obstructive Lung Disease (BOLD) study reported the prevalence of spirometry based COPD from Pune, Mumbai and Srinagar as 6.25%, 6.8% and 16.05% respectively [Citation3], while the Indian Study on Epidemiology of Asthma, Respiratory Symptoms and Chronic Bronchitis in Adults (INSEARCH) reported International Union Against Tuberculosis and Lung Disease’s (IUATLD) questionnaire-based prevalence of chronic bronchitis as 3.49% [Citation4]. The results of a spirometry-based population study in Chennai and Shillong are awaited [Citation5]. The variation across the country is wide, with ischaemic heart disease and diabetes higher in most states with higher epidemiological transition, and CRDs higher in less developed states at lower levels of the transition [Citation6].
This study estimates the prevalence of airflow obstruction using spirometry, in rural adults aged 30 years and above, from Vellore, Tamil Nadu.
Methodology
This cross-sectional survey was carried out in in 2018 in nine villages in a rural block in Vellore district. Kaniyambadi block has 82 villages, with a population of 116, 085. The main occupations are farming, animal husbandry and manual labour. The nine study villages were chosen, as two cardiovascular risk factor surveys had been conducted in these villages chosen by random sampling, in 1994 and 2011 [Citation6].
Using population proportional to size sampling, 20 clusters were selected from the 2750 households in the study area. Assuming a prevalence of 7% [Citation7], design effect of 1.5 and 20% drop out for spirometry, the sample size was determined to be 1220. In each cluster, around 55 households were selected, with the number selected per street proportional to the number of households. A random number was used to obtain the initial household, followed by selection of one adult per household randomly selected using a Kish sampling grid, from the household list of adults ≥ 30 years [Citation8]. At least two visits were made to contact selected individuals to minimise non-response bias. Participants were interviewed at their homes, by trained field workers.
A questionnaire based on the INSEARCH study [Citation4], developed from the IUATLD questionnaire [Citation9], was used with permission from the lead author of INSEARCH. Questionnaire based asthma was defined as wheezing or whistling from the chest in the last 12 months, waking up with chest tightness or breathlessness AND having had acute asthma symptoms or used metered dose inhalers/dry powder inhaler/nebulizer/other treatment for breathlessness, in the past 12 months. Chronic bronchitis was defined as phlegm on most mornings for three consecutive months, for two or more years. Severity of symptoms of COPD was graded using the COPD Assessment Test (CAT) score [Citation10]. Dyspnoea was graded using the modified Medical Research Council (mMRC) grading, as recommended by the Global Initiative for Chronic Lung Disease (GOLD) [Citation11]. Ever smoking was defined as having smoked for one or more years. Predominant fuel used for cooking was ascertained to obtain exposure to biomass fuels (wood, coal, cow dung, crop residues). Modified BG Prasad scale was used to assess socioeconomic class, based on per capita monthly income [Citation12].
Spirometry was done by a graduate respiratory therapist, using a portable spirometer (EasyOne Air, ndd Medizintechnik AG, Switzerland), a battery-operated device chosen for its accuracy, portability and calibration stability [Citation13]. Height was measured using the SECA 213 stadiometer and weight using a digital scale (Phoenix, Nitiraj Engineer Ltd., India). Spirometry was performed according to American Thoracic Society criteria [Citation14]. Forced Expiratory Volume in one second (FEV1) and Forced Vital Capacity (FVC) were measured. Post bronchodilator readings were taken 15 min after administering 400 mcg of salbutamol from a Metered Dose Inhaler [Citation14]. At least three acceptable trials were performed, ensuring that FEV1 and FVC of the two best trials were within 150 mL of each other, to fulfil reproducibility criteria. A senior staff respiratory therapist at the referral lab subsequently verified that spirometry recordings had been performed as per standards.
Spirometry definitions
Airflow obstruction was defined as pre-bronchodilator FEV1/FVC < Lower Limit of Normal (LLN, below fifth percentile) [Citation14]. The following spirometry prediction equations were used, with permission from the lead author (S.K. Chhabra) of a Multicentric Study of Pulmonary Function in Normal Adults in India: Development of Reference Standards for Spirometry, Static Lung Volumes and Single Breath Diffusion Capacity, 2015, derived from a sample of 407 healthy non-smoking adults (males 275, females 132) of a Southern Indian population in 2016-17, under the aegis of the Indian Council of Medical Research (unpublished):
FEV1/FVC males: 102.56-0.756*age in years + 0.00488*age in years [Citation2]; SEE (Standard Error of the Estimate): 5.03
FEV1/FVC females: 95.916-0.267*age in years; SEE: 5.28
FEV1 males: −3.713–0.027*age in years + 0.050*height in cm - 0.010*weight in kg; SEE: 0.356
lnFEV1 females: −1.413 + 0.0163*ht-0.0096*age in years; SEE: 0.108
FVC males: −5.959–0.019*age in years + 0.067*height in cm −0.012*weight in kg; SEE: 0.411
lnFVC females: −1.409 + 0.0165*ht-0.0065*age; SEE: 0.098
For comparison, post-bronchodilator FEV1/FVC < LLN to indicate fixed airflow obstruction [Citation15] and the GOLD definition of airflow obstruction (post-bronchodilator FEV1/FVC < 70%) [Citation11] were also used. Secondary analysis was performed to estimate the agreement between diagnosis of airflow obstruction (pre-bronchodilator FEV1/FVC < LLN) using the above south Indian equations and the Global Lung Function Initiative (GLI) – 2012 reference equations, using ‘Other’ category for ethnicity [Citation16].
Severity of airflow obstruction (based on FEV1) [Citation17]:
mild (z score: ≥ −2)
moderate (−2.5 ≤ z score < −2)
moderately severe (−3.0 ≤ z score < −2.5)
severe (−4.0 ≤ z score < −3.0)
very severe (z score < −4.0)
The severity grading based on percent of predicted FEV1 (GOLD staging) was also used for comparison [Citation11]. The syndrome of asthma was defined as symptoms suggestive of asthma confirmed by post bronchodilator variability of ≥12% and ≥ 200 mL in FEV1, in those with airflow limitation (FEV1/FVC < LLN), according to the Global Initiative for Asthma guidelines [Citation18]. The clinical syndrome of COPD was defined as respiratory symptoms with persistent airflow obstruction (post-bronchodilator FEV1/FVC < LLN). Restrictive disease was defined as pre-bronchodilator FVC < LLN (with FEV/FVC ≥ LLN) and compared with the fixed criteria definition of FEV1/FVC ≥70% and FVC <80% of predicted values [Citation19].
Statistical methods
Data entry was done using Epi Data version 3.1 (The EpiData Association, Odense, Denmark) and analysis using SPSS version 24 for Windows (SPSS Inc. Chicago, Illinois). Categorical variables were summarised using frequencies and 95% confidence intervals (CI), and continuous variables using mean and standard deviation or median. Adjusted Odds Ratios (ORs) were obtained by logistic regression, adjusting for factors with a chi-square p value < 0.05.
The study was conducted in accordance with the amended Declaration of Helsinki and written informed consent was obtained from all participants. Approval was obtained from the Ethics Committee and Institutional Review Board of the concerned tertiary health care institution (IRB number: 10858 – OBSERVE, 27.09.2017).
Results
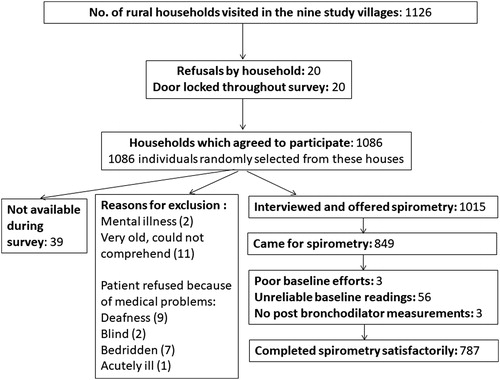
Of the 1126 households visited, 1086 (96.4%) agreed to participate. Of the 1015 interviewed individuals 787 (77.5%) completed acceptable spirometry (). Of the 1015 participants, 574 (56.6%) were females and the mean age was 51.5 years (standard deviation: SD:12.1 years). Overall 26.3% of males had history of ever smoking (), of whom 14.6% were current smokers, and 11.7% were ex-smokers. The prevalence of current tobacco use was 15.5%. Among those who did not undergo spirometry or did not complete spirometry satisfactorily (228 out of 1015 interviewed participants), mean age was 51.3 years (SD: 12.9 years), 55.7% were females, and 25.7% of the males had a history of smoking, indicating that sociodemographic characteristics were similar to responders.
Table 1. Descriptive characteristics of the rural study participants.
The prevalence of previously diagnosed Chronic Respiratory Disease (CRD) was 1.8% (95% CI: 0.9%–2.6%), . Only 12 (66.7%) were on regular treatment, with 15 (93.8%) reporting symptoms in the last 12 months. Of those with previously diagnosed CRD (18), 17 underwent spirometry, of which 11 were confirmed to have airflow obstruction (FEV1/FVC < LLN), one had restrictive disease (FVC < LLN and FEV1/FVC ≥ LLN), while five (29.4%) had normal spirometry.
Table 2. Prevalence of respiratory symptoms & history of Chronic Respiratory Disease (CRD).
Symptoms of chronic bronchitis and asthma were seen in 2.0% and 2.7% respectively (). Of those with chronic bronchitis, 52.9% were found to have airflow limitation on spirometry as compared to only 8.0% of those without chronic bronchitis (Fisher’s exact test p value < 0.001). Those who were questionnaire positive for asthma, were more likely to have spirometry suggestive of asthma (29.2%, 7/24), compared to individuals with no such history (3.1%, 24/764, Fisher’s exact test p value < 0.001).
The prevalence of airflow obstruction (pre-bronchodilator FEV1/FVC < LLN) was 9.0.% (95% CI: 6.9%–11.0%, n = 71), while the prevalence of airflow obstruction based on GOLD criteria (post bronchodilator FEV1/FVC < 70%) was 4.1% (95% CI: 2.7%–5.5%, n = 32), . The agreement between airflow obstruction by the LLN and GOLD definitions for airflow obstruction was moderate, (kappa 0.578.) Of 71 subjects with airflow obstruction (pre-bronchodilator FEV1/FVC < LLN), only 31 (43.7%) were also GOLD Stage 1 or higher, indicating that 40 were missed by the GOLD criterion. Of the 32 who were GOLD criteria positive, 31 (96.9%) were abnormal by pre-bronchodilator LLN criteria also. Of the 41 individuals with discordant results, one was positive only by GOLD criteria (male aged 59 years) and 40 only by LLN criteria (87.5% females; mean age: 46.9 years, SD:10.6).
Table 3. Spirometry based prevalence of chronic respiratory disease.
Secondary analysis using the GLI-2012 reference equations for ‘Other’ ethnic group [Citation16], yielded a prevalence of airflow obstruction (pre-bronchodilator FEV1/FVC < LLN) of 7.8% (95% CI: 5.9%–9.7%, n = 62), similar to 9.0% using the local south Indian equations, kappa = 0.778 (95% CI: 0.697–0.858), showing substantial agreement between the equations. Agreement between the GOLD criteria and FEV1/FVC < LLN of GLI-2012 was moderate (kappa = 0.640, 95% CI: 0.526–0.754), with GOLD missing 50% (31) of the airflow obstruction diagnosed by FEV1/FVC < LLN of GLI-2012.
The mean z scores (SD) for FEV1/FVC, FEV1 and FVC (pre-bronchodilator values) in the whole study population, were -0.099 (1.080), 0.632 (1.03) and 0.021 (0.949) for males, and 0.074 (1.000), −0.468 (0.667) and 0.036 (0.877) for females, respectively.
Using the definition of post bronchodilator FEV1/FVC < LLN15 (South Indian equations), the prevalence of spirometric “COPD” or chronic fixed airflow obstruction was 4.6% (95% CI: 3.1%–6.1%, n = 36). The agreement between diagnosis of airflow obstruction using pre bronchodilator FEV1/FVC (definition used in this analysis) and post-bronchodilator FEV1/FVC was moderate, (kappa 0.592). Overall, 23 individuals (2.9%) were diagnosed to have chronic fixed airflow obstruction by both post-bronchodilator FEV1/FVC < LLN, as well as GOLD criteria.
The prevalence of moderate or severe airflow obstruction in the study population, defined as z score for FEV1 lower than −2.0 was 3.3% (95% CI:2.0%–4.6%) compared to 2.9% (95% CI: 1.7%–4.1%) of GOLD Stage 2 or higher categories, .
The median age of those with airflow obstruction (FEV1/FVC < LLN) was 54.1 years (range: 30–82 years; SD: 13.1). Of these, only 11 (15.5%) had been previously diagnosed to have a lung disease and ten of these were on treatment. Of those with mild airflow obstruction, 7.7% (2/26) were on treatment, compared to 17.8% (8/45) of those with moderate and severe grades of airflow obstruction.
Among those with airflow obstruction, 42.3% (30) had at least one symptom in the last 12 months, resulting in a prevalence of symptomatic obstructive lung disease (symptoms + airflow obstruction by spirometry), of 3.8% (95% CI: 2.4%–5.2%), . The prevalence of the clinical syndrome of asthma (symptoms of asthma, airflow obstruction and significant increase in post-bronchodilator FEV1) was 0.9% (95% CI: 0.2%–1.6%, n = 7). The prevalence of the clinical syndrome of COPD (symptoms, post-bronchodilator FEV1/FVC < LLN) was 2.03% (95% CI: 1.03%–3.03%, n = 16).
The prevalence of symptoms was 30.8.% among those with mild airflow obstruction, compared to 48.9% among those with more severe grades of obstruction (chi-square p value 0.212). Exertional breathlessness was the most common symptom, seen in 38.0% (27/71) of those with airflow obstruction, with 7.0% (7/71) reporting moderate to severe dyspnoea (mMRC grading). Conversely, only 1.9% (14/717) of those without airflow obstruction on spirometry had moderate to severe dyspnoea (Fisher’s exact test p value 0.022). Among those with symptomatic airflow obstruction, the median CAT score was 17.50 (Interquartile Range: 10.00-22.00), with 35.7% (1028) having scores of >20, indicating high impact of symptoms on their health.
The prevalence of restrictive lung disease (pre-bronchodilator FVC < LLN, FEV1/FVC ≥ LLN) was 20.9% (95% CI: 18.0%–23.8%). The agreement between diagnosis of restrictive disease by LLN criteria and fixed percent criteria (<80% of predicted) was good (kappa: 0.73). Considering restrictive pattern as post-bronchodilator FEV1/FVC < LLN [Citation3], the prevalence of restrictive disease was 14.4% (95% CI: 12.9% - 15.8%).
After adjusting for confounders, factors significantly associated with airflow obstruction (pre-bronchodilator FEV1/FVC < LLN) were female gender (OR: 2.56, 95% CI: 1.35–4.76) and smoking for more than 20 years (OR: 6.66, 95% CI: 2.61–16.97), .
Table 4. Factors associated with airflow obstruction (FEV1/FVC < LLN) among rural adults.
Those without complete spirometry data (228 individuals) were less likely to have a history of wheezing (non-responders: 1.8% vs responders: 6.6%, chi-square p: 0.005) and exertional breathlessness (non-responders: 5.7% vs responders: 11.6%, chi-square p: 0.010), compared to the 787 participants with both clinical and spirometry data. However, there was no difference in prevalence of symptoms of chronic bronchitis (non-responders: 1.3% vs responders: 2.2%, chi-square p: 0.591).
Discussion
To the authors’ knowledge this is the first community-based study to report the prevalence of spirometry based airflow obstruction from a rural Southern Indian population, while others reported prevalence based on symptoms alone, or a combination of symptoms followed by spirometry only in symptomatic individuals [Citation4]. In this rural population, 26.3% of males were ever smokers and 14.6% were current smokers in 2018. History of ever smoking seems to have declined from 47.4% in 1994, with the current rate being similar rate to 2011 (25.9%) [Citation6]. This rural population in the state of Tamil Nadu, has reported literacy rates, tobacco use, household use of clean fuel for cooking and availability of electricity comparable to the southern states of Andhra Pradesh, Karnataka and Telangana (Census 2011) [Citation20].
The prevalence of persistent airflow obstruction (post-bronchodilator FEV1/FVC < LLN) was lower in males (4.2%) compared to females (4.9%), unlike the results from Pune from the Burden of Obstructive Lung Disease (BOLD) study, using the same criterion (males 5.7%, females 1.2%)Citation3 .However, use of the fixed ratio GOLD criteria led to higher rates of airflow obstruction in males (5.7%) compared to females (2.9%).
While a systematic review reported the prevalence of chronic bronchitis as 6.5%-7.5% in rural India [Citation7], our study found only 2.0% of rural adults ≥ 30 years with symptoms of chronic bronchitis. The INSEARCH study had reported 3.5% prevalence of chronic bronchitis among those aged ≥35 years [Citation4].
The prevalence of symptoms of chronic bronchitis was lower (2.0%) in the current study, compared to the prevalence of fixed airflow obstruction based on spirometry (4.6%, post-bronchodilator FEV1/FVC < LLN), indicating that symptom-based diagnosis alone may tend to under-estimate the prevalence of COPD. In usual clinical practice, only symptomatic individuals are advised spirometry. This coupled with the limited availability of spirometry facilities in the country may be responsible for the huge burden of undiagnosed COPD. Epidemiological investigations are known to pick up asymptomatic cases based on spirometry alone. A Danish cohort study reported a higher incidence of exacerbations, pneumonia and all-cause mortality among those with such undiagnosed, asymptomatic screen-detected COPD, compared to those without COPD [Citation21]. However, the merit in population wide screening remains unclear, due to insufficient evidence to suggest that detection of COPD by spirometry-based screening will improve outcomes [Citation22].
Our study primarily used the definition of airflow obstruction based on the FEV1/FVC < LLN criteria, comparing it to the GOLD criteria (FEV1/FVC < 70%). The differences between fixed and LLN criteria have been often debated and as seen here can have large effects on the estimation of prevalence of airflow obstruction (9.0% using pre-bronchodilator FEV1/FVC < LLN compared to 4.1% using GOLD criteria). While LLN seems to better accommodate regional variations, it must also be considered that in populations where restrictive lung function is highly prevalent, as in Indians [Citation3], the normal FEV1/FVC ratio as well as the lower limit thereof, may be distorted and shifted to a higher value. This would then lead to increased diagnosis of airflow obstruction in such populations. This could be interpreted as either unmasking of the underlying obstruction, hidden by the restriction, or as false positives. We are inclined towards the former interpretation since airway disease risks and symptoms are both elevated in IndiansCitation3 .There was substantial agreement between the local south Indian reference equations used in this study and the GLI-2012 reference for ‘Other’ ethnic group, with GOLD missing 50-56% of the airflow obstruction diagnosed using these equations for LLN based diagnosis.
The use of the fixed ratio definition is known to result in underestimation of COPD in younger individuals and women, with over estimation of COPD in older participants [Citation23,Citation24]. This was also confirmed in our study, as the LLN definition categorised more younger participants as having airflow limitation, missed by the GOLD criterion (40 individuals, mainly females under 50 years) [Citation24]. However, only one person was overdiagnosed by GOLD (one male, 59 years), unlike other reports [Citation23].
The prevalence of symptoms of asthma was 2.7% in rural Vellore (age ≥30 years), compared to 1.3% in Chennai in the INSEARCH study (age ≥15 years) [Citation4]. Overall 7.0% of participants from Chennai (rural and urban) in the INSEARCH study had one or more respiratory symptoms [Citation4], compared to 14.7% in this study which was limited to a rural area in the same state (Tamil Nadu).
Of those with airflow obstruction, only 15.5% had been previously diagnosed to have a lung disease with 84.5% having undiagnosed disease, similar to China (65%) [Citation21]. This situation did not seem to be different even in subjects with more severe disease, indicating a large gap in diagnosis and treatment in the community. However, only 42.3% of those with airflow obstruction were symptomatic in the last 12 months, including only 48.9% of those with moderate-severe obstruction, which may be the reason for not seeking treatment. In clinical settings, only those with symptoms undergo spirometry or receive medication. A review article on underdiagnosis listed limited access to health care and spirometry for diagnosis, asymptomatic affected younger individuals and poor awareness as possible explanations for undiagnosed COPD, especially in low resource settings [Citation23].
Overdiagnosis of ‘false-positive COPD’ is also a concern, with 1.9% overdiagnosis in low to middle income countries and 4.9% in high income countries [Citation25]. In the current study, 29.4% of those with previously diagnosed CRD had normal spirometry, indicating either overdiagnosis leading to unnecessary treatment, or that these individuals were asthmatics with normal lung function tests and no demonstrable bronchodilator reversibility at the time of testing.
As seen previously, airflow obstruction was associated with a prolonged history of smoking. However, quitting smoking is potentially advantageous for reducing COPD burden even among long term chronic smokers, as disease free former smokers will have lower incidence of COPD compared to those who continue to smoke [Citation26].
Given the high burden of COPD and respiratory symptoms in rural India, capacity building of the primary health care system is required, to provide essential services for those with CRDs [Citation27]. With declining tobacco use, the contribution of other risk factors to the burden of COPD is rising, with rising prevalence in females. Of all the DALYs lost due to COPD in India, in 2016, 53.7% was attributable to air pollution, 25.4% to tobacco and 16.5% to occupational exposures [Citation2]. Rising urban air pollution and continuing exposure to biomass fuels especially in rural areas, need to be urgently addressed. While the government has undertaken commendable steps such as monitoring air quality and decreasing urban vehicular and industrial emissions [Citation28], there remains the need to reduce indoor air pollution in rural areas, by decreasing the use of solid fuels [Citation2].
Data sharing
Data is available from the corresponding author on reasonable request.
Declaration of interest
There were no conflicts of interests for any of the authors.
Author contributions
DJC is the guarantor and takes responsibility for the integrity of the content of the article, study processes and data. AMO takes responsibility for the integrity of the data analysis and is the corresponding author. AMO, DS and DJC had full access to the data, were involved in study implementation, data analysis and interpretation. KG, AA and BT were involved in study design, data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript.
Acknowledgments
The study was partially funded by the Pulmonary Medicine departmental research fund, Christian Medical College, Vellore. Dr Anurag Agrawal acknowledges Wellcome Trust DBT India Alliance Senior Fellowship and funding by CSIR MLP5502 grant.
Additional information
Funding
References
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706.
- India State-Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6(12):e1363–e1374.
- Burney P, Jithoo A, Kato B, et al. Burden of Obstructive Lung Disease (BOLD) Study. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty-a BOLD analysis. Thorax. 2014;69(5):465–473. doi:10.1136/thoraxjnl-2013-204460.
- Jindal SK, Aggarwal AN, Gupta D, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tuberc Lung Dis. 2012;16(9):1270–1277. doi:10.5588/ijtld.12.0005.
- Rajkumar P, Pattabi K, Vadivoo S, et al. A cross-sectional study on prevalence of chronic obstructive pulmonary disease (COPD) in India: rationale and methods. BMJ Open. 2017;7(5):e015211. doi:10.1136/bmjopen-2016-015211.
- Oommen AM, Abraham VJ, George K, et al. Rising trend of cardiovascular risk factors between 1991-94 and 2010–12: a repeat cross-sectional survey in urban and rural Vellore. Indian Heart J. 2016;68(3):263–269. doi:10.1016/j.ihj.2015.09.014.
- McKay AJ, Mahesh PA, Fordham JZ, et al. Prevalence of COPD in India: a systematic review. Prim Care Respir J. 2012;21(3):313–321. doi:10.4104/pcrj.2012.00055.
- World Health Organisation. STEPwise approach to noncommunicable disease risk factor surveillance. Available from: https://www.who.int/ncds/surveillance/steps/en/
- Burney PG, Laitinen LA, Perdrizet S, et al. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. Eur Respir J. 1989;2(10):940–945.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. Sepdoi:10.1183/09031936.00102509.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers. Available from: https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
- Pandey V, Aggarwal P, Kakkar R. Modified BG Prasads Socio-economic Classification-2018: the need of an update in the present scenario. Indian J Community Health. 2018; 30(1):82–84.
- Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006; 51:1167–1171.
- Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Vollmer WM, Gíslason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34(3):588–597. doi:10.1183/09031936.00164608.
- Quanger PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012; 40(6):1324–1343. doi:10.1183/09031936.00080312.
- Quanger PH, Pretto JJ, Brazzale DJ, et al. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J. 2014;43:505–512. doi:10.1183/09031936.00086313.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Revised 2014 Vancouver, GINA, 2014. Available from: www.ginasthma.org
- Johnson JD, Theurer WM. A stepwise approach to the interpretation of pulmonary function tests. Am Fam Physician. 2014;89(5):359–366.
- Government of India, Ministry of Home Affairs. Census of India 2011. Available from: http://www.censusindia.gov.in/2011census/dchb/DCHB.html
- Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/S2213-2600(17)30119-4.
- Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: a systematic evidence review for the U.S. preventive services task force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. (Evidence Syntheses, No. 130.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK361185/
- Ho T, Cusack RP, Chaudhary N, et al. Under- and over-diagnosis of COPD: a global perspective. Breathe. 2019;15(1):24–35. doi:10.1183/20734735.0346-2018.
- Miller MR, Levy ML. Chronic obstructive pulmonary disease: missed diagnosis versus misdiagnosis. BMJ. 2015; 351:h3021doi:10.1136/bmj.h3021.
- Sator L, Horner A, Studnicka M, et al. Overdiagnosis of COPD in subjects with unobstructed spirometry: a BOLD analysis. Chest. 2019;156(2):277–288.
- Terzikhan N, Verhamme KM, Hofman A, et al. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792. doi:10.1007/s10654-016-0132-z.
- Kalkana T, Moitra S, Jindal SK, et al. Increasing burden of COPD in rural India: an example why India warrants primary healthcare reforms. ERJ Open Res. 2016;2(2):00032-2016. doi:10.1183/23120541.00032-2016.
- Government of India, Ministry of Environment, Forest and Climate Change. Environment, air pollution. Available from: http://moef.gov.in/environment/pollution/