?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The flammability of cellulosic material restricts its use in insulation and packaging applications. This study examined carbon-based additives to protect cellulose paper against thermal degradation. Carbon nanotubes (CNT) and expandable graphite (EG) were used to modify paper material. Thermal analysis was performed to examine the different modification systems. Our results showed consistently that the addition of EG resulted in an increased fire resistance in the mini fire tube (MFT) test, limited oxygen index (LOI) measurements, and mass loss calorimeter (MLC). In combination with CNT, the heat-release rate could be drastically reduced. Thermal analysis (DSC with TG analysis) revealed that EG had a longer resistance to thermal degradation. The developed composite material can be used, depending on the needs of the industry, as part of thin panel material, partition, or fireproof cladding in construction, railways, transport, automotive, and shipbuilding industries.
摘要
纤维素材料的易燃性限制了其在绝缘和包装应用中的使用. 这项研究检测了碳基添加剂,以保护纤维素纸免受热降解. 采用碳纳米管(CNT)和可膨胀石墨(EG)对纸张材料进行改性. 进行热分析以检查不同的改性系统. 我们的结果一致表明,在迷你火管(MFT)测试、有限氧指数(LOI)测量和质量损失量热计(MLC)中,EG的添加导致耐火性增加. 与CNT结合,可以显著降低热释放速率. 热分析(DSC和TG分析)显示EG具有较长的热降解抗性. 根据行业需求,开发的复合材料可以用作建筑、铁路、运输、汽车和造船行业的薄板材料、隔板或防火覆层的一部分.
Introduction
Cellulose is used in many industries, such as pharmaceuticals, electronics, biofuels, adsorbents production, and many others. This is due to its high mechanical properties and its being the most common natural and renewable polymer on Earth (Chandrasekaran et al. Citation2023; Miyashiro, Hamano, and Umemura Citation2020). It has high biocompatibility and biodegradability, and its surface can be easily chemically functionalized to change and improve its properties. Cellulose is a polysaccharide, which is a carbohydrate polymer consisting of several thousand monosaccharide units. All common polysaccharides contain glucose as the monosaccharide unit; therefore, polysaccharides are used by plants, animals, and humans as a source of food, structural support, or metabolizable energy. As a sustainable and biodegradable resource, cellulose has properties that are attractive to science and technology. These properties are high tensile strength, low bending strength, high specific surface area, and low density (Fangueiro and Rana Citation2016). However, the flammability of cellulose limits its use in many areas, particularly in construction as an insulation material. Cellulose, which is thermally decomposed in a series of successive dehydration reactions, leading to water, carbon dioxide, and carbon formation, can develop a char layer (Mazela and Broda Citation2015; Shen and Gu Citation2009). Modifying cellulose fibers with organic and inorganic compounds may decrease their flammability properties (Salmeia et al. Citation2016; Zheng, Li, and Ek Citation2017). One critical parameter affecting cellulose’s thermal degradation properties is its degree of crystallinity. Thermal degradation of cellulose was observed to start in the amorphous regions and propagate to its more crystalline domains (Zheng, Li, and Ek Citation2018). Expandable graphite (EG) flake is a radically different intumescent additive used in many flame-retardant applications. It can expand up to 100 times its original thickness. As a result, the graphite flake generates a more significant isolative layer than many intumescents. In addition, unlike the carbon char layer formed with chemical intumescent, the graphite char formed from expandable flake retains the superior heat resistance of graphite (Zabel and Solin Citation2013). EG is a low-density carbon material with extraordinary thermophysical, mechanical, and electrical properties. EG is an intumescent additive that improves the fire retardancy properties of various materials, particularly polyurethane foam (Duquesne et al. Citation2003; Mazela, Batista, and Grześkowiak Citation2020). EG is achieved through the intercalation of graphite flakes with concentrated sulfuric acid () in the presence of other strong oxidizers, such as nitric acid (
) or potassium permanganate (
); top-down methods synthesize flake graphene from graphite or other carbon sources (Focke et al. Citation2014; Jin et al. Citation2013; Smith et al. Citation2019). The oxidation of graphite leads to graphite oxide, consisting of multiple layers of graphene oxide with a hexagonal carbon structure, including a hydroxyl group, alkoxy, carbonyl, and acids group (Smith et al. Citation2019). The production methods of EG are similar to that of graphite oxide (Focke et al. Citation2014). However, it is indicated that these production methods are environmentally hazardous. Graphite structure comprises layers in which six-membered aromatic cyclic systems are conjugated. The carbon atoms in the graphite layer are covalently bound, while weak van der Waals forces bind the layers. Graphite is characterized by highly anisotropic properties: excellent electrical and thermal conductivity and a low coefficient of thermal expansion. Moreover, it resists high temperatures (Thostenson, Li, and Chou Citation2005). Graphite has a very high melting point, which is about 3727°C at high pressure, while under atmospheric pressure, it sublimes at 2727°C (Smędowski and Muzyka Citation2013). In their work, Seo et al (Seo et al. Citation2016). used various carbon additives for the surface protection of MDF boards. They produced a carbon-based coating composite containing EG, carbon nanotubes (CNT), and graphene nanoparticles with improved fire properties. The thermogravimetric (TG) analysis also shows the thermal stability of coatings.
One of the most promising nanomaterials as a source of additional carbon is CNTs. CNTs can be used in polymer composites to improve their mechanical, thermal, and electrical characteristics (Maria and Mieno Citation2017). CNTs are a popular addition to polymer composites to enhance the mechanical properties and fire resistance. CNTs dispersed in a matrix of polystyrene and polymethylmethacrylate and can be used as a flame retardant, reducing the mass loss rate (MLR) and peak heat release rate (pHRR) (Cipiriano et al. Citation2007; Kashiwagi et al. Citation2005). Fu et al. (Fu et al. Citation2010) showed that CNT-based coatings reduced the flammability of composites. For example, CNT-coated PETI330/T650 (polyimide (PETI-330) with T650 carbon fabric) reduced pHRR and total weight loss and increased the time to ignition (Tti). Anderson et al. (Anderson et al. Citation2010) made a multifunctional cellulose/CNT composite paper that exhibits very low flammability. TG analysis of the phenol-formaldehyde/cellulose composite showed that thermal stability increased slightly by adding 1.0 wt. polyhedral (MW) CNTs. Cotton fabrics cross-linked with polyvinyl phosphonic acid showed retarded ignition and reduced burn rate after MWCNT incorporation (Gashti and Almasian Citation2013). Although research on cellulose/CNT composites is not new, only a few studies have described composites (wood-based materials) mixed with CNTs (Łukawski et al. Citation2019). Several studies have focused on carbon nanomaterials and wood-based products, especially on wood-plastic composites made of wood flour and polymer. It has been shown that adding CNT to a composite reduces its flammability and increases mechanical strength. Polypropylene/wood flour with 2 wt% CNT showed a 25% decrease in pHRR and total heat released (THR) and a significant decrease in carbon oxide yield (Farsheh et al. Citation2011). In Maria and Mieno (Maria and Mieno Citation2017), a CNT/gelatine suspension was used in cellulose paper sheets in weight ratios of CNT/cellulose of 1/30, 1/15, and 1/10. TG/DT analysis experiments showed a heating rate of 10°C/min to 800°C/min in an air atmosphere. The cellulose and CNT/cellulose sheets began to lose weight at similar temperatures of 332 and 355°C, respectively. The second step of thermolysis occurred at 409 and 507°C. They concluded that there was an interaction between cellulose and CNTs.
Due to their strength, charring ability, and biodegradability, cellulose-based reinforced polymers have received considerable attention (Fox et al. Citation2009). However, the burning characteristics of cellulosic fibers are significantly different from one another and are based on their chemical composition (Salmeia et al. Citation2016). Although cellulose is usually overlooked as a flame-retardant for polymers due to its low thermal stability, the potentially high char yield could be advantageous in reducing the available fuel and peak-heat release in the event of an actual fire (Fox et al. Citation2009). The work of Suttipintu et al. (Suttipintu et al. Citation2022) showed the preparation and flammability properties of cellulose-based paper treated with sodium silicate. The UL94 test measured the fire properties of treated paper and showed an increase in combustion time compared to untreated samples.
In general, the fire protection mechanism of polymers works by promoting a char layer between the heat source and the protected substrate. The char layer, protecting the underlying structure, should combine the following characteristics: low thermal conductivity in the thickness direction, high thermal conductivity in the plane, high stability, low heat absorption, high heat capacity, and compact structure; the structure of the char layer largely depends on the porosity and permeability of the material. These two properties are believed to limit the mass loss (ML) in the thickness direction (Zhuge Citation2012).
The main goal of this study was to produce innovative-modified cellulosic materials with increased fire resistance through modification using various carbon sources and characterization of the fire properties of new materials. The research aimed to determine the flammability properties of the composite based on Kraft cellulose encrusted with EG and/or CNTs. The aim was also to determine which of the additives has the most significant impact on the flammability characteristics of the material and whether there is an interaction between the individual components of the composite. The scope of the research included variants of composites differentiated in terms of component proportions and the final product’s thickness. The flammability properties were characterized by four test methods: mini fire tube (MFT), limited oxygen index (LOI), mass loss calorimeter (MLC), and differential scanning calorimetry with thermogravimetric analysis (DSC/TG).
Materials and methods
EG (ES 100, Krapfmull GmbH, Germany) and multi-wall technical nanotubes NC7000™ (CNT) were provided by NanocylⓇ (France) and were used as carbon additives to the cellulose fibers. Bleached softwood Kraft fibers (Södra Black R) were used as the base material and referred to as cellulose (C) in this study.
Production of cellulose sheets
Ten grams of ground Södra Black cellulose was soaked for 24 h in 100 ml of deionized water. After 24 h, the cellulose was milled in a blender with 400 ml of deionized water. The milled pulp was transferred to the Rapid-Koethen apparatus (Labormex, Poland), where it was diluted to a volume of 6.7 L. Cellulosic sheets were formed once the pulp was mixed and filtered on a screen. The sheet was transferred to allow the drying of excess water. The method of distribution of carbon additives in the pulp was modified. The graphite-only variants were produced by soaking the graphite in water for 24 h and adding it to the partially ground pulp. The EG stock was then milled for 10 s and transferred to the Rapid-Koethen apparatus. The variants with the addition of CNT were made the same way as with graphite by soaking the CNT and CNT/EG mixture in water and then homogenizing for 30–45 s using a homogenizer with a speed of 0–4000 rpm. The obtained pulp was transferred to the partially milled pulp and milled in the same manner as the C and EG samples. Ten sheets of each variant were made. Among the sheets made, five similar masses were isolated, three of which were randomly selected and cut using a bookbinding guillotine into test samples of dimensions corresponding to the selected research methods (). Individual variants and components’ average thickness and content are listed in .
Figure 1. Changes in the appearance of the variants of unmodified and modified cellulose sheets after production before cutting on sample size (the best variants).
Table 1. Selected variants for flammability tests with thickness and grammage of samples.
Methods
Mini fire tube
The MFT method was adapted and modified according to ASTM E69 (2002) (ASTM E69–02 Citation2002). A profile tube made of aluminum (20 × 20 mm) with a stand was placed on a laboratory balance. The heat source was a flame from a gas burner mounted on a tripod with an adjustable flame height of 1.5 cm, placed directly below the sample. The exhausted gas temperature was measured at the pipe outlet using a type-K thermocouple display for the temperature range −50–1200°C. The MFT method measured the sample’s ML during the test and the temperature of the emitted exhaust gases. The duration of one fire test was 60 s, and the measured values were registered every second. For tests using MFT, 10 samples were randomly selected from each variant with dimensions of 100 × 10 mm and thickness given in (Grześkowiak Citation2017; Grześkowiak et al. Citation2021; Zeinali, Koalitis, and Schmid Citation2018).
Limited oxygen index
LOI measurements were performed under standard conditions according to ASTM D 2863–77 (ASTM D-Citation2863) and ISO 4589–2:1999 (ISO Citation4589–2: 1999) standards. The test method determined the minimum oxygen concentration supporting combustion and was recorded as LOI in percentage. Accordingly, a reaction to fire classification can be assigned to the material (Gilman Citation1999; ISO Citation4589–1: 2017; Janowska, Przygocki, and Włochowicz Citation2007; Zeinali, Koalitis, and Schmid Citation2018).
LOI [%] fire classification of materials:
≤23 combustible/flammable material
24–28 partially fire retardant/fire retardant/self-extinguishing material
29–35 flame-retardant/fire-retardant/self-extinguishing material
Therefore, 15 samples of each variant with dimensions of 100 × 10 mm and thickness given in were randomly taken for testing. The flow rate of the mixture of oxygen and nitrogen was set at 200 l/h ±10 l/h. After placing the sample in the column, the top was ignited using a gas igniter with a flame height of 1 cm. Ignition of the sample lasted 30 s. After removing the fire source, the time until the burnt sample expired or reached the boundary of 70 mm was measured (Zeinali, Koalitis, and Schmid Citation2018).
Mass Loss Calorimeter (MLC)
Flammability measurements were made using an MLC calorimeter Fire Testing Technology following EN ISO 13,927 standards (ISO 13927: Citation2015). Tests were performed at a heat flux of 35 kW/m2 and a distance from the cone of 25 mm. Three samples of each variant were tested with dimensions of 100 × 100 mm and thickness given in . Time to ignition (Tti) and time to flameout (Ttf), heat release rate (HRR), THR, MLR, and maximum peak HRR were measured.
Methods of thermal analysis (TG/DSC)
The thermal analysis was performed using a thermal analyzer (Netzsch STA 449 F1 JupiterⓇ, Germany) with an autosampler, under the same measurement conditions: the heating rate, atmosphere, and pressure. The measurement (TG and DSC) was performed with a temperature range of 40–1200°C and a heating rate of 10°C min−1. A gas mixture corresponding to air was used, having 20 ml/min of nitrogen as a protective gas, 35 ml/min nitrogen, and 15 ml/min oxygen as a purge gas. Open crucibles of aluminum oxide (Al2O3) were used with the sample carrier type S. Pure materials in a loose form (EG and CNT) and microcrystalline cellulose (ProUmid MCC012, batch 56,112,191,210, Germany) were used to determine the calibration curves using an amount of 6 ± 2 mg per sample. In addition, samples of tested materials were milled, and due to the cellulose content, they were in the form of shredded fibers.
Results and discussion
MFT
MFT tests showed the greatest mass loss (ML) (98.8%) for control samples (C) containing 10 g of cellulose. The C variant reached the highest exhaust gas temperature value after 18 s of measurement at 408.4°C. Of all tested variants, the highest ML was recorded for the variant C+CNT (1:1) (48%), with a maximum temperature of 311.1°C after 25 s. The lowest MLs and temperature range during 60 s of the test were obtained for variants C+EG+CNT (1:1:1) with an ML of 5.3% and temperature of 114.9°C, C+EG (1:2) with an ML of 6.35% and temperature 111.36°C, and C+EG (1:1.5) with an ML of 7.91% and temperature 102.6°C ().
Table 2. Results of mass losses (MLs) and maximum temperature of exhaust gases obtained after the mini fire tube (MFT) test.
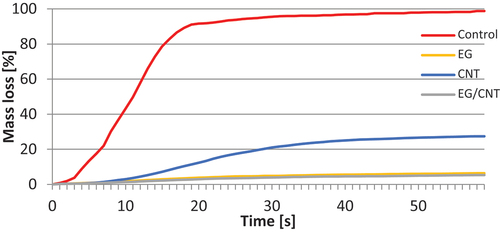
By analyzing the MLs by the various content of cellulose and carbon additives, it was possible to find an improvement in fire retardant properties in the case of variants containing the EG/CNT mixture in all configurations, regardless of the number of ingredients used, except for the 1:1 ratio of cellulose to additives. Similarly, it was possible to find an improvement in fire retardant properties in the case of all variants in comparison with control samples. For example, shows an exemplary course of ML for variants containing 33.33 wt% of cellulose and 66.67 wt% of carbon additives.
LOI of modified cellulose paper
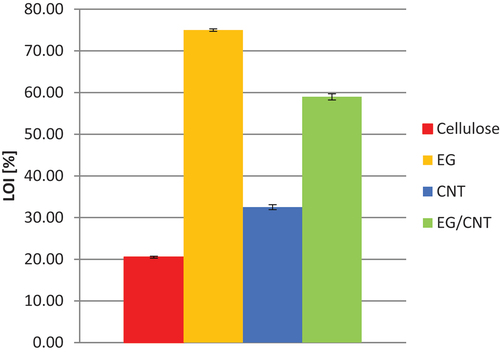
Analysis of the results obtained by the LOI method indicated a high efficiency of most of the tested variants, except for the C variant. With the increase in individual additives’ content, the LOI value increased significantly. The highest value of the LOI was 75% (variant C+EG (1:2)), and the minimum was 29.27% for the C+CNT (1:1) variant. All the tested cellulosic materials had an LOI higher than 29%, simultaneously obtaining the fire-retardant/self-extinguishing material classification. The C variant showed an LOI of 20.5%, corresponding to flammable material (Gilman Citation1999; ISO Citation4589–1: 2017; Janowska, Przygocki, and Włochowicz Citation2007). shows sample LOI values for all variants containing 33.33 wt% of cellulose and 66.67 wt% of carbon additives, which showed the best results.
Results of MLC tests
In the MLC tests, the untreated samples (C) had the highest HRR. In contrast, the EG and EG/CNT variants (C+EG+CNT (1:1:1) and C+EG (1:2)) showed the lowest values with pHRR of 22.82 kW/m2 and 38.06 kW/m2. The variant containing CNT and cellulose in a ratio of 2:1 exhibited the longest Tti, which was 18 s, followed by the variant containing EG/CNT and cellulose in a ratio of 2:1, which took 15.7 s. The samples from the C+EG (1:1) variant ignited the fastest, in just 6.7 s. shows the average results and standard deviations of pHRR, Tti, Ttf, and ML after the MLC tests. shows a representative HRR development for variants that contained cellulose and carbon additives in a 1:2 ratio.
Figure 4. Heat release rate (HRR) measured by cone calorimeter test for variants containing carbon additives in the weight ratio cellulose:additive 1:2.
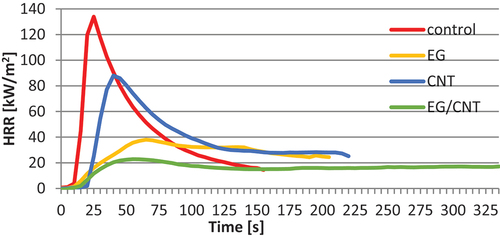
Table 3. Average values and standard deviations of the flammability properties of materials obtained by the ML calorimeter method.
Results of DSC/TG
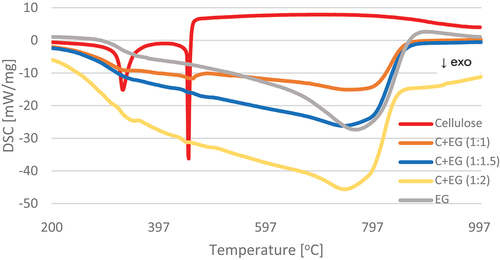
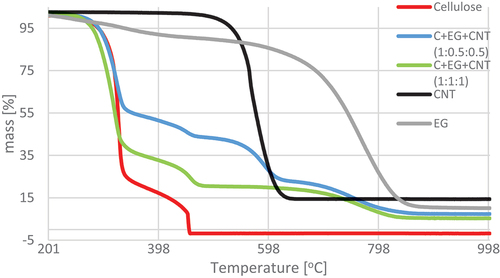
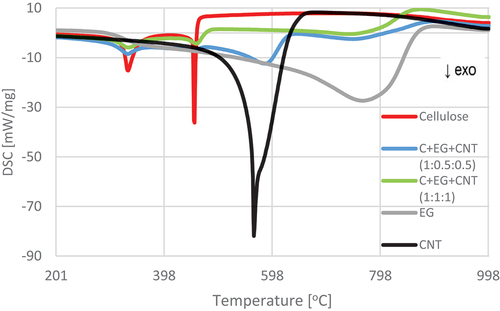
The results of thermogravimetric analysis indicate slower thermal degradation of the cellulose composite containing EG compared to the variants with pure cellulose and with the addition of CNT. From 600°C upward, CNT-treated material was almost fully degraded, while EG-treated material persisted. The course of the TG and DSC curves () showed two temperature regions of the thermal decomposition of the control sample. The first region covered the temperature range of 265.5–394°C, and the second was 427.16–472.72°C (). These temperature ranges are associated with the thermal decomposition of cellulose. ML of the tested material accompanied the process of thermal decomposition. It was 78.64% for the first temperature region and 13.27% for the second designated region. The total ML at the assumed temperature of the final analysis (1200°C) was 100%.
Figure 5. Thermogravimetric analysis (TG) curves for pure materials in the temperature range 200–1000°C.
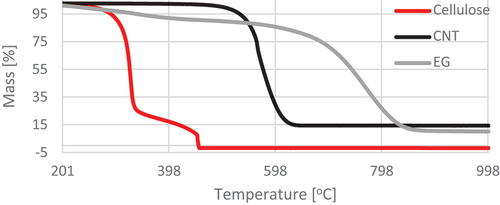
Figure 6. Differential scanning calorimetry (DSC) curves for pure materials in the temperature range 200–1000°C.
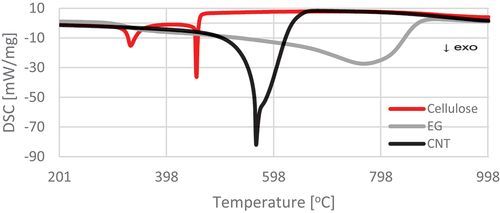
Table 4. Results of thermogravimetric analysis of selected variants.
The reference materials (EG, CNT) showed one temperature region of thermolysis, amounting to 557.89–866.83°C and 501.12–641.15°C, respectively, with MLs in this region of 76.83% and 85.32%. As a result, the final ML was 89.76% and 85.01%, respectively ().
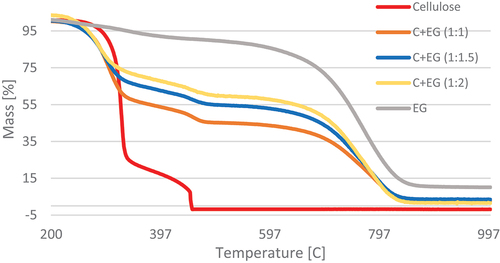
By analyzing the material modified with the EG in a different weight ratio to cellulose, three temperature regions of distribution were determined. Generally, the first two are related to the base material (cellulose) presence, which were characterized by similar temperature and ML values, whereas the third is related to the EG content. In the case of the first regions, a narrowing of the thermolysis temperature region was observed. On the other hand, a decrease in ML was recorded for the second temperature region. The third region characterizing the share of EG in the material occurred in the temperature range of 594.9–843.1°C. The lowest ML was determined in the case of variant C+EG (1:1.5) (; ).
Figure 7. TG curves of material containing cellulose and expandable graphite (EG) in the temperature range 200–1000°C.
By analyzing the variants containing the addition of CNT in the pulp, it can be concluded that with the increase in CNT content, the material’s decomposition temperature range increased, compared to variant C, and the ML in respective regions decreased. Three regions of active thermolysis characterized all three variants. For the first temperature region, its beginning was shifted to the following temperatures (ML): 271.38°C (33.44%) (C+CNT (1:1)), 296.85°C (25.97%) (C+CNT (1:1.5)), and 307.66°C (24.52%) (C+CNT (1:2)). The end of this region in all three variants was similar and was 352.11–356.93°C. The second of the designated regions started in a similar temperature range. However, the ends of these regions increased. Also, in this case, there was a decrease in MLs with an increase in the CNT content in the mass. In the case of the third region of active thermolysis, its beginning was at the same level, whereas the end of this region for variant C+CNT (1:1) was almost 70°C lower than the others. The final MLs decreased with increasing CNT content in the mass (; ). The thermal decomposition regions and temperatures of cellulose and cellulose/CNT sheets were similar to those of Maria and Mielo (Maria and Mieno Citation2017). Their values depend on the amount of CNTs in cellulose.
Figure 9. TG curves of material containing cellulose and carbon nanotubes (CNTs) in the temperature range 200–1000°C.
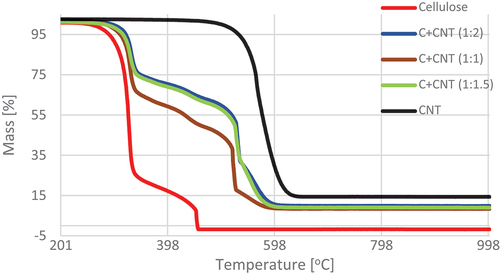
Figure 10. DSC curves of material containing cellulose and CNTs in the temperature range 200–1000°C.
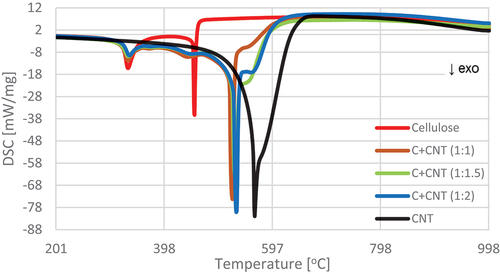
In the case of variants containing both EG and CNT in the pulp, a significant variation was found in the number of thermolysis temperature regions and MLs in these regions. Four regions of thermolysis were determined for variant C+EG+CNT (1:0.5:0.5). The first two were related to the content of cellulose, the third to the presence of CNT in pulp, and the fourth to the presence of EG. In the case of the first two regions, the temperature shifted to higher ranges, and the MLs in these regions were reduced compared to variant C. A different course was observed for variant C+EG+CNT (1:1:1), where three regions of active thermolysis were noted. The first two refer to the cellulose content, and the third probably refers to the EG and CNT content. No transparent region associated with CNT content was shown. The beginning of the first thermolysis region was shifted toward lower temperatures. It amounted to 271.38°C, and the end of this region was similar to the region in variant C+EG+CNT (1:0.5:0.5). Also, the beginning of the second region had a lower temperature (422.31°C), and the courses were the same as in variant C+EG+CNT (1:0.5:0.5). The third temperature region started at a significantly lower temperature (614.86°C) compared to variant C+EG+CNT (1:0.5:0.5), and the end of this region was at a lower temperature (833.99°C) (; ).
Conclusions
This work shows an effective and simple method of producing cellulose:carbon additive sheets with increased fire-retardant properties.
The content of EG significantly improved the flammability parameters of the manufactured composites, regardless of the amount and testing method used.
Variants containing equal amounts of CNTs and EG showed the best flame-retardant properties when tested by MLC, MFT, and LOI methods. In addition, cellulose:additive weight ratios of 1:1.5 and 1:2 showed the best fire-retardant properties of the materials.
The use of an equal amount of EG and CNTs in the cellulose material, tested by the MLC and the MFT method, showed an increase in the fire-resistant properties of the material, which may indicate a positive interaction of both components.
The results of the thermogravimetric analysis indicated slower thermal degradation of cellulose composites containing EG compared to the variants with pure cellulose or with the addition of CNT, leading to a shift toward higher temperatures for the thermal decomposition of the material.
Highlights
The developed composite material can be used, depending on the needs of the industry, as part of thin, light panel material, partition, or fireproof cladding in construction, railways, transport, automotive, and shipbuilding industries.
This work shows an effective and simple method of producing cellulose:carbon additive sheets with increased fire-retardant properties.
The addition of expandable graphite (EG) resulted in an increased fire resistance in the mini fire tube (MFT) test, limited oxygen index (LOI) measurements, and mass loss calorimeter (MLC).
The use of an equal amount of EG and CNTs (carbon nanotubes) in the cellulose material, tested by the MLC and the MFT method, showed an increase in the fire-resistant properties of the material, which may indicate a positive interaction of both components.
The results of the thermogravimetric analysis indicated slower thermal degradation of cellulose composites containing EG compared to the variants with pure cellulose or with the addition of CNT, leading to a shift toward higher temperatures for the thermal decomposition of the material.
Acknowledgments
The study was financed within the framework of the National Center for Research and Development, III edition of EEA and Norway grants; The Program ‘Applied Research’ in the frame of Norway Grants 2014–2021/POLNOR 2019 (NOR POLNOR/CellMat4ever/0063/2019-00)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Anderson, R. E., J. Guan, M. Ricard, G. Dubey, J. Su, G. Lopinski, G. Dorris, O. Bourne, and B. Simard. 2010. “Multifunctional Single-Walled Carbon Nanotube–Cellulose Composite Paper.” Journal of Materials Chemistry 20 (12): 2400–14. https://doi.org/10.1039/b924260k.
- ASTM D-2863. “Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index).”
- ASTM E69-02. 2002. Standard Test Method for Combustible Properties of Treated Wood by the Fire-Tube Apparatus.
- Chandrasekaran, S., A. Cruz-Izquierdo, R. Castaing, B. Kandola, and J. L. Scott. 2023. “Facile Preparation of Flame-Retardant Cellulose Composite with Biodegradable and Water Resistant Properties for Electronic Device Applications.” Scientific Reports 13 (1): 3168. https://doi.org/10.1038/s41598-023-30078-0.
- Cipiriano, B. H., T. Kashiwagi, S. R. Raghavan, Y. Yang, E. Grulke, K. Yamamoto, J. R. Shields, and J. F. Douglas. 2007. “Effects of Aspect Ratio of MWNT on the Flammability Properties of Polymer Nanocomposites.” Polymer 48 (20): 6086–6096. https://doi.org/10.1016/j.polymer.2007.07.070.
- Duquesne, S., M. L. Bras, S. Bourbigot, R. Delobel, H. Vezin, G. Camino, B. Eling, C. Lindsay, and T. Roels. 2003. “Expandable Graphite: A Fire Retardant Additive for Polyurethane Coatings.” Fire and Materials 27 (3): 103–117. https://doi.org/10.1002/fam.812.
- Fangueiro, R., and S. Rana. 2016. “Natural Fibres: Advances in Science and Technology Towards Industrial Applications.” RILEM Bookseries 12. https://doi.org/10.1007/978-94-017-7515-1_1.
- Farsheh, A. T., M. Talaeipour, A. H. Hemmasi, H. Khademieslam, and I. Ghasemi. 2011. “Investigation on the Mechanical and Morphological Properties of Foamed Nanocomposites Based on Wood Flour/pvc/multi-Walled Carbon Nanotube.” Bio Resources 6 (1): 841–852. https://doi.org/10.15376/biores.6.1.841-852.
- Focke, W. W., H. Muiambo, W. Mhike, H. J. Kruger, and O. Ofosu. 2014. “Flexible PVC Flame Retarded with Expandable Graphite.” Polymer Degradation and Stability 100 (1): 63–69. https://doi.org/10.1016/j.polymdegradstab.2013.12.024.
- Fox, D. M. L. Jieun, E. Ford, E. Balsley, M. Zammarano, S. Matko, and J. W. Gilman. 2009 POSS modified cellulose for improving flammability characteristics of polystyrene. 10th International Conference on Wood & Biofiber Plastic Composites and Cellulose Nanocomposites Symposium May 11-13, 2009 (Forest Products Society) Monona Terrace Community & Convention Center, Madison, Wisconsin, USA.
- Fu, X., C. Zhang, T. Liu, R. Liang, and B. Wang. 2010. “Carbon Nanotube Buckypaper to Improve Fire Retardancy of High-Temperature/high-Performance Polymer Composites.” Nanotechnology 21 (23): 235701–xxx. https://doi.org/10.1088/0957-4484/21/23/235701.
- Gashti, M., and A. Almasian. 2013. “UV Radiation Induced Flame Retardant Cellulose Fiber by Using Polyvinylphosphonic Acid/Carbon Nanotube Composite Coating.” Composites Part B: Engineering 45 (1): 282–289. https://doi.org/10.1016/j.compositesb.2012.07.052.
- Gilman, J. W. 1999. “Flammability and Thermal Stability Studies of Polymer Layered – Silicate (Clay) Nanocomposites.” Applied Clay Science 15 (1–2): 31–49. April. https://doi.org/10.1016/S0169-1317(99)00019-8.
- Grześkowiak, W. Ł. 2017. “Effectiveness of New Wood Fire Retardants Using a Cone Calorimeter.” Journal of Fire Sciences 35 (6): 565–576. https://doi.org/10.1177/0734904117737464.
- Grześkowiak, W. Ł., K. Moliński, M. Molińska-Glura, and G. Cofta. 2021. “Assessment of the Impact of the Storage Time of Fire Retardant and Heating of the Protected Wood on the Effectiveness of Fireproofing.” Drewno 64 (207): 145–157. https://doi.org/10.12841/wood.1644-3985.371.07.
- ISO 13927:2015. 2015. “Plastics — Simple Heat Release Test Using a Conical Radiant Heater and a Thermopile Detectora.”
- ISO 4589-1:2017. 2017. “Plastics一determination of Burning Behaviour by Oxygen Index - Part 1: Guidance.”
- ISO 4589-2:1999. 1999. “Plastics - Determination of Burning Behaviour by Oxygen Index - Part 2: Ambient-Temperature Test.”
- Janowska, G., W. Przygocki, and A. Włochowicz. 2007. Palność polimerów i materiałów polimerowych. Warszawa: Wydawnictwo Naukowo – Techniczne.
- Jin, H., J. Yuan, H. Hao, Z. Ji, M. Liu, and S. Hou. 2013. “The Exploration of a New Adsorbent As MnO2 Modified Expanded Graphite.” Materials Letters 110:69–72. https://doi.org/10.1016/j.matlet.2013.07.042.
- Kashiwagi, T., F. Du, K. I. Winey, K. M. Groth, J. R. Shields, S. P. Bellayer, H. Kim, and J. F. Douglas. 2005. “Flammability Properties of Polymer Nanocomposites with Single-Walled Carbon Nanotubes: Effects of Nanotube Dispersion and Concentration.” Polymer 46 (2): 471–481. https://doi.org/10.1016/j.polymer.2004.10.087.
- Łukawski, D., W. Grześkowiak, D. Dukarska, B. Mazela, A. Łękawa-Raus, and A. Dudkowiak. 2019. “The Influence of Surface Modification of Wood Particles with Carbon Nanotubes on Properties of Particleboard Glued with Phenol-Formaldehyde Resin.” Drewno 62 (203): 93–105. https://doi.org/10.12841/wood.1644-3985.265.03.
- Maria, K. H., and T. Mieno. 2017. “Production and Properties of Carbon Nanotube/Cellulose Composite Paper.” Journal of Nanomaterials 2017:6745029. https://doi.org/10.1155/2017/6745029.
- Mazela, B., A. Batista, and W. Grześkowiak. 2020. “Expandable Graphite As a Fire Retardant for Cellulosic Materials—A Review.” Forests 11 (7): 755. https://doi.org/10.3390/f11070755.
- Mazela, B., and M. Broda. 2015. “Natural Polymer-Based Flame Retardants for Wood and Wood Products.” Proceedings of the 11th meeting of the Northern European Network for Wood Sciences and Engineering (WSE), 146–155. Poland. Poznan University of Life Sciences. 14–15 September 2015.
- Miyashiro, D., R. Hamano, and K. Umemura. 2020. “A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes.” Nanomaterials: Overview and Historical Perspectives 10 (2): 186. https://doi.org/10.3390/nano10020186.
- Salmeia, K. A., M. Jovic, A. Ragaisiene, Z. Rukuiziene, R. Milasius, D. Mikucioniene, S. Gaan. 2016. “Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy.” Polymers 8 (8): 293. https://doi.org/10.3390/polym8080293.
- Seo, H. J., S. Kim, W. Huh, K.-W. Park, D. R. Lee, D. W. Son, Y.-S. Kim, et al. 2016. “Enhancing the Flame-Retardant Performance of Wood-Based Materials Using Carbon-Based Materials.” Journal of Thermal Analysis and Calorimetry 123 (3): 1935–1942. https://doi.org/10.1007/s10973-015-4553-9.
- Shen, D., and S. Gu. 2009. “The Mechanism for Thermal Decomposition of Cellulose and Its Main Products.” Bioresource Technology 100 (24): 6496–6504. https://doi.org/10.1016/j.biortech.2009.06.095.
- Smędowski, Ł., and R. Muzyka. 2013. “Grafen–metody otrzymywania a zastosowanie i właściwości.” Karbo 2: 79–87 http://sbc.org.pl/dlibra/publication/edition/272061.
- Smith, A. T., A. M. La Chance, S. Zeng, B. Liu, and L. Sun. 2019. “Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites.” Nano Materials Science 1 (1): 31–47, ISSN 2589–9651. https://doi.org/10.1016/j.nanoms.2019.02.004.
- Suttipintu, T., S. Lhosupasirirat, T. Osotchan, and T. Srikhirin. 2022. “Development of Flame Retardant Property on Sodium Silicate Treated Paper Based Materials.” Journal of Physics: Conference Series 2175 (1): 012035. https://doi.org/10.1088/1742-6596/2175/1/012035.
- Thostenson, E. T., C. Li, and T.-W. Chou. 2005. “Nanocomposites in Context.” Composites Science and Technology 65 (3): 491–516. https://doi.org/10.1016/j.compscitech.2004.11.003.
- Zabel, H., and S. A. Solin. 2013. Graphite Intercalation Compounds I: Structure and Dynamics, 5–6. Vol. 14. Berlin, Germany: Springer Science & Business Media.
- Zeinali, D., D. Koalitis, and J. Schmid. 2018. Guide for Obtaining Data from Reaction to Fire Tests. Switzerland: ETH Zürich.
- Zheng, C., N. Li, and M. Ek. 2017. “Cellulose-Fiber-Based Insulation Materials with Improved Reaction-To-Fire Properties.” Nordic Pulp and Paper Research Journal 32 (3): 466–472. https://doi.org/10.3183/npprj-2017-32-03-p466-472.
- Zheng, C., N. Li, and M. Ek. 2018. “Mechanism and Kinetics of Thermal Degradation of Insulating Materials Developed from Cellulose Fiber and Fire Retardants.” Journal of Thermal Analysis and Calorimetry 135 (6): 3015–3027. https://doi.org/10.1007/s10973-018-7564-5.
- Zhuge, J. 2012. “Fire Retardant Polymer Nanocomposites: Materials Design and Thermal Degradation Modeling.” Electronic Theses and Dissertations, 2004-2019 2174. https://stars.library.ucf.edu/etd/217433.