ABSTRACT
Cartilage and joint damage easily degenerates cartilage and turns into osteoarthritis (OA), which seriously affects human life and work, and has no cure currently. The temporal and spatial changes of multiple microenvironments upon the damage of cartilage and joint are noticed, including the emergences of inflammation, bone remodeling, blood vessels, and nerves, as well as alterations of extracellular and pericellular matrix, oxygen tension, biomechanics, underneath articular cartilage tissues, and pH value. This review summarizes the existing literatures on microenvironmental changes, mechanisms, and their negative effects on cartilage regeneration following cartilage and joint damage. We conclude that time-dependently rebuilding the multiple normal microenvironments of damaged cartilage is the key for cartilage regeneration after systematic studies for the timing and correlations of various microenvironment changes.
Introduction
The biocomposite formed by articular cartilage, calcified cartilage (their combination is cartilage) and subchondral bone is called osteochondral unit,Citation1 in which cartilage damage can be caused by several conditions including accidents such as a tear to the anterior cruciate ligament, injury, slow degeneration over time (aging), excessive activity (overuse), excessive weight (obesity), poor alignment of joint, necrosis, and other diseases of osteoarthritis (OA) and rheumatoid arthritis etc. Cartilage has a minimal ability to repair itself in terms of structure, function, and strength for its avascular nature and poor capability for adult chondrocytes to secret extracellular matrix. To be worse, the appeared mesenchymal stromal cells (MSCs) show a changed phenotype, which is susceptible to hypertrophy, matrix metalloproteinase-13 (MMP-13) release and osteogenesis.Citation2 Generally, cartilage damage is healed by fibrocartilage different from normal cartilage, which is difficult to integrate with surrounding tissues, and ultimately degenerated and disintegrated over time. Cartilage damage usually leads to serious medical consequences, in which constant and severe pain, inflammation, and some degree of disability are typical.
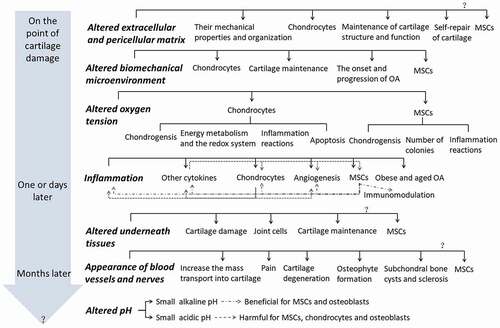
A “seed and soil theory” is raised by Stephen Paget in 1880, which implies that the initiation and progression of disease such as cancer focus not only on the cell itself, “the seed,” but also on “the soil,” in which it derives its nutrients, oxygen, and signals for growth and development.Citation3 The importance of microenvironment is postulated thereafter. This review combs the time-dependently appeared microenvironmental changes and their mechanism following cartilage or joint damages, and the influences of one microenvironmental change on relative tissues, cells, cytokines, and other microenvironments as well as their interplays, focusing on MSCs for their promise in cartilage regeneration, which is summarized in . We conclude that systematic studies for the timing and correlations of various microenvironmental changes and time-dependently rebuilding the multiple normal microenvironments of damaged joint may be the key to cartilage regeneration.
Figure 1. The time-dependently (in the gray arrow) altered microenvironments (the bold italic letters) and their influences on relative tissues, cells, cytokines, and other microenvironments (normal black letters) as well as their interplays (as shown in Inflammation, positive effects are gray line and dot lines, the negative effects are dot lines) after cartilage or joint damage.
Extracellular and pericellular matrixes microenvironment
Time-dependently altered extracellular and pericellular matrixes after cartilage and joint damage
After cartilage damage, the concentrations of matrix degradation enzymes, such as MMP-3,Citation4 MMP-13, and dsRNA-dependent protein kinase PKR increase,Citation5 resulting in loss of proteoglycan and type II collagen on the fifth day, loss of fixed charged density,Citation6 change of collagen fiber orientation and increase of cartilage thickness in two weeks.Citation7 In addition, the type I collagen, fiberonectin, and their degradation fragments increase in 20 days.Citation8,Citation9 Significant proteoglycan loss and increase of type II collagen orientation angle and content exist in the middle and deep zones of cartilage tissue in four weeks.Citation10 The ratio of collagen degradation to synthesis increases after 6 months.Citation4 The fibrillation expands to the radial zone at eight months.Citation11 With the development of OA, the staining for type VI collagen and perlecan changes progressively from compact to weakened and less focalized.Citation12 Laminin is depleted from the pericellular matrix before collagen type IV.Citation13 In fact, the microscale spatial pericellular matrix and local extracellular matrix in middle and superficial zones of cartilage are softened before the structural changes in macroscale tissue properties appear.Citation14 On the other hand, a small leucine-rich proteoglycan of decorin is found to slightly increased ten weeks after OA initiation.Citation15
The negative effects of altered extracellular and pericellular matrixes on cartilage maintenance and regeneration
The normal components of extracellular and pericellular matrixes are important for maintenance of structure and function in osteochondral tissues and cells, as well as for their self-repair capability. For example, collagen provides an important microenvironment for human OA chondrocytes in response to mechanical stimulation.Citation16 Type III collagen is a key regulator of collagen fibrillar structure and biomechanics of articular cartilage. Reduction of type III collagen leads to increased heterogeneity and mean thickness of collagen fibril diameter, as well as reduced modulus, and these effects become more pronounced with skeletal maturation. Type III collagen also has a marked contribution to the micromechanics of the pericellular matrix.Citation17 The proteoglycan-4 secreted by synovial fibroblasts and superficial zone chondrocytes plays important autocrine role in modulating OA synoviocyte proliferation and expression of matrix degrading enzymes.Citation18 Perlecan is required for the chondrogenic differentiation of synovial MSCs.Citation19 Laminins and nidogen-2 in pericellular matrix drive chondrogenic progenitor cells toward chondrogenesis.Citation20 Retention of the native chondrocyte pericellular matrix improves its matrix production.Citation21 Decorin could increase the adhesion between aggrecan and aggrecan molecules, and between aggrecan molecules and collagen II fibrils, as well as increase the retention of aggrecan in the neo-matrix of chondrocytes. Decorin delays cartilage matrix degeneration and fibrillation,Citation22 but decorin-null cartilage substantially reduces aggrecan content.Citation23
The altered extracellular and pericellular matrixes lead to changes in mechanical properties and organization in themselves. Decrease of equilibrium modulus, dynamic modulus and phase angle in cartilage two weeks after OA induction,Citation7 as well as impair of stiffness in collagen network and increase of permeability after four weeks are found.Citation24 The elastic moduli in extracellular and pericellular matrixes are decreased for 45% and 30% on the medial condyle after OA.Citation25 The stiffnessCitation26 and mean Young’s modulusCitation12 of pericellular matrix are also decreased. However, chondrocyte shear strain is increased for 30% in early-OA model.Citation14 Loss of organization in pericellular matrix, including perforation, decrease and destruction of local micro-fibrillar collagen intensity, regional collagen type VI signal weakness or absence are observed in early OA.Citation27
The altered extracellular and pericellular matrixes in cartilage affect joint cells. Cartilage wear particles result in a significant increase in DNA and collagen content as well as nitric oxide (NO) and prostaglandins E2 synthesis of synoviocytes.Citation28 The fragments from degraded extracellular matrix may have catabolic properties, such as fibronectin fragments (Fn-fs) induce expression of proinflammatory cytokines, NO, and other inflammatory mediators as well as proteinases in joint, acting as proinflammatory mediators in a positive feedback loop and therefore promoting inflammation and cartilage destruction in OA.Citation29 However, its effect on MSCs is unclear.
Mechanical microenvironment
Time-dependent altered mechanical microenvironment after cartilage and joint damage
Mechanical loading to the articular joint induces a series of mechanical forces, including tension, hydrostatic pressure, shear and compression on cartilage. Due to the damage, the mechanical properties of cartilage are changed. The viscoelastic properties of articular cartilage, especially around the tidemark are decreased in two weeks after OA initiation and degraded as cartilage degeneration progressed.Citation30 The equilibrium and dynamic moduli of cartilage are lowered for up to three times four weeks after OA initiation.Citation10 The cartilage stiffnessCitation31 and storage modulusCitation32 of OA patients are reduced. But the exercise-induced cartilage strain is increased.Citation33 OA cartilage has a higher capacity to hold water.Citation32
As to subchondral bone, the stiffness is reduced four weeks after OA initiationCitation34 and the modulus is decreased in mild OA patients.Citation35 The shear modulus and radial strain ratio are reduced. But the energy dissipation increases.Citation36 The hyperelastic material constants, the structural parameters,Citation37 and the reduction in cartilage stiffness correlate with OA grade.Citation31 The detailed time-dependent alterations in mechanical microenvironment after cartilage and joint damage need further studies.
The negative effects of altered biomechanical microenvironment on chondrocytes and MSCs, as well as effects on OA
It is widely accepted that mechanical forces of a relevant magnitude are essential for maintaining cartilage homeostasis within a healthy joint and playing a vital role in cartilage development. However, excess mechanical stress and strain are experienced by the damaged chondrocytes, which may promote apoptosisCitation38 and anabolic function, increase reactive oxygen species (ROS) production, and decrease uncoupling protein 2 (UCP2) in chondrocytes.Citation39 High fluid shear stress of 20 dyn/cm2 experienced after cartilage damage induces the mRNA expression of MMP-12. In fact, the micromechanical environment changes harm chondrocyte activities are before the macro-scale degradations at the tissue level become apparent.Citation40
The external mechanical environment has potent capability in regulation of MSC differentiation compared with other two known powerful means of biological factors and substrate stiffness.Citation41 Suitable biomechanical stimulation promotes the proliferation and chondrogenesis of MSCs.Citation42 However, the higher or lower mechanical environment could induce MSCs to other cell types, i.e. osteogenic or adipogenic cells.Citation43
Abnormal mechanical loading also lead to onset and progression of OA.Citation44 Increased matrix stiffness disrupts the homeostatic balance between chondrocyte catabolism and anabolism, consequently eliciting OA pathogenesis. Enhancement of matrix cross-linking also augments surgically induced OA pathogenesis. While suppressing these events effectively inhibits OA.Citation40
Oxygen tension microenvironment
Altered oxygen tension microenvironment after cartilage and joint damage
Oxygen tensions within cartilage are strongly affected by many factors, including oxygen concentrations in synovial fluid, cartilage thickness, cell density, and cellular oxygen consumption rates, and supply of oxygen from the subchondral bone.Citation45 Normally, the oxygen tension of cartilage is estimated to be an average of ~5%, with levels slightly higher (around 6%) at the surface and lower (2 ~ 3%) in the depth,Citation46 whilst that of bone marrow is at 7%.Citation47,Citation48 However, the oxygen tension of cartilage would be changed after damage. The deep and low oxygen tension survival chondrocytes have possibility to contact with oxygen rich synovial fluid due to cartilage lesion. And the blood vessels evolved in subchondral bone or even tidemark with the progression of OACitation49 increase mass transport from bone to cartilage,Citation49 which provides opportunity for high oxygen tension in damaged cartilage. On the other hand, the inflammation could induce hypoxia.Citation50 The increased mineralization of calcified cartilage may reduce its oxygen tension.Citation49
The negative effects of altered oxygen tension microenvironment on chondrocytes, MSCs, and inflammation reactions
Physiological oxygen tension profits, however, the lower or higher oxygen tension is harmful for chondrocyte chondrogenesis. For example, compared with physiological oxygen tension, 21% O2 promotes dedifferentiation of chondrocytes toward an MSC phenotype,Citation51 and inhibits the dynamic compression stimulated glycosaminoglycan synthesis.Citation52 1% O2 reduces hyaluronan synthesis in chondrocytes compared with 21% O2.Citation53 Oxygen affects energy metabolism and the redox system in normal and OA chondrocytes. In detail, compared with 20% O2, 1% O2 significantly reduces, but 5% O2 significantly increases ATP levels.Citation54 Less than 1% O2 leads to reduction in cell viability, glutathione (GSH): oxidized glutathione (GSSG) ratio and superoxide dismutase 1 (SOD1) and SOD2 protein expressions, increase in glycosaminoglycan release, and alteration in mitochondrial membrane potential and ROS levels in 48 h compared with condition of 5% O2.Citation55,Citation56
Physiological oxygen tension profits, however, the higher oxygen tension is harmful for MSC chondrogenesis. For example, compared with physiological oxygen tension, MSCs from 20% O2 responsive donors have decreased glycosaminoglycan and type II collagen content, and induced hypertrophic markers.Citation57 Similarly, human MSCs express typical articular cartilage biomarkers and established inhibitors of hypertrophic differentiation under 2.5% O2. In contrast, 21% O2 prevents the expression of these inhibitors and is associated with increased hypertrophic differentiation.Citation58 Furthermore, 21% O2-mediated isolation and expansion of MSCs reduce the number of colonies, the chondrogenic capacity, the mRNA expression of hypoxia inducible factor-2ɑ, but increase the mRNA expression of collagen X.Citation59 20% O2 enhances the inhibitory effect of inflammation factors on the chondrogenic differentiation of MSCs.Citation60
The oxygen tension sensitivity of MSCs is dependent on their differentiative potentials. MSC preparations and articular cartilage progenitors clones of high intrinsic chondrogenicity produce abundant matrix both at 20% and 2% O2, while poorly chondrogenic cells demonstrate a significant fold-change matrix increase only at 2% but not 20% O2. Both high and low intrinsic chondrogenicity groups upregulate chondrogenic genes; however, only high intrinsic chondrogenicity groups have a concomitant decrease in hypertrophy-related genes and increase in many common hypoxia-responsive genes at 2% O2, while low intrinsic chondrogenicity groups downregulate most of these genes. High intrinsic chondrogenicity groups produce comparable type II collagen but less type I collagen at 2% O2 than at 20% O2. Type X collagen is detectable in some articular cartilage progenitors pellets at 20% O2 but reduced or absent at 2% O2. In contrast, type X collagen is detectable in all MSC preparations at 2% and 20% O2.Citation61
Oxygen affects inflammation reactions in articular chondrocytes, where 5% O2 is optimal compared with 1% and 20% O2. IL-1 induced reduction in protein translation, increase in autophagy and no effects on senescence are only found at 20% O2 but not at 1% and 5% O2. The NO donor induced reduction in ATP levels is observed at all oxygen tensions, but increase of phospho-adenosine monophosphate-activated protein kinase (pAMPK) and senescence is only shown at 5% O2, and decrease of pAMPK, protein translation and autophagy is only appeared at 1% O2.Citation54
Inflammatory microenvironment
Appearance of and time-dependently altered inflammatory microenvironment after cartilage and joint damage
Following joint damage, macrophage phenotypes accumulate and localize.Citation62 The synovial macrophages, fibroblasts and chondrocytes secret inflammatory factors,Citation63 which increase their concentrations in synovial fluid, including interleukin-1ß,6,8, (IL-1ß,6,8), tumor necrosis factor-ɑ (TNF-ɑ), and C-reactive protein in one dayCitation64 and IL-10 in 2 weeks.Citation63 After acute phase, their concentrations generally drop down, but some are reported to maintain elevated levels than normal conditions for months to years, especially under OA progression.
The negative effects of the inflammatory microenvironment on chondrocytes and MSCs, as well as effects on other cytokines, angiogenesis, and OA
The inflammatory factors elicit catabolism in joint. Inflammation inhibits the synthesis of proteoglycans and type II collagen,Citation65 and results in apoptosis of chondrocytes.Citation66 In detail, IL-1ß decreases mRNA of type II collagen, aggrecan core protein and MMP-9, while increases MMP-1, 2, 3 and tissue inhibitors of matrix metalloproteinases (TIMPs) in chondrocytes.Citation67 TNF-α increases MMP activity, induces MMP-13 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-5) gene expression, and reduces dynamic compression induced glycosaminoglycan synthesis in chondrocytes dose-dependently in 48 h.Citation52 While NO generated in joint induces apoptosis of human articular chondrocytes within 24 h.Citation68 NO also inhibits respiration and synthesis of adenosine triphosphate (ATP) and proteoglycans without affecting cell viability, but increases cellular levels of heat-shock protein 70 (hsp70) and heme oxygenase 1 (HO-1)Citation69 with cartilage protective effects.Citation70,Citation71 Prostaglandin E2 and F2 generate synergistic effects on the expression of MMP-12.Citation72 While the inflammation induced MMPs, ADAMTS, and other cytokines are known to cause cartilage matrix degradation and angiogenesis.Citation29,Citation73,Citation74
Inflammation affects proliferation, differentiation, and angiogenesis of MSCs. Exposure of MSCs to proinflammatory cytokines reduces the viability and differentiation abilities to osteogenic, adipogenic and chondrogenic cells,Citation75 in addition to their expression levels of SOX-9, TGF-β1, aggrecan, and type II collagen.Citation76 The expression of VEGF in MSCs is upregulated by OA synovial fluid.Citation77 TNF-α activated MSCs promote endothelial progenitor cell homing and angiogenesis.Citation78
Inflammation affects immunomodulation and inflammation modulation of MSCs. Inflammatory cytokines authorize the immunomodulatory capabilities of MSCs, which can orchestrate local and systemic innate and adaptive immune responses through the releaseCitation79 and interventionCitation80 of various mediators. Even nonviable MSCs can exert beneficial immunomodulatory effects, but apoptotic MSCs show immunosuppressive functions.Citation79 For example, inflammatory environment impairs galectin expression in MSCs, which may limit MSC tissue repair properties following intra-articular administration, for galectins are a family of β-galactoside binding proteins regulating inflammation, adhesion and cell migration in diverse cell types.Citation80 Lipopolysaccharide (LPS) and IL-1β increase the expression of pro-inflammatory cytokines, i.e. TNF-α and IL-1β, 6, 8 in MSCs.Citation77 On the other hand, MSCs secreted factors under inflammatory environment cause multiple anti-inflammatory effects.Citation81 In special, MSC exosomes suppress IL-1β-induced NO and MMP13 production in chondrocytes.Citation82
The net outcomes of MSC activation might vary depending on the levels and the types of inflammationCitation83 and MSC sources. Factors secreted by pro-inflammatory macrophages substantially increase MSC attachment and migration, whereas those released by anti-inflammatory macrophages enhance MSC osteogenic activity as well as cell migration.Citation83 Inflammatory synovial environment may not be enough to activate MSC immunomodulatory potential when they are intraarticular administered, yet inflammatory priming enhances MSC immunomodulatory potential,Citation84 where inflammatory cytokine primed-MSCs produce less cytotoxic allo-antibodies directed against donor’s leukocyte antigen than naïve-MSCs in secondary intraarticular administration.Citation85 IL-1β stimulates more IL-8, oncostatin M (OSM), the angiogenic proteins of vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1, VEGF-A, VEGF-C, the proteases of MMP-1 and 3, and the protease inhibitor TIMP3 in naïve MSCs than in MSCs undergoing chondrogenic differentiation in 48 h.Citation86
The inflammatory factors promote angiogenesis in joint. Chondrocytes in fracture model play a key role in angiogenesis, which is transcription factor forkhead box-O 1 (FOXO1) dependent and that FOXO1 in chondrocytes regulates a potent angiogenic factor, VEGF-A.Citation87 Inflammation drives synovial angiogenesis.Citation88 Fibroblast changes under chronic synovial inflammation condition induce angiogenesis under hypoxic conditions.Citation89 TNF-α induces the secretion of leucine-rich-alpha-2-glycoprotein 1 in human umbilical vein endothelial cells, which could increase angiogenesis and recruit MSCs in the subchondral bone of OA joints.Citation66 NO and prostaglandins also contribute to angiogenesis.Citation90,Citation91
The inflammatory factors induce the production of other cytokines that aggravate the inflammation condition in joint. For instance, IL-1ß induces IL-1ß, 6, 8, 11, and TNF-α expression,Citation92 in addition to NO and prostaglandins E2 production in chondrocytes.Citation54,Citation67 TNF-α increases NO and prostaglandins E2 dose-dependently.Citation52
Inflammation may involve in the obesity and aging induction of OA. Obese people and animals show a higher systematic level of serum TNF-α, IL-1β, and 6.Citation65 Similarly, aged people are characterized by the presence of a low-grade systematic proinflammatory status.Citation93 Highly vascularized synovial tissue, cartilage surface and subchondral bone could expose the obese and aged cartilage to the systemic influences of low grade inflammation and suffer from the negative effects.Citation93
Underneath articular cartilage tissues microenvironment
Time-dependently altered underneath articular cartilage tissues and appearance of bone remodeling microenvironment after cartilage and joint damage
Cartilage damage induces changes in tissues underneath articular cartilage. The thickness of human calcified cartilage decreases with age and the number of tidemarks increases particularly over age of sixty, and these processes are accelerated with increasing age.Citation94 The calcified cartilage is extremely hypermineralized, especially in superior regions, which is translocated into subchondral bone and articular cartilage in human end-stage OA.Citation95
The subchondral bone changes in damaged cartilage are widely studied for its hypothesized key role in OA progression. The bone remodeling and loss are increased in early-stage, but the remodeling is slowed and subchondral bone is densified in late stage, which are important spatial and temporal pathogenetic process of OA subchondral bone.Citation96 In addition, the fibril pattern has changed from a regular periodic banding pattern with apatite crystals parallel to the long axis of the fibrils in grade I OA, to a random and undulated arrangement with nonhierarchical intrafibrillar mineralization and higher calcium to phosphorus ratios accompanied by a circular oriented pattern of apatite crystals in grade IV OA.Citation97
The osteophyte bone is emerged in OA patients.Citation98 The number and volume of subchondral bone cysts are associated with lateral OA severity, lateral joint space narrowing, alignment, and sex. Greater cyst number and volume are associated with higher bone mineral density.Citation99 However, lateral-compartment osteophytes are not associated with biomechanically weaker cartilage or with more-advanced histological signs of degeneration of lateral-compartment cartilage in knees with varus arthritis.Citation100
The negative effects of altered underneath articular cartilage tissues microenvironment on cartilage and joint cells
Normal tissues and cells underneath articular cartilage profit cartilage maintenance. Normal healthy osteoblasts stimulate an initial increase in the number and later osteogenic differentiation of MSCs, and osteocytes are more influential than osteoblasts in osteogenesis of MSCs.Citation101 Normal healthy osteocytes enhance the IL-17-induced osteogenesis in MSCs through critically orchestrating the activation and differentiation of both osteoblasts and osteoclasts.Citation102
The altered tissues underneath articular cartilage promote cartilage damage. Abnormalities in subchondral bone of OA promote joint pain generation and articular cartilage degeneration.Citation103 Increased subchondral bone remodeling-induced microstructural damage aggravates OA, which is preceded by osteoporosis.Citation104 Osteoporosis may also accelerate OA progression because its aberrant bone remodeling leads to an inferior microstructure and worsening biomechanical properties, which potentially affect transmission of loading stress from the cartilage to the subchondral bone.Citation105
The altered tissues underneath articular cartilage disadvantage joint cells. The pathology of primary OA is accompanied by bone MSC exhaustion, a hallmark of aging.Citation106 The MLOY4 osteocytic cells cultured on extracellular matrix secreted from primary human subchondral bone osteoblasts of OA patients show a decrease of IL-β1 expression and deactivation of focal adhesion kinase (FAK) cell signaling pathway, which subsequently affect the initial osteocytic cells’ attachment and functions, including morphological abnormalities of cytoskeletal structures, focal adhesions, increased apoptosis, altered osteocyte specific gene expression and increased MMP-2 and -9 expression compared with MLOY4 osteocytic cells cultured on normal extracellular matrix.Citation107 However, the effect of altered tissues underneath articular cartilage on MSCs is unclear.
Blood vessels and nerves microenvironment
Appearance of blood vessels and nerves microenvironment after cartilage and joint damage
The cartilage is avascular without nerve. However, H-type vessels are increased in subchondral bone of six months OA model and aged mice.Citation108 The chondrocytes expressing vascular endothelial growth factor (VEGF), the proliferation of endothelial cell and vascular density in articular cartilage are increased, which are associated with subchondral bone marrow replacement by fibrovascular tissue expressing VEGF, and increased nerve growth factor expression within vascular channels.Citation109 The tidemark is breached by vascular channels along with sympathetic and sensory nerves present within in samples from patients received total knee joint replacement surgery. Perivascular and free nerve fibers and nerve trunks are observed within the subchondral bone marrow and the marrow cavities of osteophytes.Citation110 Osteochondral vascularity is associated with the severity of OA cartilage changes and clinical disease activity.Citation111
The negative effects of the appeared blood vessels and nerves microenvironment on cartilage maintenance and regeneration
The invaded blood vessels and nerves to subchondral bone, calcified cartilage or even to tidemark increase the mass transport into cartilageCitation49 and contribute to tibiofemoral pain in OA across a wide range of structural disease severity,Citation110 in addition to cartilage degeneration, osteophyte formation, subchondral bone cysts and sclerosis, and synovitis.Citation112 For instance, VEGF destructs condylar cartilage, profits the proliferation of their hypertrophic cells, decreases levels of proteoglycan, and increases expression of VEGF receptor 2 (VEGFR2), MMP-9, MMP-13, and TNF-related activation-induced cytokine.Citation113 On the contrary, the specific angiogenesis inhibitor PPI-2458 reduces synovial and osteochondral angiogenesis, synovial inflammation, joint damage, and pain behavior of induced OA animals.Citation114 Research shows that sole blockade of angiogenesis in MSCs is sufficient for inducing MSC chondrogenic differentiation.Citation115 However, its effect on MSCs is unclear.
pH value microenvironment
Altered pH value microenvironment after cartilage and joint damage
Normally the pH value in joint is 7.4. However, the pH value of synovial fluid is lowered after inflammationCitation50 and obesity.Citation116 The pH value of OA synovial fluid is age- and gender-dependent ranging from 6.80 to 7.68 with a mean of 7.78,Citation117 and lower pH value in female,Citation118 which is changed to 7.60 after revision aseptic operation, and to 7.55 after revision septic operation.Citation117 However, the detailed time-dependent pH change after cartilage and joint damage is unclear.
The negative effects of the altered pH microenvironment on joint cells
Small but not large alkaline pH may be beneficial for MSCs and osteoblasts. pH values of 7.4 and 8.0 upregulate MSC proliferation,Citation119 mineralization.Citation120 Weak alkaline conditions (<8.0) stimulate osteogenic differentiation, but inhibit the osteoclasts formation of osteoporotic rat MSCs.Citation121 While pH value of 8.5 inhibits proliferation and promotes senescence of MSCs.Citation119 Small alkaline pH (<8.4) is beneficial for the proliferation, differentiation and ATP content in developing osteoblasts.Citation122 The influence of alkaline pH on chondrocytes is not clear.
Small acidic pH may be harmful for MSCs, chondrocytes and osteoblasts. pH values of 6.3 and 6.7 inhibit proliferation and promote senescence of MSCs.Citation119 48 h of exposure to pH of 6.6–7.5 decreases the alkaline phosphatase activity and collagen I synthesis of MSCs.Citation123 pH affects elements of the redox system in normal articular chondrocytes. pH 6.2 leads to reduction in cell viability, GSH:GSSG ratio and SOD1 and SOD2 protein expressions, but increase in glycosaminoglycan release, alteration in mitochondrial membrane potential and ROS levels in 48 h compared with condition of pH 7.2.Citation55 pH also affects elements of the redox system in human osteoarthritic chondrocytes.Citation56 An acidic pH environment (>6.4) increases cell death and pro-inflammatory cytokine release in osteoblasts.Citation124 However, irrigation fluid with pH of 6.4 but not 7.6 facilitates intra-articular fibrous cartilage formation and cartilage healing after microfracture procedure.Citation125
Future directions
As discussed above, the sequential and related microenvironment changes, i.e. extracellular and pericellular matrix, biomechanics, oxygen tension, inflammation, underneath tissues, blood vessels, and nerves, as well as pH value emerge after cartilage damage, and are not fit for the normal growth and differentiation of chondrocytes, as well as the recruitment, growth, and chondrogenic differentiation of stem cells, which in turn lead to negative feedback loops and contribute to the eventual failure of cartilage repair as shown in Inflammation of . Accordingly, we suggest that time-dependently rebuilding the normal multiple microenvironments of damaged cartilage is the key for cartilage regeneration, though one specific microenvironment modification such as inhibition of inflammation or bone remodeling already shows benefit for cartilage regeneration through enzymatic disruption and removal or disruption of function of the factors induced microenvironment changes,Citation126–128 which is not the focus of our review here.
Considering the microenvironment changes and their negative feedbacks, in order to successfully regenerate cartilage, several questions below exist and are needed to study. (i) The time-dependent microenvironment changes are not understood clearly and systematically, especially the pH and oxygen tension. (ii) The correlations among various microenvironment changes are deficient. The pivotal microenvironmental factors affecting cartilage regeneration are needed to disclose. In particular, the previous appeared microenvironment change affects the subsequent microenvironment change, so as to reveal the influence of the control for previous appeared microenvironment change on the subsequent microenvironment change during cartilage regeneration. For example, whether the newly formed osteophyte is resulted from the hyperoxia, overload and vascularization microenvironments in damaged cartilage for these microenvironments profit osteogenic but not chondrocyte differentiation of MSCs? Furthermore, whether revision of hyperoxia, overload, and vascularization microenvironments could inhibit the osteophyte formation?
Abbreviations
Author contributions
Danyang Yue, Lin Du, Bingbing Zhang, Huan Wu, and Qiong Yang help collecting reference papers and write draft. Min Wang helps collecting information on clinics and discussing on cartilage regeneration. Jun Pan contributes and summarizes the ideas and writes the manuscript.
Acknowledgments
This study was supported by grants from NSF of China (No. 31470902; 11272366; 10972243), the Fundamental Research Funds for the Central Universities of China (2020CDCGSW051, 2018CDPTCG0001/46, 106112015CDJZR238803), Innovation Team in University of Chongqing Municipal Government (CXTDX201601002) and the Chongqing Graduate Student Research Innovation Project (CYS19055).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–44. doi:https://doi.org/10.1038/nrrheum.2016.148.
- Jayasuriya CT, Hu N, Li J, Lemme N, Terek R, Ehrlich MG, Chen Q. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci Rep. 2018;8(1):7044. doi:https://doi.org/10.1038/s41598-018-25395-8.
- Pucci M, and Lauriola M. Chapter 18 - Resistance to EGFR targeting treatments in colorectal cancer Oncogenomics 1 . In: Dammacco F, and Silvestris F, editors. Oncogenomics: Franco Dammacco and Franco Silvestris, 281 ; 2019.
- Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, Luc-Harkey BA, Ulici V, Marshall SW, Jordan JM, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–97. doi:https://doi.org/10.1002/jor.23534.
- Ma CH, Wu CH, Jou IM, Tu YK, Hung CH, Hsieh PL, Tsai KL. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018;14:72–81. doi:https://doi.org/10.1016/j.redox.2017.08.011.
- Ojanen SP, Maj F, Reunamo AE, Ronkainen AP, Mikkonen S, Herzog W, Saarakkala S, Korhonen RK. Site-specific glycosaminoglycan content is better maintained in the pericellular matrix than the extracellular matrix in early post-traumatic osteoarthritis. PLoS One. 2018;13(4):e0196203. doi:https://doi.org/10.1371/journal.pone.0196203.
- Ojanen SP, Maj F, JTA M, Saarela K, Happonen E, Herzog W, Saarakkala S, Korhonen RK. Anterior cruciate ligament transection of rabbits alters composition, structure and biomechanics of articular cartilage and chondrocyte deformation 2 weeks post-surgery in a site-specific manner. J Biomech. 2020;98:109450. doi:https://doi.org/10.1016/j.jbiomech.2019.109450.
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–45. doi:https://doi.org/10.1172/JCI119316.
- Almonte-Becerril M, Costell M, Kouri JB. Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. J Orthop Res. 2014;32(9):3366–74. doi:https://doi.org/10.1002/jor.22649.
- Mäkelä JT, Rezaeian ZS, Mikkonen S, Madden R, Han SK, Jurvelin JS, Herzog W, Korhonen RK. Site-dependent changes in structure and function of lapine articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2014;22(6):869–78. doi:https://doi.org/10.1016/j.joca.2014.04.010.
- Muraoka T, Hagino H, Okano T, Enokida M, Teshima R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of Guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007;56(10):3366–74. doi:https://doi.org/10.1002/art.22921.
- Danalache M, Kleinert R, Schneider J, Al E, Schwitalle M, Riester R, Traub F, Hofmann UK. Changes in stiffness and biochemical composition of the pericellular matrix as a function of spatial chondrocyte organisation in osteoarthritic cartilage. Osteoarthritis Cartilage. 2019;27(5):823–32. doi:https://doi.org/10.1016/j.joca.2019.01.008.
- Foldager CB, Toh WS, Gomoll AH, Olsen BR, Spector M. Distribution of basement membrane molecules, laminin and collagen type iv, in normal and degenerated cartilage tissues. Cartilage. 2014;5(2):123–32. doi:https://doi.org/10.1177/1947603513518217.
- Khoshgoftar M, Torzilli PA, Maher SA. Influence of the pericellular and extracellular matrix structural properties on chondrocyte mechanics. J Orthop Res. 2018;36:721–29.
- Dourado GS, Adams ME, Matyas JR, Huang D. Expression of biglycan, decorin and fibromodulin in the hypertrophic phase of experimental osteoarthritis. Osteoarthritis Cartilage. 1996;4(3):187–96. doi:https://doi.org/10.1016/S1063-4584(96)80015-X.
- Diao HJ, Fung HS, Yeung P, Lam KL, Yan CH, Chan BP. Dynamic cyclic compression modulates the chondrogenic phenotype in human chondrocytes from late stage osteoarthritis. Biochem Biophys Res Commun. 2017;486(1):14–21. doi:https://doi.org/10.1016/j.bbrc.2017.02.073.
- Wang C, Brisson BK, Terajima M, Li Q, Hoxha K, Han B, Goldberg AM, Sherry Liu X, Marcolongo MS, Enomoto-Iwamoto M, et al. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. 2020;85-86:47–67. doi:https://doi.org/10.1016/j.matbio.2019.10.001.
- Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther. 2017;19(1):89. doi:https://doi.org/10.1186/s13075-017-1301-5.
- Sadatsuki R, Kaneko H, Kinoshita M, Futami I, Nonaka R, Culley KL, Otero M, Hada S, Goldring MB, Yamada Y, et al. Perlecan is required for the chondrogenic differentiation of synovial mesenchymal cells through regulation of Sox9 gene expression. J Orthop Res. 2017;35(4):837–46. doi:https://doi.org/10.1002/jor.23318.
- Schminke B, Frese J, Bode C, Goldring MB, Miosge N. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am J Pathol. 2016;186(2):410–18. doi:https://doi.org/10.1016/j.ajpath.2015.10.014.
- Larson CM, Kelley SS, Blackwood AD, Banes AJ, Lee GM. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002;21(4):349–59. doi:https://doi.org/10.1016/S0945-053X(02)00026-4.
- Li Q, Han B, Wang C, Tong W, Wei Y, Tseng WJ, Han LH, Liu XS, Enomoto-Iwamoto M, Mauck RL, et al. Decorin mediates cartilage matrix degeneration and fibrillation in post-traumatic osteoarthritis. Arthritis Rheumatol. 2020;72(8):1266–77. doi:https://doi.org/10.1002/art.41254.
- Han B, Li Q, Wang C, Patel P, Adams SM, Doyran B, Nia HT, Oftadeh R, Zhou S, Li CY, et al. Decorin regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix. ACS Nano. 2019;13(10):11320–33. doi:https://doi.org/10.1021/acsnano.9b04477.
- Mäkelä JT, Han SK, Herzog W, Korhonen RK. Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J Biomech. 2015;48(12):3369–76. doi:https://doi.org/10.1016/j.jbiomech.2015.06.010.
- Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage. 2013;21(12):1895–903. doi:https://doi.org/10.1016/j.joca.2013.08.026.
- Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38(3):509–17. doi:https://doi.org/10.1016/j.jbiomech.2004.04.012.
- Felka T, Rothdiener M, Bast S, Uynuk-Ool T, Zouhair S, Ochs BG, De Zwart P, Stoeckle U, Aicher WK, Hart ML, et al. Loss of spatial organization and destruction of the pericellular matrix in early osteoarthritis in vivo and in a novel in vitro methodology. Osteoarthritis Cartilage. 2016;24(7):1200–09. doi:https://doi.org/10.1016/j.joca.2016.02.001.
- Silverstein AM, Stefani RM, Sobczak E, Tong EL, Attur MG, Shah RP, Bulinski JC, Ateshian GA, Hung CT. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage. 2017;25(8):1353–61. doi:https://doi.org/10.1016/j.joca.2017.03.015.
- Pérez-García S, Carrión M, Gutiérrez-Cañas I, Villanueva-Romero R, Castro D, Martínez C, González-Álvaro I, Blanco FJ, Juarranz Y, and Gomariz RP. Profile of matrix-remodeling proteinases in osteoarthritis: impact of fibronectin. Cells 9(1) . 2019;40.
- Nakamura S, Ikebuchi M, Saeki S, Furukawa D, Orita K, Niimi N, Tsukahara Y, Nakamura H. Changes in viscoelastic properties of articular cartilage in early stage of osteoarthritis, as determined by optical coherence tomography-based strain rate tomography. BMC Musculoskelet Disord. 2019;20(1):417. doi:https://doi.org/10.1186/s12891-019-2789-4.
- Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage. 2005;13(11):958–63. doi:https://doi.org/10.1016/j.joca.2005.06.008.
- Cooke ME, Lawless BM, Jones SW, Grover LM. Matrix degradation in osteoarthritis primes the superficial region of cartilage for mechanical damage. Acta Biomater. 2018;78:320–28. doi:https://doi.org/10.1016/j.actbio.2018.07.037.
- Owusu-Akyaw KA, Heckelman LN, Cutcliffe HC, Sutter EG, Englander ZA, Spritzer CE, Garrett WE, DeFrate LE. A comparison of patellofemoral cartilage morphology and deformation in anterior cruciate ligament deficient versus uninjured knees. J Biomech. 2018;67:78–83. doi:https://doi.org/10.1016/j.jbiomech.2017.11.019.
- Iijima H, Aoyama T, Tajino J, Ito A, Nagai M, Yamaguchi S, Zhang X, Kiyan W, Kuroki H. Subchondral plate porosity colocalizes with the point of mechanical load during ambulation in a rat knee model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2016;24(2):354–63. doi:https://doi.org/10.1016/j.joca.2015.09.001.
- Day JS, Ding M, Van Der Linden JC, Hvid I, Sumner DR, Weinans H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res. 2001;19(5):914–18. doi:https://doi.org/10.1016/S0736-0266(01)00012-2.
- Shaktivesh MF, Lee PVS, Lee PVS. Shock absorbing ability in healthy and damaged cartilage-bone under high-rate compression. J Mech Behav Biomed Mater. 2019;90:388–94. doi:https://doi.org/10.1016/j.jmbbm.2018.10.023.
- Robinson DL, Kersh ME, Walsh NC, Ackland DC, de Steiger RN, Pandy MG. Mechanical properties of normal and osteoarthritic human articular cartilage. J Mech Behav Biomed Mater. 2016;61:96–109. doi:https://doi.org/10.1016/j.jmbbm.2016.01.015.
- Huang Z, Zhou M, Wang Q, Zhu M, Chen S, Li H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch Oral Biol. 2017;84:125–32. doi:https://doi.org/10.1016/j.archoralbio.2017.09.021.
- Zhu G, Qian Y, Wu W, Li R. Negative effects of high mechanical tensile strain stimulation on chondrocyte injury in vitro. Biochem Biophys Res Commun. 2019;510(1):48–52. doi:https://doi.org/10.1016/j.bbrc.2019.01.002.
- Kim JH, Lee G, Won Y, Lee M, Kwak JS, Chun CH, Chun JS. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc Natl Acad Sci U S A. 2015;112(30):9424–29. doi:https://doi.org/10.1073/pnas.1505700112.
- Yue D, Zhang M, Lu J, Zhou J, Bai Y, and Pan J. The rate of fluid shear stress is a potent regulator for the differentiation of mesenchymal stem cells. J Cell Physiol 2019;234(9):1–8.
- Lu J, Fan Y, Gong X, Zhou X, Yi C, Zhang Y, Pan J. The lineage specification of mesenchymal stem cells is directed by the rate of fluid shear Stress. J Cell Physiol. 2016;231(8):1752–60. doi:https://doi.org/10.1002/jcp.25278.
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi:https://doi.org/10.1016/j.cell.2006.06.044.
- Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010;24(1):39–46. doi:https://doi.org/10.1016/j.berh.2009.08.008.
- Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50(12):3915–24. doi:https://doi.org/10.1002/art.20675.
- Gibson JS, Milner PI, White R, Fairfax TPA, Wilkins RJ. Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch. 2008;455(4):563–73. doi:https://doi.org/10.1007/s00424-007-0310-7.
- Girish P, Brian J, Johannes Z, Denitsa D, and Peter A. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. INT J MOL SCI 20 3 . 2019;484.
- Pattappa G, Johnstone B, Zellner J, Docheva D, and Angele P. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int J Mol Sci 20 3 . 2019;484.
- Pan J, Wang B, Li W, Zhou X, Scherr T, Yang Y, Price C, Wang L. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51(2):212–17. doi:https://doi.org/10.1016/j.bone.2011.11.030.
- Milner PI, Smith HC, Robinson R, Wilkins RJ, Gibson JS. Growth factor regulation of intracellular pH homeostasis under hypoxic conditions in isolated equine articular chondrocytes. J Orthop Res. 2013;31(2):197–203. doi:https://doi.org/10.1002/jor.22221.
- Mennan C, Garcia J, McCarthy H, Owen S, Perry J, Wright K, Banerjee R, Richardson JB, Roberts S. Human articular chondrocytes retain their phenotype in sustained hypoxia while normoxia promotes their immunomodulatory potential. Cartilage. 2019;10(4):467–79. doi:https://doi.org/10.1177/1947603518769714.
- Tilwani RK, Vessillier S, Pingguan-Murphy B, Lee DA, Bader DL, Chowdhury TT. Oxygen tension modulates the effects of TNFα in compressed chondrocytes. Inflamm Res. 2017;66(1):49–58. doi:https://doi.org/10.1007/s00011-016-0991-5.
- Hashimoto K, Fukuda K, Yamazaki K, Yamamoto N, Matsushita T, Hayakawa S, Munakata H, Hamanishi C. Hypoxia-induced hyaluronan synthesis by articular chondrocytes: the role of nitric oxide. Inflamm Res. 2006;55(2):72–77. doi:https://doi.org/10.1007/s00011-005-0012-6.
- Fermor B, Gurumurthy A, Diekman BO. Hypoxia, RONS and energy metabolism in articular cartilage. Osteoarthritis Cartilage. 2010;18(9):1167–73. doi:https://doi.org/10.1016/j.joca.2010.06.004.
- Collins JA, Moots RJ, Clegg PD, Milner PI. Resveratrol and N-acetylcysteine influence redox balance in equine articular chondrocytes under acidic and very low oxygen conditions. Free Radic Biol Med. 2015;86:57–64. doi:https://doi.org/10.1016/j.freeradbiomed.2015.05.008.
- Collins JA, Moots RJ, Winstanley R, Clegg PD, Milner PI. Oxygen and pH-sensitivity of human osteoarthritic chondrocytes in 3-D alginate bead culture system. Osteoarthritis Cartilage. 2013;21(11):1790–98. doi:https://doi.org/10.1016/j.joca.2013.06.028.
- Pattappa G, Schewior R, Hofmeister I, Seja J, Zellner J, Johnstone B, Docheva D, and Angele P. Physioxia has a beneficial effect on cartilage matrix production in interleukin-1 beta-inhibited mesenchymal stem cell chondrogenesis. Cells 8 8 . 2019;936.
- Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111(38):13954–59. doi:https://doi.org/10.1073/pnas.1410977111.
- Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3(2):9. doi:https://doi.org/10.1186/scrt100.
- Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17(10):1368–76. doi:https://doi.org/10.1016/j.joca.2009.04.023.
- Anderson DE, Markway BD, Bond D, McCarthy HE, Johnstone B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res Ther. 2016;7(1):154. doi:https://doi.org/10.1186/s13287-016-0419-8.
- Nakazawa KR, Walter BA, Laudier DM, Krishnamoorthy D, Mosley GE, Spiller KL, Iatridis JC. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18(2):343–56. doi:https://doi.org/10.1016/j.spinee.2017.09.018.
- Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825–34. doi:https://doi.org/10.1016/j.joca.2015.08.015.
- Kozijn AE, Tartjiono MT, Ravipati S, Van Der Ham F, Barrett DA, Mastbergen SC, Korthagen NM, Fpjg L, Zuurmond AM, Bobeldijk I, et al. Human C-reactive protein aggravates osteoarthritis development in mice on a high-fat diet. Osteoarthritis Cartilage. 2019;27(1):118–28. doi:https://doi.org/10.1016/j.joca.2018.09.007.
- Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi:https://doi.org/10.1016/j.cytogfr.2018.10.002.
- Wang Y, Xu J, Zhang X, Wang C, Huang Y, Dai K, Zhang X. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8(3):e2715. doi:https://doi.org/10.1038/cddis.2017.129.
- Martin G, Andriamanalijaona R, Grässel S, Dreier R, Mathy-Hartert M, Bogdanowicz P, Boumédiene K, Henrotin Y, Bruckner P, Pujol JP. Effect of hypoxia and reoxygenation on gene expression and response to interleukin-1 in cultured articular chondrocytes. Arthritis Rheum. 2004;50(11):3549–60. doi:https://doi.org/10.1002/art.20596.
- Maneiro E, López-Armada MJ, De Andres MC, Caramés B, Martín MA, Bonilla A, Del Hoyo P, Galdo F, Arenas J, Blanco FJ. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005;64(3):388–95. doi:https://doi.org/10.1136/ard.2004.022152.
- Tomita M, Sato EF, Nishikawa M, Yamano Y, Inoue M. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis Rheum. 2001;44:96–104.
- Clérigues V, Guillén MI, Castejón MA, Gomar F, Mirabet V, Alcaraz MJ. Heme oxygenase-1 mediates protective effects on inflammatory, catabolic and senescence responses induced by interleukin-1β in osteoarthritic osteoblasts. Biochem Pharmacol. 2012;83(3):395–405. doi:https://doi.org/10.1016/j.bcp.2011.11.024.
- Son YO, Kim HE, Choi WS, Chun CH, Chun JS. RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat Commun. 2019;10(1):77. doi:https://doi.org/10.1038/s41467-018-08035-7.
- Guan PP, Ding WY, Wang P. The roles of prostaglandin F2 in regulating the expression of matrix metalloproteinase-12 via an insulin growth factor-2-dependent mechanism in sheared chondrocytes. Signal Transduct Target Ther. 2018;3(1):27. doi:https://doi.org/10.1038/s41392-018-0029-2.
- Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1(1):33–47. doi:https://doi.org/10.1007/s13238-010-0002-5.
- Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. doi:https://doi.org/10.1016/j.lfs.2019.116786.
- Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, Ranera B, Zaragoza P, Martín-Burriel I, Rodellar C. Inflammation affects the viability and plasticity of equine mesenchymal stem cells: possible implications in intra-articular treatments. J Vet Sci. 2017;18(1):39–49. doi:https://doi.org/10.4142/jvs.2017.18.1.39.
- Zayed MN, Schumacher J, Misk N, Dhar MS. Effects of pro-inflammatory cytokines on chondrogenesis of equine mesenchymal stromal cells derived from bone marrow or synovial fluid. Vet J. 2016;217:26–32. doi:https://doi.org/10.1016/j.tvjl.2016.05.014.
- Vézina Audette R, Lavoie-Lamoureux A, Lavoie JP, Laverty S. Inflammatory stimuli differentially modulate the transcription of paracrine signaling molecules of equine bone marrow multipotent mesenchymal stromal cells. Osteoarthritis Cartilage. 2013;21(8):1116–24. doi:https://doi.org/10.1016/j.joca.2013.05.004.
- Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM, Lee MJ, Jang IH, Kwon SM, Lee JS, Kim JH. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832(12):2136–44. doi:https://doi.org/10.1016/j.bbadis.2013.08.002.
- Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi:https://doi.org/10.1038/s41581-018-0023-5.
- Reesink HL, Sutton RM, Shurer CR, Peterson RP, Tan JS, Su J, Paszek MJ, Nixon AJ. Galectin-1 and galectin-3 expression in equine mesenchymal stromal cells (MSCs), synovial fibroblasts and chondrocytes, and the effect of inflammation on MSC motility. Stem Cell Res Ther. 2017;8(1):243. doi:https://doi.org/10.1186/s13287-017-0691-2.
- Van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JA, Bernsen MR, Van Osch GJ. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20(10):1186–96. doi:https://doi.org/10.1016/j.joca.2012.06.003.
- Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi:https://doi.org/10.1016/j.biomaterials.2019.02.006.
- Vallés G, Bensiamar F, Maestro-Paramio L, García-Rey E, Vilaboa N, Saldaña L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):57. doi:https://doi.org/10.1186/s13287-020-1578-1.
- Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, Ranera B, Zaragoza P, Martín-Burriel I, Rodellar C. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet Immunol Immunopathol. 2016;171:57–65. doi:https://doi.org/10.1016/j.vetimm.2016.02.007.
- Barrachina L, Cequier A, Romero A, Vitoria A, Zaragoza P, Vázquez FJ, Rodellar C. Allo-antibody production after intraarticular administration of mesenchymal stem cells (MSCs) in an equine osteoarthritis model: effect of repeated administration, MSC inflammatory stimulation, and equine leukocyte antigen (ELA) compatibility. Stem Cell Res Ther. 2020;11(1):52. doi:https://doi.org/10.1186/s13287-020-1571-8.
- Bundgaard L, Stensballe A, Elbæk KJ, Berg LC. Mass spectrometric analysis of the in vitro secretome from equine bone marrow-derived mesenchymal stromal cells to assess the effect of chondrogenic differentiation on response to interleukin-1β treatment. Stem Cell Res Ther. 2020;11(1):187. doi:https://doi.org/10.1186/s13287-020-01706-7.
- Zhang C, Feinberg D, Alharbi M, Ding Z, Lu C, Jp O, Graves DT. Chondrocytes Promote Vascularization in Fracture Healing Through a FOXO1-Dependent Mechanism. J Bone Miner Res. 2019;34(3):547–56. doi:https://doi.org/10.1002/jbmr.3610.
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–98.
- del Rey MJ, Izquierdo E, Caja S, Usategui A, Santiago B, Galindo M, Pablos JL. Human inflammatory synovial fibroblasts induce enhanced myeloid cell recruitment and angiogenesis through a hypoxia-inducible transcription factor 1alpha/vascular endothelial growth factor-mediated pathway in immunodeficient mice. Arthritis Rheum. 2009;60(10):2926–34. doi:https://doi.org/10.1002/art.24844.
- Ghimire K, Altmann HM, Straub AC, Isenberg JS. Nitric oxide: what’s new to NO? Am J Physiol Cell Physiol. 2017;312(3):C254–c62. doi:https://doi.org/10.1152/ajpcell.00315.2016.
- Majima M, Amano H, Hayashi I. [Endogenous prostaglandins and angiogenesis]. Nihon Yakurigaku Zasshi. 2001;117(4):283–92. doi:https://doi.org/10.1254/fpj.117.283.
- Aida Y, Maeno M, Suzuki N, Namba A, Motohashi M, Matsumoto M, Makimura M, Matsumura H. The effect of IL-1beta on the expression of inflammatory cytokines and their receptors in human chondrocytes. Life Sci. 2006;79(8):764–71. doi:https://doi.org/10.1016/j.lfs.2006.02.038.
- Hügle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatol. 2017;56:1461–71.
- Lane LB, Bullough PG. Age-related changes in the thickness of the calcified zone and the number of tidemarks in adult human articular cartilage. J Bone Joint Surg Br. 1980;63(3):2700–10. doi:https://doi.org/10.1302/0301-620X.62B3.7410471.
- Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203(2):191–202. doi:https://doi.org/10.1046/j.1469-7580.2003.00193.x.
- Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665–73. doi:https://doi.org/10.1038/nrrheum.2012.130.
- Zuo Q, Lu S, Du Z, Friis T, Yao J, Crawford R, Prasadam I, Xiao Y. Characterization of nano-structural and nano-mechanical properties of osteoarthritic subchondral bone. BMC Musculoskelet Disord. 2016;17(1):367. doi:https://doi.org/10.1186/s12891-016-1226-1.
- Rabelo GD, Vom Scheidt A, Klebig F, Hemmatian H, Citak M, Amling M, Busse B, Jähn K. Multiscale bone quality analysis in osteoarthritic knee joints reveal a role of the mechanosensory osteocyte network in osteophytes. Sci Rep. 2020;10(1):673. doi:https://doi.org/10.1038/s41598-019-57303-z.
- Burnett WD, Kontulainen SA, McLennan CE, Hazel D, Talmo C, Wilson DR, Hunter DJ, Johnston JD. Knee osteoarthritis patients with more subchondral cysts have altered tibial subchondral bone mineral density. BMC Musculoskelet Disord. 2019;20(1):14. doi:https://doi.org/10.1186/s12891-018-2388-9.
- Waldstein W, Kasparek MF, Faschingbauer M, Windhager R, Boettner F. Lateral-compartment Osteophytes are not Associated With Lateral-compartment Cartilage Degeneration in Arthritic Varus Knees. Clin Orthop Relat Res. 2017;475(5):1386–92. doi:https://doi.org/10.1007/s11999-016-5155-y.
- Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. doi:https://doi.org/10.22203/eCM.v023a02.
- Liao C, Zhang C, Jin L, Yang Y. IL-17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol. 2020;235(5):4466–80. doi:https://doi.org/10.1002/jcp.29323.
- Yu D, Xu J, Liu F, Wang X, Mao Y, Zhu Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin Exp Clin Exp Rheumatol. 2016;34:929–34.
- Bellido M, Lugo L, Roman-Blas JA, Castañeda S, Caeiro JR, Dapia S, Calvo E, Largo R, Herrero-Beaumont G. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010;12(4):R152. doi:https://doi.org/10.1186/ar3103.
- Chu L, Liu X, He Z, Han X, Yan M, Qu X, Li X, Yu Z. Articular cartilage degradation and aberrant subchondral bone remodeling in patients with osteoarthritis and osteoporosis. J Bone Miner Res. 2020;35(3):505–15. doi:https://doi.org/10.1002/jbmr.3909.
- Čamernik K, Mihelič A, Mihalič R, Marolt Presen D, Janež A, Trebše R, Marc J, Zupan J. Increased exhaustion of the subchondral bone-derived mesenchymal stem/ stromal cells in primary versus dysplastic osteoarthritis. Stem Cell Rev Rep. 2020;16(4):742–54. doi:https://doi.org/10.1007/s12015-020-09964-x.
- Prasadam I, Farnaghi S, Feng JQ, Gu W, Perry S, Crawford R, Xiao Y. Impact of extracellular matrix derived from osteoarthritis subchondral bone osteoblasts on osteocytes: role of integrinβ1 and focal adhesion kinase signaling cues. Arthritis Res Ther. 2013;15(5):R150. doi:https://doi.org/10.1186/ar4333.
- Lu J, Zhang H, Cai D, Zeng C, Lai P, Shao Y, Fang H, Li D, Ouyang J, Zhao C, et al. Positive-feedback regulation of subchondral h-type vessel formation by chondrocyte promotes osteoarthritis development in mice. J Bone Miner Res. 2018;33(5):909–20. doi:https://doi.org/10.1002/jbmr.3388.
- Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Fransès RE, Mapp PI, Wilson D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatol. 2010;49(10):1852–61. doi:https://doi.org/10.1093/rheumatology/keq188.
- Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Da W. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–28. doi:https://doi.org/10.1136/ard.2006.063354.
- Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15(7):743–51. doi:https://doi.org/10.1016/j.joca.2007.01.020.
- Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and Its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res. 2016;31(5):911–24. doi:https://doi.org/10.1002/jbmr.2828.
- Guo K, Wang GY, Fan BT, Sah MK, Qu PY, Zhang SY. The effect of vascular endothelial growth factor (VEGF) on mouse condylar articular cartilage cultured in vitro. Int J Clin Exp Pathol. 2018;11:5194–202.
- Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63(9):2700–10. doi:https://doi.org/10.1002/art.30422.
- Marsano A, Medeiros da Cunha CM, Ghanaati S, Gueven S, Centola M, Tsaryk R, Barbeck M, Stuedle C, Barbero A, Helmrich U, et al. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Cells Transl Med. 2016;5(12):1730–38. doi:https://doi.org/10.5966/sctm.2015-0321.
- Senol O, Gundogdu G, Gundogdu K, Miloglu FD. Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol. 2019;38(5):1351–60. doi:https://doi.org/10.1007/s10067-019-04428-1.
- Milošev I, Levašič V, Vidmar J, Kovač S, Trebše R. pH and metal concentration of synovial fluid of osteoarthritic joints and joints with metal replacements. J Biomed Mater Res B Appl Biomater. 2017;105(8):2507–15. doi:https://doi.org/10.1002/jbm.b.33793.
- Roman MD, Fleaca RS, Boicean A, Bratu D, Birlutiu V, Rus LL, Cristian T, Cernusca Mitariu SI. Mitariu Sebastian ioan cernusca assessment of synovial fluid ph in osteoarthritis of the hip and knee. Revista De Chimie. 2017;68(6):1242–44. doi:https://doi.org/10.37358/RC.17.6.5649.
- Fliefel R, Popov C, Troltzsch M, Kuhnisch J, Ehrenfeld M, Otto S. Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. J Craniomaxillofac Surg. 2016;44(6):715–24. doi:https://doi.org/10.1016/j.jcms.2016.03.003.
- Simao AM, Bolean M, Hoylaerts MF, Millan JL, Ciancaglini P. Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles’ biomimetics. Calcif Tissue Int. 2013;93(3):222–32. doi:https://doi.org/10.1007/s00223-013-9745-3.
- Liu W, Wang T, Yang C, Darvell BW, Wu J, Lin K, Chang J, Pan H, Lu WW. Alkaline biodegradable implants for osteoporotic bone defects–importance of microenvironment pH. Osteoporos Int. 2016;27(1):93–104. doi:https://doi.org/10.1007/s00198-015-3217-8.
- Galow AM, Rebl A, Koczan D, Bonk SM, Baumann W, Gimsa J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem Biophys Rep. 2017;10:17–25.
- Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: implications for tissue engineering of bone. J Biomed Mater Res. 2002;60(2):292–99. doi:https://doi.org/10.1002/jbm.10050.
- Lee GH, Hwang JD, Choi JY, Park HJ, Cho JY, Kim KW, Chae HJ, Kim HR. An acidic pH environment increases cell death and pro-inflammatory cytokine release in osteoblasts: the involvement of BAX inhibitor-1. Int J Biochem Cell Biol. 2011;43(9):1305–17. doi:https://doi.org/10.1016/j.biocel.2011.05.004.
- Ulku TK, Kocaoglu B, Gereli A, Uslu S, Nalbantoglu U. Low pH irrigation fluids have positive effect on intra-articular chondral healing. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):936–41. doi:https://doi.org/10.1007/s00167-017-4796-z.
- Ji B, Zhang Z, Guo W, Ma H, Xu B, Mu W, Amat A, Cao L. Isoliquiritigenin blunts osteoarthritis by inhibition of bone resorption and angiogenesis in subchondral bone. Sci Rep. 2018;8(1):1721. doi:https://doi.org/10.1038/s41598-018-19162-y.
- Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, Xie L, Wang L, Bian Q, Qiu T. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75(9):1714–21. doi:https://doi.org/10.1136/annrheumdis-2015-207923.
- Mu W, Xu B, Ma H, Li J, Ji B, Zhang Z, Amat A, Cao L. HalofuginoneFage. Front Pharmacol. 2018;9:269. doi:https://doi.org/10.3389/fphar.2018.00269.
