ABSTRACT
By analysis of lncRNA expression profiles of macrophages in response to Mycobacterium tuberculosis (Mtb) infection, we identified novel highly expressed transcripts, unique in encompassing a protein coding transcript- Cytidine Monophosphate Kinase 2 (CMPK2) and a previously identified lncRNA- Negative Regulator of Interferon Response (NRIR). While these transcripts (TILT1, 2,3 – TLR4 and Infection induced Long Transcript) are induced by virulent Mtb as well as lipopolysaccharide (LPS) early, lack of/delayed expression in non-viable Mtb/BCG infected cells, respectively, suggest an important role in macrophage responses. The elevated expression by 3 hr in response to fast growing bacteria further emphasizes the importance of these RNAs in the macrophage infection response. Overall, we provide evidence for the presence of multiple transcripts that form a part of the early infection response programme of macrophages.
Abbreviations: IFN: Interferon; NRIR: negative regulator of interferon response; CMPK2: cytidine/ uridine monophosphate kinase; LPS: lipopolysaccharide; LAM: Lipoarabinomannan; PIMs: Phosphatidylinositol Mannosides; TILT1, 2,3: TLR4 and Infection induced Long Transcript; TLR4: Toll-like receptor 4; Mtb: Mycobacterium tuberculosis; BCG: Mycobacterium bovis BCG; MDMs: human monocyte derived macrophages
Introduction
Intracellular bacteria encounters diverse host-derived stresses and adapt to a wide ranging yet cell/ tissue-specific niche [Citation1,Citation2]. Modulating the host to subdue these potentially harmful responses is one of the strategies employed by pathogens for long term infection [Citation3,Citation4]. This plasticity requires an enormous degree of flexibility in the pathogen’s ability to not only sense the stimuli but fine tune host transcriptional programmes to suit survival inside the cells [Citation5,Citation6]. It is not surprising that gene expression patterns at the onset of infection, immediate to the initial contact with host cells, often determines the outcome and overall pathogenesis of the organism.
Table 1. List of primers used in the study
Over several years, a detailed coverage of expression has helped to identify the protein coding patterns in diverse pathogens and cells/ intracellular niches in addition to paving the way for deciphering the regulatory circuits of non-protein coding transcripts [Citation7,Citation8]. Recent evidences have delineated a definitive role for the long non-coding RNAs (lncRNA) in orchestrating the immune response by regulating gene expression [Citation9–11]. These RNAs have been implicated in the regulation of several cellular processes, immune cell functions, and response to infections [Citation12–15]. Non-coding response dynamics have helped uncover novel facets of host – pathogen interactions in several bacterial pathogens [Citation16–19]. Not surprisingly, Mtb infection of macrophages induces several of the non-coding transcripts with functional relevance in activating innate immune functions as well as modulation of these responses by the pathogens [Citation20–22]. Clinically relevant lncRNAs have recently been identified as putative biomarkers for TB patients [Citation23]. With phagocytes initiating a strong innate immune response on primary contact with invading pathogens, it is logical to assume that activation of surface TLRs would be one of the initial signals for expression of gene regulatory networks in these cells. Recent advances in high throughput deep sequencing have revealed the identity of novel transcripts that arise either out of novel ORFS, trans-splicing events, long extensions, and degradation products of previously identified transcripts [Citation24–27]. In our attempt to study the role of type I IFN response in macrophages, we discovered that NRIR, previously identified as a lncRNA regulating this pathway [Citation28] was one of the highest expressed non-coding transcript in Mtb infected macrophages. While the origins of these RNAs are not yet clearly defined, detailed evaluation of this genomic locus indicated the presence of multiple transcripts. In line with this, we identified 3 other transcripts that appear to be encompassing NRIR and the neighbouring IFN inducible gene – CMPK2. We demonstrate that these transcripts- TILT 1,2,3 are induced early in response to bacterial infection and is specific to activation of TLR4 by LPS. Expression of TILT is induced significantly in patients with active TB. Early expression following stimulation of macrophages suggests an important role for this transcript in the rapid response program of cells.
Results
The lncRNA NRIR is induced strongly in response to Mtb infection in macrophages
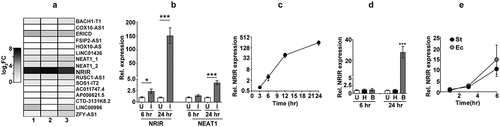
The response of macrophages to mycobacterial infection is characterized by an elaborate expression of genes that include protein-coding and non-coding transcripts. The type I IFN signalling pathway is one of the early and robust response of host macrophage to mycobacterial infection [Citation29,Citation30]. Previous studies have identified the lncRNA NRIR as a primary lncRNA regulating this response in infected cells [Citation28]. We hypothesized that NRIR, given the strong type I IFN signalling induced, would also be a crucial component of the host response to Mtb in macrophages. We also compared the expression profile of another previously identified infection induced lncRNAs-NEAT1 that regulates the inflammatory response of the host [Citation31,Citation32]. In line with our surmise, NRIR showed the highest expression levels in Mtb infected macrophages amongst a few of the well-annotated mammalian lncRNAs ()). While NRIR was expressed 6 hr post-infection with a steady increase in levels at later time points, NEAT1, was more protracted and was expressed at ~3x higher by 24 hr of infection ()). In fact, NRIR showed a steady rise in expression from the ~4x by 6 hr to a sharp increase by 24 hr of infection in excess of 200 times the values in uninfected samples ()). In response to a non-pathogenic vaccine strain, BCG, NRIR was only induced by the later time point (24 hr) in infection, albeit at a lower magnitude than Mtb infection ()). Interestingly, the response was completely abrogated in macrophages exposed to heat killed Mtb even by 24 hr implicating an active infection mediated induction of these non-coding transcripts in macrophages. The kinetic response of NRIR induction observed in Salmonella enterica subspecies enterica serovar Typhimurium (St) and Escherichia coli (Ec) infections further validated the importance of these transcripts in infection response of human macrophage. NRIR was visible by the 3 hr of infection followed by a steady increase of expression by 6 hr of infection in macrophages infected by either strain of bacteria ()).
Figure 1. NRIR is expressed in response to bacterial infection
The NRIR locus supports transcription of several other transcripts
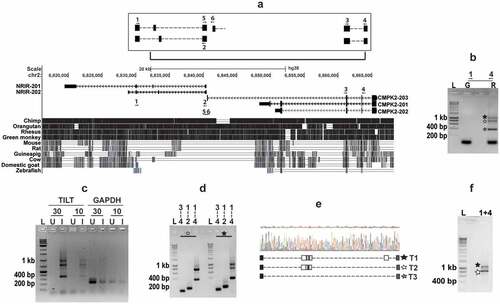
NRIR is localized in the same genomic loci with the mitochondria associated gene – CMPK2 ()). While NRIR has been characterized extensively as a viral infection induced lncRNA, recent analysis have suggested the presence of alternate non-coding transcripts in this locus [Citation28,Citation33,Citation34] ()). Given the induction of NRIR by Mtb infection of macrophages, we sought to evaluate other infection specific transcripts, by using PCR with primers mapping to the NRIR/ CMPK2 specific region (1 and 4). We observed three distinct amplicons of ~400 bp, 800 bp and 1 kb which were absent when genomic DNA was used as a template ()). Expression of these three transcripts was exclusive for infection; with 10 and 30 ng of cDNA from infected macrophages, we could amplify the transcripts as opposed to the complete absence in cDNA from uninfected cells ()). A strong amplification of the NRIR and CMPK2 specific regions verified the identity of the larger 1 kb and 800 bp fragments ()). While the largest 1kb fragment again yielded all 3 fragments, the 800bp fragment produced only the 400bp and 800bp amplicons, on amplification with primers-1 and 4. Sequencing of the amplicons revealed the presence of 3 unique splice variants with fusion of Exon 4 of CMPK2-203 and the first exon of NRIR-202 ()). To check if the transcripts were universally expressed, we analysed expression in total human blood RNA. As seen in THP1 cells, amplicons of ~800 bp and 1 kb in PCR with specific primers 1 and 4 were observed confirming the widespread presence of these transcripts ()).
Figure 2. Identification of the novel transcripts-TILT encompassing CMPK2 and lncRNA – NRIR in Mtb infected macrophages
The novel transcripts are actively induced in response to bacterial infection of macrophages
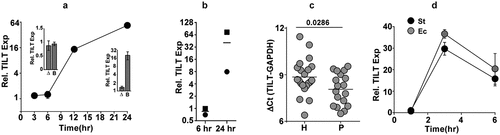
Being a critical component of the host innate defence, macrophages are fine tuned for a rapid response to infections and alter their transcriptional profile to suit the incoming insult. TILT, in contrast to NRIR, was more protracted and reduced in expression. A temporal analysis of Mtb infected macrophages revealed that expression of TILT was seen only by 12 hr of infection (14-fold) with Mtb with a further increase to 50-fold by 24 hr in these macrophages ()). The absence of TILT expression in macrophages infected with heat inactivated Mtb even by 24 hr argued for the importance of active infection in the expression of TILT. Moreover, delayed expression of these RNAs only by 24 hr in response to infection by a non-pathogenic slow growing mycobacterial strain- M. bovis BCG further corroborated the importance in the response to pathogenic infection (inset). Even in primary human macrophages (MDMs) derived from PBMCs, Mtb strongly induced TILT expression (between 7- and 70-fold across the two individuals) late in infection by 24 hr ()). The clinical relevance in infection was highlighted by the significantly enhanced expression in the blood of active TB patients in comparison to healthy individuals ()). A similar profile of enhanced expression of TILT was observed for infection with St and Ec ()), expression levels of TILT showed a strong peak (~25-40-fold) by 3 hr of infection followed by a rapid decline to around 10-fold greater than basal levels by 6 hr of infection associated with the faster growth rate of these bacteria in comparison to slow growing mycobacteria.
Figure 3. TILT is actively induced in Mtb infected macrophages
TLR4 signalling acts as the primary stimulus for TILT expression in macrophages
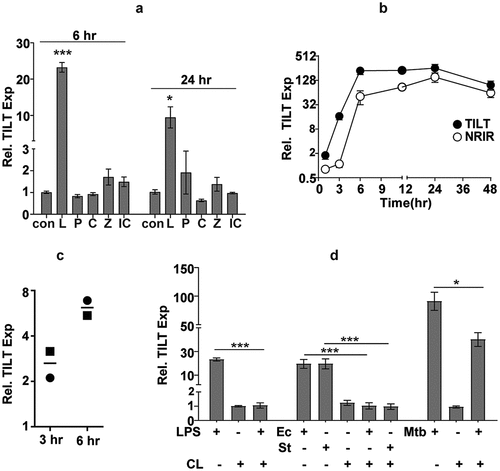
Macrophages, being the primary responders to any infection, are endowed with the capacity to recognize microbial PAMPs via innate receptors like TLRs and activate signalling mechanisms for rapid and robust neutralization of the pathogen. To identify the stimulus involved in activating this lncRNA, we analysed TILT expression in macrophages stimulated with different TLR ligands ()). Where stimulation of most TLRs resulted in minimal induction, only LPS actively enhanced TILT expression in THP1 cells by ~20-fold increase by 6 hr and a sharp decline to ~9-fold by 24 hr. In response to LPS, the two transcripts- NRIR and TILT showed distinct kinetics of expression. Contrasting with the Mtb induced profile of faster NRIR expression, TILT was strongly induced as early as 3 hr (~16-fold) reaching an excess of 200 times the basal levels by the 6th hour of LPS stimulation and stabilizing thereafter to nearly 100-folds by 48 hr. In contrast, NRIR, despite any detectable expression by 3 hr, amplified rapidly to reach ~50 times basal levels with a peak of ~150-fold at 24 hr and then stabilizing to ~60-fold by 48 hr of stimulation ()). As with infection, MDMs from human PBMCs showed a time dependent increase in TILT expression ranging from 2-3-fold by 3 hr and ~6-fold by 6 hr of stimulation with LPS ()). A complete dependence on TLR4 signalling was observed for expression of TILT in macrophages. Treatment with the TLR4 antagonist-CLI095 completely neutralized TILT expression in macrophages in response to LPS, Ec and St. In contrast, the effect of CLI095 was only partial in THP1 infected with Mtb suggesting a TLR4 independent accessory signalling for TILT expression ()).
Figure 4. TILT is induced temporally in response to LPS stimulation in THP1 macrophages
Relative expression at any given time w.r.t unstimulated/ uninfected is depicted as mean ± SEM for triplicate assays of 2–3 independent experiments.
Discussion
Macrophages have been shown to successfully respond to numerous stimuli including infections and initiate response profiles for control of the pathogen [Citation35]. Over several decades of research, the landscape of stimuli driven protein coding expression patterns have been realized in high detail [Citation18]. It is now possible to portray the layout of protein/pathway induction profiles in host macrophages as a response to infection with several bacterial pathogens [Citation36]. The discovery of several non-protein coding transcripts as key regulators has only supplemented another layer to the multi-scale pyramid of the macrophage response kinetics [Citation37–39]. In the last few years, several non-coding transcripts and networks have been identified with important regulatory roles in infection with the help of powerful omics-untargeted/specific-targeted approaches [Citation34,Citation40–43].
By using simple molecular techniques, we have identified novel transcripts (TILT1-3) encompassing a previously identified lncRNA (NRIR) and a mitochondrial resident gene (CMPK2). We demonstrate the presence of multiple transcripts comprising of different exons from the NRIR and CMPK2 genomic locus implying a higher degree of splicing based regulation of gene expression in this region. Our finding of TILT in human blood cells and in monocyte derived macrophages also argues for the presence of these transcripts in myeloid cell populations. Surprisingly, this arrangement of NRIR with CMPK2 is only observed in human cells and is absent from murine cells suggestive of a unique regulatory network to fine tune macrophage response kinetics [Citation44].
Despite a differential amplitude and kinetics of expression of the two transcripts (NRIR and TILT) in response to different stimuli, the rapid and sustained levels of expression over 24–48 hr of stimulus highlights the importance of this locus in the response of macrophages. Increased expression of TILT in sera of active TB patients and in response to infection in THP1 cells/ MDMs only signifies the importance of this response in TB infections. Enhanced expression in response to infection with E. coli and Salmonella further emphasizes the overall significance of these transcripts in the macrophage infection-response program. Given the importance of TLR4 mediated signalling in macrophage responses, it is not surprising that TILT expression is significantly augmented upon LPS stimulation. While TLR4 signalling by LPS is a dominant response in infection with Ec and St, the active TLR4 agonist is not yet defined in the case of Mtb, which primarily lacks LPS. While complex glycolipids of the Mtb cell wall like LAM, its precursor or derivatives or PIMs have been shown to activate TLR4 responses, it is clear that Mtb has evolved the ability to activate multiple layers of macrophage signalling cascades for optimal infection [Citation45,Citation46]. It is well established that macrophage response to Mtb is not primarily dependent on TLR4 signalling and depends on a combinatorial requirement for other TLRs- (TLR2), NLR, RLR mediated signalling [Citation47,Citation48]. In line with these observations, we also observed a partial reduction in TILT expression by inhibition of TLR4 signalling with CLI095 treatment in Mtb infected macrophages.
Given the presence of NRIR transcripts in the same genomic locus, it can be argued that these novel transcripts form a part of this complex. While we did not find a promoter like element different from the annotated promoter of CMPK2 (Data not shown), separate promoter like elements were observed upstream of NRIR indicative of an uncoupled expression of this lncRNA from CMPK2 and TILT. Moreover, the differential expression kinetics of NRIR and TILT argue strongly for the novelty of these transcripts; contrasting with NRIR, TILT expression is earlier with LPS stimulation and more protracted and of a lower magnitude in response to Mtb infection. In fact, similar to the higher induction of TILT in LPS stimulated macrophages, infection with St or Ec, strains that prominently activate TLR4 with their LPS, also induce TILT strongly at an early time point in macrophages. This is in line with reports that suggest transient activation of macrophages by LPS followed by a decline in response kinetics in an effort to control prolonged inflammation in cells [Citation49,Citation50]. Abrogation of TILT expression following treatment with CLI095 in LPS stimulated/ Ec/ St infections only substantiates the absolute requirement of TLR4 mediated signalling for TILT expression in macrophages. Failure of heat killed Mtb or the protracted kinetics with an avirulent vaccine strain- BCG again suggests the importance of these transcripts in macrophage response to active infection.
Several studies have hinted at the differential response of macrophages to BCG and Mtb or to heat killed Mtb in activating immune signalling mechanisms (bioRxiv doi: https://doi.org/10.1101/594655) [Citation51,Citation52]. Type I IFN response is one of the immediate responses that is induced in macrophages infected with Mtb with a similar differential profile in BCG vs Mtb vs heat inactivated Mtb [Citation30]. With both CMPK2 and NRIR, dependent on type I IFN response signalling, one can envisage an important role for this response in the expression of these transcripts as well. Identifying the regulatory circuit and the consequence of this control in macrophage/ immune cell function would provide important insights into the diversity and complexity of mammalian innate responses.
Material and methods
Bacterial strains and growth conditions
Mycobacterium tuberculosis strain Erdman was grown in 7H9 Middlebrook enriched with Middlebrook ADC (BD Biosciences, USA). E. coli and Salmonella enterica subspecies enterica serovar Typhimurium were grown in LB media (BD Biosciences, USA) at 37°C.
Macrophage culture and infection
RPMI 1640-GlutaMAX (Himedia laboratories, Mumbai, India) supplemented with 10% foetal bovine serum (Himedia laboratories, Mumbai, India,) and 1 mM sodium pyruvate (Himedia laboratories, Mumbai, India) was used to culture THP-1 monocytes. Differentiation of THP1 monocytes to macrophages was done using 100 nM phorbol myristate acetate (PMA) (Sigma Aldrich, USA) for 24 hr followed by a rest of 48 hr without PMA prior to infection/ stimulation. For human monocyte derived macrophages (MDMs), 10 ml blood from healthy individuals were collected in EDTA containing tubes and peripheral blood mononuclear cells (PBMCs) were isolated using Hisep (Himedia Laboratories, Mumbai, India) according to manufacturer’s protocol. 3 × 105 cells per well were seeded in a 24 well and stimulated with 50 ng/ml granulocyte-macrophage colony-stimulating factor (Invitrogen Corporation, California, USA) for 7 days. Mtb, E. coli and Salmonella typhimurium were grown to mid-log phase at 37°C, washed twice with phosphate buffered saline (PBS) containing 0.05% Tween80, finally suspended in PBS and centrifuged at 800 rpm for 10 min to get a uniform single cell suspension. This uniform single cell suspension was diluted with complete RPMI 1640 to the required cell density and then used to infect the differentiated THP-1 macrophages and MDMs at multiplicity of infection (MOI) of 5. At different time intervals, cells were harvested in RNAzol (Sigma Aldrich, USA) for analysis. LPS, CLI095, poly I:C, and Pam3CSK2 were purchased from InvivoGen, Toulouse, France. Zymosan (Sigma Aldrich, USA) was used according to the concentration mentioned.
RNA isolation and qRT-PCR
RNA isolation was done as per standard RNAzol protocol. cDNA synthesis was performed from 1 µg RNA using verso cDNA synthesis kit (Thermo Scientific, USA). The expression level was checked by DyNAmo Flash SYBR Green qPCR kit (Thermo Scientific, USA) in Roche LC480 system. GAPDH was used as internal control. Ct values were normalized with uninfected control.
Genome locus and PCR
Genome locus was analysed using UCSC web (https://genome.ucsc.edu/.)
Whole blood collection
Confirmed cases of pulmonary TB patients between ages of 5 and 15 years were included in the study with prior consent as per the Institutional ethical committee guidelines. BCG vaccination, primary or secondary infection and single or co-infection status were recorded. HIV positive and any non-respiratory major disease patients were excluded from the study. The control samples were obtained from individuals without any overt clinical manifestation of disease. 2–3 ml blood was collected in RNAgard blood tubes (Biomatrica, USA) and stored in −80°C until RNA precipitation. RNA was isolated from frozen blood samples as per the manufacturer’s recommendations.
Graphs
Graphs were generated using Graphpad prism or R. Student's t-test was used for statistical analysis of the data.
Author contributions
PA, VR were involved in conceptualizing and design of the work, MS and RL were instrumental in sampling of blood from TB patients, the work was performed by PA and manuscript was written by VR and PA.
Ethics statement
All human samples were used according to approved protocols by the Institutional human ethics committee- CSIR-IGIB/IHEC/2017-18/No.15 and CSIR-IGIB/IHEC/2015-16/No.10.
Acknowledgments
The authors thank CSIR (VR-BSC0123) and STS0016 for funding and facility maintenance and PA- CSIR-BSC0124, CSIR SRF/ RA for fellowship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hardison RL, Heimlich DR, Harrison A, et al. Transient nutrient deprivation promotes macropinocytosis-dependent intracellular bacterial community development. mSphere. 2018;3(5):e00286-18.
- Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12:101–114.
- Passalacqua KD, Charbonneau ME, O’Riordan MXD. Bacterial metabolism shapes the host-pathogen interface. Microbiol Spectr. 2016;4. DOI:10.1128/microbiolspec.VMBF-0027-2015
- Cameron A, Huynh S, Scott NE, et al. High-frequency variation of purine biosynthesis genes is a mechanism of success in campylobacter jejuni. mBio. 2015;6:e00612–15.
- Eisenreich W, Rudel T, Heesemann J, et al. To eat and to be eaten: mutual metabolic adaptations of immune cells and intracellular bacterial pathogens upon infection. Front Cell Infect Microbiol. 2017;7:316.
- Cornejo E, Schlaermann P, Mukherjee S. How to rewire the host cell: a home improvement guide for intracellular bacteria. J Cell Biol. 2017;216:3931–3948.
- Hayward JA, Mathur A, Ngo C, et al. Cytosolic recognition of microbes and pathogens: inflammasomes in action. Microbiol Mol Biol Rev. 2018;82. DOI:10.1128/MMBR.00015-18
- Winchell CG, Steele S, Kawula T, et al. Dining in: intracellular bacterial pathogen interplay with autophagy. Curr Opin Microbiol. 2016;29:9–14.
- Spurlock CF 3rd, Crooke PS 3rd, Aune TM. Biogenesis and transcriptional regulation of long noncoding RNAs in the human immune system. J Immunol. 2016;197:4509–4517.
- Imamura K, Akimitsu N. Long non-coding RNAs involved in immune responses. Front Immunol. 2014;5:573.
- Yu AD, Wang Z, Morris KV. Long noncoding RNAs: a potent source of regulation in immunity and disease. Immunol Cell Biol. 2015;93:277–283.
- Xie Y, Wang M, Tian J, et al. Long non-coding RNA expressed in macrophage co-varies with the inflammatory phenotype during macrophage development and polarization. J Cell Mol Med. 2019;23:6530–6542.
- Robinson EK, Covarrubias S, Carpenter S. The how and why of lncRNA function: an innate immune perspective. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194419.
- Menon MP, Hua KF. The long non-coding RNAs: paramount regulators of the NLRP3 inflammasome. Front Immunol. 2020;11:569524.
- Ouyang J, Zhu X, Chen Y, et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe. 2014;16:616–626.
- Gomez JA, Wapinski OL, Yang YW, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell. 2013;152:743–754.
- Wang Y, Zhong H, Xie X, et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci U S A. 2015;112:E3883–92.
- Roy S, Schmeier S, Kaczkowski B, et al. Transcriptional landscape of Mycobacterium tuberculosis infection in macrophages. Sci Rep. 2018;8:6758.
- Yan H, Xu R, Zhang X, et al. Identifying differentially expressed long non-coding RNAs in PBMCs in response to the infection of multidrug-resistant tuberculosis. Infect Drug Resist. 2018;11:945–959.
- Yang X, Yang J, Wang J, et al. Microarray analysis of long noncoding RNA and mRNA expression profiles in human macrophages infected with Mycobacterium tuberculosis. Sci Rep. 2016;6:38963.
- Huang S, Huang Z, Luo Q, et al. The expression of lncRNA NEAT1 in human tuberculosis and its antituberculosis effect. Biomed Res Int. 2018;2018:9529072.
- Sharbati S, Ravon F, Einspanier R, et al. Mycobacterium smegmatis but not mycobacterium avium subsp. hominissuis causes increased expression of the long non-coding RNA MEG3 in THP-1-derived human macrophages and associated decrease of TGF-beta. Microorganisms. 2019;7. DOI:10.3390/microorganisms7030063
- Chen ZL, Wei LL, Shi LY, et al. Screening and identification of lncRNAs as potential biomarkers for pulmonary tuberculosis. Sci Rep. 2017;7:16751.
- Kalam H, Fontana MF, Kumar D. Alternate splicing of transcripts shape macrophage response to Mycobacterium tuberculosis infection. PLoS Pathog. 2017;13:e1006236.
- Jackson R, Kroehling L, Khitun A, et al. The translation of non-canonical open reading frames controls mucosal immunity. Nature. 2018;564:434–438.
- Agirre X, Meydan C, Jiang Y, et al. Long non-coding RNAs discriminate the stages and gene regulatory states of human humoral immune response. Nat Commun. 2019;10:821.
- Srikumar S, Kroger C, Hebrard M, et al. RNA-seq brings new insights to the intra-macrophage transcriptome of salmonella typhimurium. PLoS Pathog. 2015;11:e1005262.
- Kambara H, Niazi F, Kostadinova L, et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42:10668–10680.
- Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188:6205–6215.
- Novikov A, Cardone M, Thompson R, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–2547.
- Imamura K, Imamachi N, Akizuki G, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–406.
- Ma H, Han P, Ye W, et al. The long noncoding RNA NEAT1 exerts antihantaviral effects by acting as positive feedback for RIG-I signaling. J Virol. 2017;91. DOI:10.1128/JVI.02250-16
- Barriocanal M, Fortes P. Long non-coding RNAs in hepatitis C virus-infected cells. Front Microbiol. 2017;8:1833.
- Lagarde J, Uszczynska-Ratajczak B, Carbonell S, et al. High-throughput annotation of full-length long noncoding RNAs with capture long-read sequencing. Nat Genet. 2017;49:1731–1740.
- Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203.
- Denzer L, Schroten H, Schwerk C. From gene to protein-how bacterial virulence factors manipulate host gene expression during infection. Int J Mol Sci. 2020;21. DOI:10.3390/ijms21103730
- Aune TM, Spurlock CF 3rd. Long non-coding RNAs in innate and adaptive immunity. Virus Res. 2016;212:146–160.
- Duval M, Cossart P, Lebreton A. Mammalian microRNAs and long noncoding RNAs in the host-bacterial pathogen crosstalk. Semin Cell Dev Biol. 2017;65:11–19.
- Ahmad I, Valverde A, Ahmad F, et al. Long noncoding RNA in myeloid and lymphoid cell differentiation, polarization and function. Cells. 2020;9. DOI:10.3390/cells9020269
- Fan H, Lv Z, Gan L, et al. A novel lncRNA regulates the toll-like receptor signaling pathway and related immune function by stabilizing FOS mRNA as a competitive endogenous RNA. Front Immunol. 2019;10:838.
- Menard KL, Haskins BE, Colombo AP, et al. Toxoplasma gondii manipulates expression of host long noncoding RNA during intracellular infection. Sci Rep. 2018;8:15017.
- Moon HG, Yang J, Zheng Y, et al. miR-15a/16 regulates macrophage phagocytosis after bacterial infection. J Immunol. 2014;193:4558–4567.
- Liu W, Huang L, Wei Q, et al. Microarray analysis of long non-coding RNA expression profiles uncovers a Toxoplasma-induced negative regulation of host immune signaling. Parasit Vectors. 2018;11:174.
- Breschi A, Gingeras TR, Guigo R. Comparative transcriptomics in human and mouse. Nat Rev Genet. 2017;18:425–440.
- Queval CJ, Brosch R, Simeone R. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis. Front Microbiol. 2017;8:2284.
- Chai Q, Wang L, Liu CH, et al. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17:901–913.
- Faridgohar M, Nikoueinejad H. New findings of toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog Glob Health. 2017;111:256–264.
- Kleinnijenhuis J, Oosting M, Joosten LA, et al. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310.
- Cheng Z, Taylor B, Ourthiague DR, et al. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci Signal. 2015;8:ra69.
- Perkins DJ, Rajaiah R, Tennant SM, et al. Salmonella typhimurium co-opts the host type I IFN system to restrict macrophage innate immune transcriptional responses selectively. J Immunol. 2015;195:2461–2471.
- Upadhyay S, Mittal E, Philips JA. Tuberculosis and the art of macrophage manipulation. Pathog Dis. 2018;76. DOI:10.1093/femspd/fty037
- Butler RE, Brodin P, Jang J, et al. The balance of apoptotic and necrotic cell death in Mycobacterium tuberculosis infected macrophages is not dependent on bacterial virulence. PLoS One. 2012;7:e47573.
