?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Incorporating antibrowning agents into the edible coating is a conventional processing technique for fresh-cut preparation. The main quality attributes of fresh-cuts, i.e., appearance color, taste and texture may be affected by coating formulation and its additives at different and sometime contradictory rates. In this study, the impacts of using alginate coating alone or in combination with citric acid and/or ascorbic acid were studied on browning index, microbial load, textural properties, and some taste and nutritional parameters of apple fresh-cut. Lowering weight loss, decline in browning index, increasing acidic taste and changes in some textural properties (hardness, chewiness, and springiness) in response to adding citric acid to the coating formula can be attributed to the competition of H+ ions with calcium cations and therefore physical alteration of forming gel features. On the other side, adding ascorbic acid, as an active antioxidant compound, led to elevated antioxidant capacity, lowered browning index, and least rates of electrolyte leakage. Totally, after 7 days of storage at 4°C, lowest values of weight loss (0.33%), microbial load (2.95 log CFU/g), electrolyte leakage (35.5%) and highest rates of lightness (96.11) and antioxidant capacity (11.03 mmol Fe2+/Kg) were detected when the coating formulation was enriched with both citric acid and ascorbic acid compounds.
Introduction
The production and marketing of fresh-cut products have experienced a rapid development over the last years due to the increasing demand for convenient and healthy foods as well as improvements in their preparation technology. The routine process of fresh-cut preparation i.e. peeling, cutting, slicing, chopping and shredding, expose the tissue into water loss, microbial contamination and biochemical reactions which eventually lead to textural deterioration, color change and spoilage of the produce (Velderrain-Rodríguez et al., Citation2015).
In terms of fresh-cut quality, surface color and appearance play a critical role in market acceptability. The widespread color modification happens as a consequence of enzymatic browning of phenolic compounds, when the cell sap exposed to the oxygen. This reaction is catalyzed by polyphenol oxidases (PPOs) and initiated by oxidation of mono-phenols into ο-diphenols and then ο-diphenols into quinones (He and Luo, Citation2007). Currently, apple, pineapple, melon, grape and pear are the most common fruits that are used for fresh-cut production. Among them, the fruit of apple is known as a rich source of polyphenol compounds, which makes it highly susceptible to enzymatic browning. Surface browning as a consequence of polyphenols oxidation has been reported for a wide range of apple cultivars (Burke, Citation2010; Holderbaum et al., Citation2010).
Along with visual appearance and flavor, the other food quality criterion with a great impact on consumer acceptability is the texture. Generally, the texture of a fruit, as a plant organ, relates to the cell structure and condition including primary cell wall, the middle lamella and the degree of cell turgidity created by osmotic pressure (Terefe and Versteeg, Citation2011). In the case of fresh-cuts, deterioration of texture is triggered upon fruit peeling and cutting and exposing the tissue to the water loss. However the textural quality of foods are commonly explained subjectively by the consumers but the most accurate instrumental test for quantification of different food texture identities is the texture profile analysis (TPA). In TPA test, the fruit samples with equal size are compressed twice to simulate chewing mechanism and obtained data are used to calculate hardness, cohesiveness, springiness and so on properties (Di Monaco et al., Citation2008).
Edible coating is a well-established approach to alleviate both physical and biochemical deterioration of fresh-cuts. A thin layer of edible material (proteins, lipids and polysaccharides) acts as a partial barrier to maintain moisture and protect the tissue against oxidative reactions during product-to-consume period (Treviño-Garza et al., Citation2017). Alginate, a polysaccharide-based gelling agent, can be used as an edible coating due to its colloidal properties and forming strong gels in the presence of polyvalent metal ions such as calcium (Featherstone, Citation2015). Edible coatings may potentially serve as carrier for various additives to improve their effectiveness in keeping fresh-cut quality. The positive effects of incorporating different anti-browning agents such as citric and ascorbic acid (Chiabrando and Giacalone, Citation2012), oxalic acid (Suttirak and Manurakchinakorn, Citation2010), L-cysteine and Glutathione (Techavuthiporn and Boonyaritthonghai, Citation2016), 4-hexylresorcinol (Rojas‐Graü et al., Citation2008) and honey (McLellan et al., Citation1995) have been reported previously. Acidic-based compounds such as citric acid and ascorbic acid are the most commercially available compounds to function as anti-browning agents. These compounds commonly act by interfering the activity of phenylalanine ammonia lyase (PAL) and maintain the cut-surface pH to inappropriate level for polyphenol oxidase (PPO) activity (Ibrahim et al., Citation2004).
The objectives of present work were to follow the alteration in surface color, textual properties, microbial load and some quality attributes of fresh-cut Fuji apple slices upon coating with alginate alone or in combination with some antibrowning agents including citric acid and ascorbic acid.
Materials and Methods
Plant Materials
Fresh apple fruits at commercial stage of maturity (Malus domestica Borkh. cv. Fuji) were obtained from a local market (North-Western Azerbaijan, Iran), packed in corrugated boxes and transferred to the lab. The fruits of uniform size and maturity stage, with no physical damage were selected for further processing.
Preparation of Samples and Coating Solutions
All fruits, containers, knives and cutting boards were washed and sanitized by immersion in a 0.2% (w/v) sodium hypochlorite solution for 2 min, rinsed and dried prior to cutting operations. Subsequently, fruits were manually peeled and each apple was divided into eight equal pieces. The prepared slices were collected, and then randomly divided into five groups to receive one of below coating treatments. Three trays, each containing 10 apple slices were considered as replicates.
Control (distilled water without any coating)
Alginate+glycerol+CaCl2 (Alg)
Alginate+glycerol+CaCl2+ citric acid 1% w/v (Alg/CitA)
Alginate+glycerol+CaCl2+ ascorbic acid 0.5% w/v (Alg/AscA)
Alginate+glycerol+CaCl2+ citric acid 1% wv+ ascorbic acid 0.5% w/v (Alg/CitA/AscA)
To make coating solutions, a 2-liter glass beaker was put on a hot plate and filled with distilled water. After heating water to around 70°C, sodium alginate salt (VWR Chemicals, Netherland) was added to prepare 1% w/v solution by continuous stirring until the solution became clear. After cooling down to room temperature, 1.5% v/v glycerol (Aldrich, Germany) was added as a plasticizer. At the same time, five similar containers were prepared, one filled with d-water as control and the rest were used to prepare 2% w/v calcium chloride (Merck, Germany) solution (Ca2+ is used to induce cross-linking of carbohydrate polymers). Ascorbic acid and citric acid (Merck, Germany) were added at 0.5% w/v and 1% w/v concentrations, respectively to make above-mentioned coatings.
To perform coating treatments, among five prepared groups of apple slices, one group was dipped in distilled water (as control) and the rest four groups were immersed separately in the prepared alginate solution for 2 min. After 1 min draining off the residual, samples submerged in dedicated calcium chloride solutions twice to assure forming a layer of gel on the whole fresh-cut surface. After 30 minutes drying out, three perforated Polyethylene Terephthalate (PETE) trays were considered for replicates of each treatment and 10 apple slices were placed in each tray. The treated samples were stored at 4 ± 1°C.
Quality Assessment
Weight Loss (WL)
To determine the effectiveness of alginate coatings as moisture-barriers, the WL of apple slices were calculated by comparing the weights of samples during seven days of storage with their initial weights using a digital balance. The results are presented as percent.
Total Soluble Solid (TSS) and Titratable Acidity (TA)
The flesh of 4–5 slices of each tray was mixed. Three grams of the fruit flesh homogenate were extracted with 30 mL of distilled water using mortar and pestle. The extract was centrifuged at 6000 rpm for 15 min and supernatant was used for TSS and TA analysis. TSS was assessed with a digital hand-held refractometer (Atago, 3810 PAL-a, Japan) and expressed as percent. To measure TA, the extract was titrated against 0.01 N sodium hydroxide solution until pH 8.1 and results were presented as percent equivalents of citric acid (Ebrahimi et al., Citation2019).
Antioxidant Capacity (AC)
One gram of fruit flesh homogenate was put in a falcon tube and 10 mL of 80% v/v methanol was added to it. After shaking vigorously for 2 h, the mixture was centrifuged at 6000 rpm for 15 min and the supernatant was used for AC determination using FRAP assay (Benzie and Strain, Citation1996). The principle of this method is based on the reduction of a ferric-tripyridyltriazine (Fe3+-TPTZ) complex to ferrous (Fe2+) colored form in the presence of antioxidants. Briefly, 80 μL of supernatant was mixed with 3.6 mL FRAP prepared reagent (the 1:1:10 mixture of 20 mM FeCl3, 10 mM TPTZ, and 0.3 M acetate buffer at pH 3.6) and then 400 μL distilled water was added to the mixture. After incubation for 50 min at 37 °C, its absorbance was measured at 593 nm against a FRAP blank using Shimadzu UV-1201 UV-VIS spectrophotometer. Aqueous solutions of known Fe2+ concentrations in the range of 100–1000 μM (FeSO4.7H2O) were used for calibration. Results expressed in mmol Fe2+ equivalents per g sample.
Electrolyte Leakage (EL)
Five flesh discs (0.8 mm diameter, 0.7 mm length) were taken from each sample box and incubated at 23°C in 50 mL glass bottles containing 35 mL deionized water. During incubation, samples were agitated using a shaker at a speed of 200 rpm. Electrical conductivity of the bathing solution was measured after 1 min (E1) and 180 min (E180) of incubation using a conductivity meter (Bante510, China). The samples were then autoclaved at 121°C for 15 min, and total conductivity (ET) of bathing solution was measured after cooling. EL was calculated from the following equation (Fan and Sokorai, Citation2005):
Surface Color
Minolta CR-400 colorimeter was used to determine L*, a*, and b* values. Along with L* component, which represents lightness degree (in the range of 0–100), the values of a* and b* were used to calculate browning index (BI) using following equation (Subhashree et al., Citation2017):
where,
Microbial Load
Three apple slices per experimental unit were mixed under sterile condition. Twenty-five grams of the mixture were diluted with 100 mL sterile D-water, homogenized and filtered to make the stock solution and serial decimal dilutions (10−1-10−4). From prepared solution, 250 µL were poured in 9 cm plates containing Rose-Bengal Chloramphenicol Agar. After incubating the plates at 37°C for 48 h, the number of mold and yeast colonies were counted and presented as Log CFU/g FW.
Texture Profile Analysis (TPA) Test
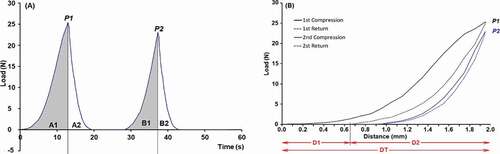
TPA test, known also as two-bite test, was done to mimic the activity of biting food in the mouth. To do that, an Instron Universal Testing Machine (QTS 230) equipped with 100 N load cell and a 75-mm flat plunger was used. For each experimental unit, the apple slices were trimmed with a sharp cutter to make cube samples of 10 × 10 × 10 mm. The cubic apple samples were placed under the plunger and subjected to a double-compression test by a downward-moving probe at the speed of 30 mm.min−1 until 20% deformation of sample. Force-time () and force-distance () graphs for each test point were analyzed with Excel software using CNC Farnell extension. The method of Alvarez et al. (Citation2002) with some modifications was used to calculate number of textual attributes including:
Hardness: the initial deformation force = P1 value.
Chewiness: required energy to chew a solid food =
Gumminess: required energy to disintegrate a semisolid food =
Springiness (Elasticity): the rate at which a deformed food reforms =
Results and Discussion
Weight Loss
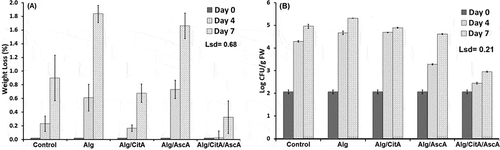
For all treatments, the rate of weight loss increased significantly throughout the storage period. Among coated samples and after 7 days of cold storage, the lowest weight loss was observed in samples which contained citric acid in their coating formulation (0.68 and 0.33% for Alg/CitA and Alg/CtA/AsA treatments, respectively). The other two coating treatments, i.e., Alg and Alg/AsA presented higher weight loss than uncoated samples (Control). At the end of experiment, the apple slices which were coated with calcium alginate alone had the highest weight reduction (1.84%) which were almost two times greater than control samples (0.90%) ().
Figure 2. Weight loss (a) and microbial load (mold/yeast) (b) of apple fresh-cut samples throughout 7 days keeping at 4°C. The means are significantly different at P ≤ 0.05, when their difference is greater than the LSD
The primary mechanism of moisture loss from fresh-cuts is transferring water in the form of vapor from product tissue into the surrounding air (Díaz-Mula et al., Citation2012). A simple physiological behavior of fruits, known as wound healing, explains the lower rate of weight loss of uncoated apple slices rather than some coated samples (Alg and Alg/AsA). Shortly after cutting, a thick exudate appears at the wounded surface which in 24 hours, it forms a layer of sclerified parenchyma that can retard further water loss within cut surface (Walter et al., Citation1990). Alginate gel itself is a hydrocolloid compound which has a poor water vapor barrier property due to its hydrophilic nature. In routine approaches of making alginate gel, the alginate solution is exposed directly to the ionic solution, where Ca2+ reacts irreversibly with carboxylic groups of guluronic acid residues to form a less dense gel matrix with large pore sizes. Therefore, Alg alone cannot perform very well as weight loss barrier with high water-activity foods. Incorporating other compounds into the alginate-based film can improve its barrier properties for water vapor. The significant effect of adding citric acid to the alginate (Alg/CtA) in lowering weight loss is attributed to the role of pH in gel-forming quality. Adding citric acid as an H+ releasing agent to the ionic solution, can lead to less strength but more homogeneous matrix due to the displacement of Ca2+ by H+ (Parreidt et al., Citation2018). The immediate covering of the cut surface with the more homogenous coating can be considered as the main reason of lowering weight loss in Alg/CitA and Alg/CitA/AscA treatments.
Decay
As shown in , in all applied treatments, yeast and mold contamination of apple fresh-cuts increased gradually within storage time from the initial value of 2.06 log CFU/g. After 7 days storing at 4°C, maximum microbial load was detected in apple slices which were coated with alginate alone (5.31 log CFU/g). However there were no significant differences between the control and Alg treatments. In contrast, the lowest contamination rate was observed in Alg/CitA/AscA coating treatment (2.95 log CFU/g). Comparing the results of citric acid and ascorbic acid additives, revealed that however their separate use could reduce the decay incidence but the more effective control of microbial load progress was observed when the coating treatment included both of them.
The physicochemical properties of fresh-cut i.e. juices rich in nutrients and high water content make them a potentially suitable place for growth of different microorganisms. Due to consumer concerns, one of the main expected function of fresh-cut coatings is its antimicrobial property. Based on our data, incorporating citric acid and ascorbic acid into alginate-based coating could successfully lower the yeast and mold contamination. Addition of organic acids to foods as a preservative approach has been known for years, since survival and growth of most food microorganisms can be adversely affected by the concentration of hydrogen ions (pH) (Raybaudi‐Massilia et al., Citation2009). In addition, the pH can impact on gel viscosity and the more uniform coatings on the fresh-cut sample can induce an internal-modified atmosphere which interfere with pathogens growth and consequently product spoilage (Olivas and Barbosa-Cánovas, Citation2005). Moreover, the highest rate of microbial contamination in samples which were coated with alginate alone (Alg), can be attributed to its gelling property which usually contain sufficient nitrogenous compounds and salts. The growth of bacteria or molds on the alginate-based coating may cause depolymerization as well as contamination and spoiling of any product in which the alginate is used (McHugh, Citation1987). Lee et al. (Citation2003) tested various types of carbohydrate polymers for fresh-cut apple coating and reported that there were many situations where coatings actually increased the incidence of decay.
Surface Color
Keeping the surface color against enzymatic browning is critical in fresh-cut quality preservation. In this experiment, the surface color was evaluated by measuring degree of lightness (L) and browning index (BI). The pattern of changes in L and BI are presented in , respectively. The degree of L decreased in almost all treatments but at different rates. The highest rate of decline in L was recorded for control samples which were not received any coating. During the first three days of storage at 4°C, L parameter reduced sharply from 97.24 to 87.61 and then continuously lowered to 86.70 to the end of test. This can be attributed to the breaking the cell walls after cutting the tissue, liberating of polyphenol oxidases (PPOs) and eventually tissue browning as a result of direct contact of PPO’s and their substrates with the air (Garcia and Barrett, Citation2002). There were no significant differences between Alg, Alg/CitA and Alg/AscA treatments in terms of L parameter. It means that any coating can alleviate flesh browning by limiting the exposure to oxygen of the air. The best results and highest L values at all analysis intervals were recorded for the samples which were coated with Alg/CitA/AscA, in a way that after 7 days of storage, L decreased by around 1% only. Citric acid and ascorbic acid are both known as anti-browning agents that act with different mechanisms against browning. Ascorbic acid is reported to reduce formed brown quinones to the original colorless di-phenols. It also mentioned as a compound that negatively interferes the function of PPO’s in the oxidation of polyphenol compounds (Ali et al., Citation2015). On the other side, acidic compounds such as citric acid can lower the activity of PPO’s either by reducing the pH or chelating the copper at the enzyme active site (Sedaghat and Zahedi, Citation2012). The synergistic effect of these anti-browning agents was seen when the alginate coating was incorporated with 1% citric acid and 0.5% ascorbic acid.
Figure 3. Lightness (a) and browning index (b) of apple fresh-cut samples throughout 7 days keeping at 4°C. The means are significantly different at P ≤ 0.05, when their difference is greater than the LSD
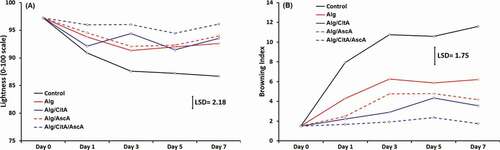
The browning index changes were in reverse of L changes and increased gradually for all treatments. The significant anti-browning effect of Alg/CitA/AscA treatment is clearly showed in when BI increased from the initial value of 1.51 to 1.75 after 7 days of storage. Within the same time, the BI of control samples (with no coating) increased to 11.58 which was almost twice of Alg treatment. This data reveals that even in Alg treatment, where no anti-browning agent was present in the coating formula, the ability of the coating to work as a barrier to oxygen, could effectively lessen the rate of browning. Some other reports attribute this effect to the presence of chloride ion in alginate coating which interacts with copper at the PPO active site (Garcia and Barrett, Citation2002).
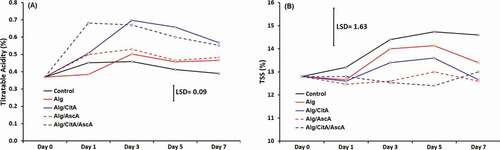
Titratable Acidity (TA) and TSS
Changes in apple slices TA based on malic acid are presented in . For those treatments which citric acid was part of their coating formula (Alg/CitA and Alg/CitA/AscA), TA experienced a sharp increase during days 1 to 3 after treatment, then stayed almost constant within the rest of storage time. This pattern reveals the role of adding 1% citric acid to the coating formula in elevation of TA from the initial value of 0.37% to around 0.70% in mentioned treatments. This finding was in agreement with Amiri et al. (Citation2017), where incorporating citric acid to the coating of apple fresh-cut led to higher level of TA. However, in this research, the increase in TA in Alg/CitA and Alg/CitA/AscA treatments is likely attributed to acidification of product rather than internal changes of apple slices but in some reports it is claimed that increase in sample acidity could be associated either to the deceleration of the respiration rate or to the presence of the citric acid that contributes to preserve the fruit freshness (Cofelice et al., Citation2019). In other treatments (Control, Alg and Alg/AscA), acidity followed a steady and non-significant alterations within storage time. Acidity is a key eating quality criteria of fruit fresh-cuts which plays its role in relation to sugar content. The ratio of TSS/TA is used as a quality index for some horticultural products (Medina-Torres et al., Citation2004). As presented in , changes in TSS values were significant only for control samples (1.8% in 7 days), while the variation of TSS for other treatments were ranged between 0 and 0.8%. Edible coating, as a semi-permeable film around the fresh-cut, can modify the internal atmosphere of fruit and induce slower degradation of polymers to simple sugars by diminishing flesh respiration and metabolic activities (Olivas and Barbosa-Cánovas, Citation2005). The effect of coating on keeping fruit TSS changes at a negligible rate is considered positive because of keeping the natural fruit sweetness. TSS content is mostly determined by water-soluble sugar compounds. The increase in TSS of control samples might be possible due to metabolic activity and conversion of complex compound such as starch or carbohydrates into simple sugars (Islam et al., Citation2013).
Antioxidant Capacity (AC)
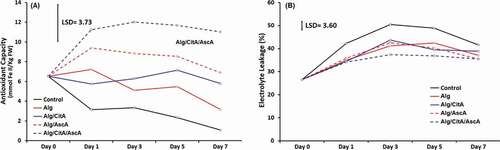
Antioxidant capacity represents the cumulative ability of all antioxidant compounds present in a fruit to scavenge free radicals. The trend of AC changes of apple fresh-cuts based on reducing of Fe3+ to Fe2+ were distinct for each coating treatment (). The samples which didn’t receive any coating had a downward trend of AC during storage time from 6.52 to 1.08 mmol Fe2+/Kg FW. Applying Alg and Alg/CitA coating treatments had no significant effects on AC of fresh-cut apple slices. Compared to control, the considerable increase in AC was detected in Alg/Asc and Alg/CitA/AscA coating treatments which both had ascorbic acid as one of their components. Ascorbic acid is considered itself as an active antioxidant compound and similar findings on the increasing effect of adding AscA to the coating formula on the level of product antioxidants have been reported for fresh-cut mangoes (Robles-Sánchez et al., Citation2013) and fresh-cut kiwifruit (Antunes et al., Citation2010).
Electrolyte Leakage (EL)
EL refers to the function of membrane permeability and releasing new electrolytes (mostly K+) out of the plant cell. As demonstrated in , EL of all coating treatments significantly increased in the course of storage. The increasing rate was sharp within first three days and then stayed constant or reduced for the rest of analysis time. The highest leakage rate values were found in control (apples with no coating) samples and EL increased from the initial value of 26.52% to 50.50% at day three. The rapid rising of EL in fresh-cuts is known as a wound stress and a physiological response to the physical damage of cell membranes (Iakimova and Woltering, Citation2018). Alleviating effect of applying all coating treatments on EL can be explained by the positive role of putting a protective layer on the cut surface on lowering oxidative reactions. Similar results were obtained by Jiang (Citation2013) who found that membrane permeability of alginate coated mushrooms was much lower than uncoated samples. Among all applied treatments, those fruits which received Alg/CitA/AscA treatment, had lowest EL values at all analysis intervals. It seems that the antioxidant property of AscA was effective in lowering processing stress and eventually leaking ions out of cell membrane.
Textural Analysis
Among applied treatments, the fresh-cuts which were coated with alginate alone had the highest hardness rates in almost all analysis intervals (). Maximum hardness value of this treatment was 28.71 N at day 3 which was dropped gradually to 25.63 N at day 7 of storage. For Alg/CitA and Alg/CitA/AscA treatments, hardness index didn’t change significantly and followed a constant rate. The decreasing trend of hardness was detected only in control (with no coating) samples in a way that reached to 15.36 N at the end of experiment. Lack of coating in control samples could be mentioned as the main cause of quick sample deformation and losing their hardness. Moreover, calcium ion, as a coating ingredient, could make a chemical bond with cell wall pectin and increase the stability of sample’s texture (Cybulska et al., Citation2012). Gumminess, the other textural feature which is described as the required energy to disintegrate food sample before swallowing, decreased in control treatment from 1.13 to 0.61 within 7 days of storage. The fluctuations of gumminess for coated treatments did not follow a certain pattern and were not significant at P < .05.
Table 1. The results of texture profile analysis (TPA) on hardness, chewiness, gumminess and springiness of fresh-cut apple samples throughout storage at 4°C. If the difference between two means is greater than the LSD, then those are significantly different at P ≤ 0.05
The data pertaining to chewiness showed that the only decreasing pattern belonged to control treatment. In Alg/CitA and Alg/CitA/AscA treatments, chewiness quantities were consistent and in Alg/AscA and Alg coatings, a sharp increase was observed during storage period (). Chewiness refers to the required energy (of chewing) to reduce the sample to grind a solid product to a state ready for swallowing, therefore, the lower chewiness value, the easer swallowing of the product (Bourne, Citation2002). On the other hand the mouthfeel depends highly on product tissue chewiness. Lowest recorded chewiness values for control samples can be attributed to the rapid deterioration of un-coated samples because of their direct contact to air, losing water, high oxidative reactions and stimulating senescence process. On the other side, high chewiness of samples that were coated with Alg alone or Alg/AscA can be related to the lack of H+ source (citric acid) in the coating formula (earlier discussed) and consequently forming a more rigid gel around the tissue. Springiness, the textural property related to sample elasticity, is calculated based on to the recovering food height during the end of first bite and the start of the second bite (Chandra and Shamasundar, Citation2014). Changes of springiness were almost similar to chewiness and coatings which didn’t contain citric acid in their formula (Alg and Alg/AscA) had increasing alterations, and other treatments stayed steady in terms of springiness.
Conclusions
Considering the results of this study, it can be concluded that microbial load, eating quality and textural properties of apple fresh-cuts were considerably affected by applying alginate-based coating. Alginate-based coating with no additives induced water loss and microbial contamination and adding both citric acid and ascorbic acid compounds led to more uniform covering of cut surfaces and consequently slowing down of weight loss and microbial contamination. In terms of surface color quality, best results were observed in samples which were exposed to Alg/CitA/AscA treatment, and in contrast lowest lightning and highest browning rate were found in the control samples which didn’t receive any coating. Adding CitA and AscA couldn’t change total soluble solids considerably but the acidic effect of CitA resulted to higher titratable acidity of samples. Moreover, adding AscA to the alginate-based coating let to higher antioxidant activity of apple fresh-cuts. Electrolyte leakage, as an index of stress, was alleviated under applying all coatings especially when it was enriched with both CitA and AscA. Textural profile analysis of samples demonstrated that adding CitA and AscA to the alginate-based gel could effectively preserve hardness index and chewiness for longer time. Lowest hardness and gumminess in control samples are attributed to rapid deterioration of un-coated fruit tissue. In the absence of citric acid (the source of H+) in the coating formula, springiness and chewiness followed higher rates as a consequence of forming more rigid gels. Since, different and sometimes contradictory results obtained for changes of eating quality parameters, appearance acceptance, and textural properties in response to additives to the coating, more studies are needed to investigate the single and combined effects of different additives on biochemistry and biophysics of fresh-cut quality.
Acknowledgments
This work was supported by a research grant from Shahid Chamran University of Ahvaz. The authors would like to thank Dr Mehrabi Koushki for his technical support to do the microbial test and all lab technicians who provided us the equipment and materials for fruit quality analysis.
References
- Ali, H.M., A.M. El-Gizawy, R.E. El-Bassiouny, and M.A. Saleh. 2015. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. Int. J. Food Sci. Technol. 52(6):3651–3659. doi: https://doi.org/10.1007/s13197-014-1437-0.
- Alvarez, M., W. Canet, and M. López. 2002. Influence of deformation rate and degree of compression on textural parameters of potato and apple tissues in texture profile analysis. Eur. Food Res. Technol. 215(1):13–20. doi: https://doi.org/10.1007/s00217-002-0515-0.
- Amiri, S., H. Akhvan, N. Zare, and M. Radi. 2017. Effect of gelatin-based edible coatings incorporated with aloe vera and green tea extracts on the shelf-life of fresh-cut apple. Ital. J. Food Sci. 30(1):1–11. doi: https://doi.org/10.14674/1120-1770-IJFS699.
- Antunes, M.D., S. Dandlen, A.M. Cavaco, and G. Miguel. 2010. Effects of postharvest application of 1-MCP and postcutting dip treatment on the quality and nutritional properties of fresh-cut kiwifruit. J. Agric. Food Chem. 58(10):6173–6181. doi: https://doi.org/10.1021/jf904540m.
- Benzie, I.F., and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239(1):70–76. doi: https://doi.org/10.1006/abio.1996.0292.
- Bourne, M.C. 2002. Food texture and viscosity (Second Edition), concept and measurement. Food science and technology. University Geneva, New York, USA. doi: https://doi.org/10.1016/B978-012119062-0/50001-2.
- Burke, A. 2010. Quantifying flesh browning, polyphenoloxidase, total phenolic content and vitamin C in select apple varieties and progeny. Cornell University, Ithaca, New York, Dissertation type. 133 p.
- Chandra, M.V., and B.A. Shamasundar. 2014. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 18(3):572–584. doi: https://doi.org/10.1080/10942912.2013.845787.
- Chiabrando, V., and G. Giacalone. 2012. Effect of antibrowning agents on color and related enzymes in fresh‐cut apples during cold storage. J. Food Process. Pres. 36(2):133–140. doi: https://doi.org/10.1111/j.1745-4549.2011.00561.x.
- Cofelice, M., F. Lopez, and F. Cuomo. 2019. Quality Control of Fresh-Cut Apples after Coating Application. Foods 8(6):189. doi: https://doi.org/10.3390/foods8060189.
- Cybulska, J., P.M. Pieczywek, and A. Zdunek. 2012. The effect of Ca 2+ and cellular structure on apple firmness and acoustic emission. Eur. Food Res. Technol. 235(1):119–128. doi: https://doi.org/10.1007/s00217-012-1743-6.
- Di Monaco, R., S. Cavella, and P. Masi. 2008. Predicting sensory cohesiveness, hardness and springiness of solid foods from instrumental measurements. J. Texture Stud. 39(2):129–149. doi: https://doi.org/10.1111/j.1745-4603.2008.00134.x.
- Díaz-Mula, H.M., M. Serrano, and D. Valero. 2012. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Tech. 5(8):2990–2997. doi: https://doi.org/10.1007/s11947-011-0599-2.
- Ebrahimi, H., S.M.H. Mortazavi, M.E. Khorasani Ferdavani, and M. Mehrabi Koushki. 2019. The impact of two‐sided ultraviolet radiation and long‐term freezing on quality of date fruit at rutab stage. J. Food Process. Pres. 43(10):1–10. doi: https://doi.org/10.1111/jfpp.14128.
- Fan, X., and K.J. Sokorai. 2005. Assessment of radiation sensitivity of fresh-cut vegetables using electrolyte leakage measurement. Postharvest Biol. Tec. 36(2):191–197. doi: https://doi.org/10.1016/j.postharvbio.2004.12.004.
- Featherstone, S. 2015. Ingredients used in the preparation of canned foods, p. 147–211. In: FeatherstoneS. (ed.). A complete course in canning and related processes: Volume 2: Microbiology, Packaging, HACCP and Ingredients. Elsevier: Amsterdam, The Netherlands, 147–21. doi: https://doi.org/10.1016/B978-0-85709-678-4.00008-7
- Garcia, E., and D.M. Barrett. 2002. Preservative treatments for fresh-cut fruits and vegetables, p. 267–304. In: LamikanraO. (ed.). Fresh-cut fruits and vegetables: Science, technology, and market. CRC Press. Boca Raton, Florida, USA. doi: https://doi.org/10.1201/9781420031874.ch9
- He, Q., and Y. Luo. 2007. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 3(6):1–7. doi: https://doi.org/10.2212/spr.2007.6:3.
- Holderbaum, D., T. Kon, T. Kudo, and M. Guerra. 2010. Enzymatic browning, polyphenol oxidase activity, and polyphenols in four apple cultivars: Dynamics during fruit development. HortScience 45(8):1150–1154. doi: https://doi.org/10.21273/HORTSCI.45.8.1150.
- Iakimova, E.T., and E.J. Woltering. 2018. The wound response in fresh-cut lettuce involves programmed cell death events. Protoplasma 255(4):1225–1238. doi: https://doi.org/10.1007/s00709-018-1228-y.
- Ibrahim, R., A. Osman, N. Saari, and R.A. Rahman. 2004. Effects of anti-browning treatments on the storage quality of minimally processed shredded cabbage. J. Food Agric. Environ. 2(2):54–58.
- Islam, M., M.Z.H. Khan, M.A.R. Sarkar, N. Absar, and S.K. Sarkar. 2013. Changes in acidity, TSS, and sugar content at different storage periods of the postharvest mango (Mangifera indica L.) influenced by Bavistin DF. Int. J. Food Sci. 1–8. doi: https://doi.org/10.1155/2013/939385.
- Jiang, T. 2013. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol. Tec. 76:91–97. doi: https://doi.org/10.1016/j.postharvbio.2012.09.005.
- Lee, J.Y., H.J. Park, C.Y. Lee, and W.Y. Choi. 2003. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT 36(3):323–329. doi: https://doi.org/10.1016/S0023-6438(03)00014-8.
- McHugh, D.J. 1987. Production, properties and uses of alginates, p. 58–115. In: D.J. McHugh (ed.). Production and utilization of products from commercial seaweeds. FAO Fisheries Technical Paper. Food and Drug Administration of the United Nations. Campbell, Australia, 288, 58–115.
- McLellan, M.R., R.W. Kime, C.Y. Lee, and T.M. Long. 1995. Effect of honey as an antibrowning agent in light raisin processing. J. Food Process. Pres. 19(1):1–8. doi: https://doi.org/10.1111/j.1745-4549.1995.tb00273.x.
- Medina-Torres, R., S. Salazar-García, and J.R. Gómez-Aguilar. 2004. Fruit quality indices in eight nance (Byrsonima crassifolia L.) selections. HortScience 39(5):1070–1073. doi: https://doi.org/10.21273/HORTSCI.39.5.1070.
- Olivas, G.I., and G.V. Barbosa-Cánovas. 2005. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 45(7–8):657–670. doi: https://doi.org/10.1080/10408690490911837.
- Parreidt, T., K. Müller, and M. Schmid. 2018. Alginate-based edible films and coatings for food packaging applications. Foods 7(10):1–38. doi: https://doi.org/10.3390/foods7100170.
- Raybaudi‐Massilia, R.M., J. Mosqueda‐Melgar, R. Soliva‐Fortuny, and O. Martín‐Belloso. 2009. Control of pathogenic and spoilage microorganisms in fresh‐cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 8(3):157–180. doi: https://doi.org/10.1111/j.1541-4337.2009.00076.x.
- Robles-Sánchez, R.M., M.A. Rojas-Graü, I. Odriozola-Serrano, G. González-Aguilar, and O. Martin-Belloso. 2013. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT 50(1):240–246. doi: https://doi.org/10.1016/j.lwt.2012.05.021.
- Rojas‐Graü, M.A., R. Soliva‐Fortuny, and O. Martín‐Belloso. 2008. Effect of natural antibrowning agents on color and related enzymes in fresh‐cut fuji apples as an alternative to the use of ascorbic acid. J. Food Sci. 73(6):267–272. doi: https://doi.org/10.1111/j.1750-3841.2008.00794.x.
- Sedaghat, N., and Y. Zahedi. 2012. Application of edible coating and acidic washing for extending the storage life of mushrooms (Agaricus bisporus). Food Sci. Technol. Int. 18(6):523–530. doi: https://doi.org/10.1177/1082013211433075.
- Subhashree, S.N., S. Sunoj, J. Xue, and G.C. Bora. 2017. Quantification of browning in apples using colour and textural features by image analysis. Food Qual. Saf. 1(3):221–226. doi: https://doi.org/10.1093/fqsafe/fyx021.
- Suttirak, W., and S. Manurakchinakorn. 2010. Potential application of ascorbic acid, citric acid and oxalic acid for browning inhibition in fresh-cut fruits and vegetables. WJST 7(1):5–14. doi: https://doi.org/10.2004/wjst.v7i1.47
- Techavuthiporn, C., and P. Boonyaritthonghai. 2016. Effects of anti-browning agents on wound responses of fresh-cut mangoes. Int. Food Res. J. 23(5):1879–1886.
- Terefe, N.S., and C. Versteeg. 2011. Texture and microstructure of fruits and vegetables, p. 89–115. In: R.M.S. Cruz (ed.). Practical food and research, (part I), Food science and technology series. Nova Science Publishers, New York, United State.
- Treviño-Garza, M.Z., S. García, N. Heredia, M.G. Alanís-Guzmán, and K. Arévalo-Niño. 2017. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Tec. 128:63–75. doi: https://doi.org/10.1016/j.postharvbio.2017.01.007.
- Velderrain-Rodríguez, G.R., A.E. Quirós-Sauceda, G.A. González Aguilar, M.W. Siddiqui, and J.F. Ayala Zavala. 2015. Technologies in Fresh-Cut Fruit and Vegetables, p. 79–103. In: M. Siddiqui and M. Rahman (eds.). Minimally processed foods. Food engineering series. Springer, Cham. doi: https://doi.org/10.1007/978-3-319-10677-9_5.
- Walter, W.M., B. Randall-Schadel, and W.E. Schadel. 1990. Wound healing in cucumber fruit. J Am. Soc. Hortic. Sci. 115(3):444–452.