ABSTRACT
Macroautophagy/autophagy mediates the degradation of ubiquitinated aggregated proteins within lysosomes in a process known as aggrephagy. The cargo receptor SQSTM1/p62 condenses aggregated proteins into larger structures and links them to the nascent autophagosomal membrane (phagophore). How the condensation reaction and autophagosome formation are coupled is unclear. We recently discovered that a region of SQSTM1 containing its LIR motif directly interacts with RB1CC1/FIP200, a protein acting at early stages of autophagosome formation. Determination of the structure of the C-terminal region of RB1CC1 revealed a claw-shaped domain. Using a structure-function approach, we show that the interaction of SQSTM1 with the RB1CC1 claw domain is crucial for the productive recruitment of the autophagy machinery to ubiquitin-positive condensates and their subsequent degradation by autophagy. We also found that concentrated Atg8-family proteins on the phagophore displace RB1CC1 from SQSTM1, suggesting an intrinsic directionality in the process of autophagosome formation. Ultimately, our study reveals how the interplay of SQSTM1 and RB1CC1 couples cargo condensation to autophagosome formation.
Macroautophagy (hereafter autophagy) contributes to cellular homeostasis by removing harmful material from the cytoplasm. This is achieved through the encapsulation of this material, also referred to as cargo, within double-membrane vesicles named autophagosomes. The selectivity of autophagy towards specific cargoes is mediated by cargo receptors which couple the cargo to the nascent autophagosomal membrane, also known as the phagophore. SQSTM1 is a cargo receptor mediating the selective degradation of misfolded and aggregated proteins in a process known as aggrephagy. Among the multiple cellular functions of SQSTM1 are the phase separation of ubiquitinated proteins into larger condensates and the tethering of these condensates to the phagophore membrane by binding to membrane-attached Atg8-family proteins. However, the molecular mechanism of how cargo condensation and autophagosome formation are coupled has remained unclear.
Figure 1. Model for cargo-induced autophagosome formation. The cargo receptor SQSTM1 directly binds to RB1CC1 and recruits it to the cargo. RB1CC1, a component of the ULK1 complex, is necessary for the recruitment of the ATG12–ATG5-ATG16L1 complex, which in turn supports the conjugation of Atg8-family proteins to the growing phagophore. Concentrated Atg8-family proteins on the phagophore bind to the SQSTM1 LIR motif, thereby displacing RB1CC1 from the receptor and confining it to regions of the cargo where the phagophore has not yet formed.
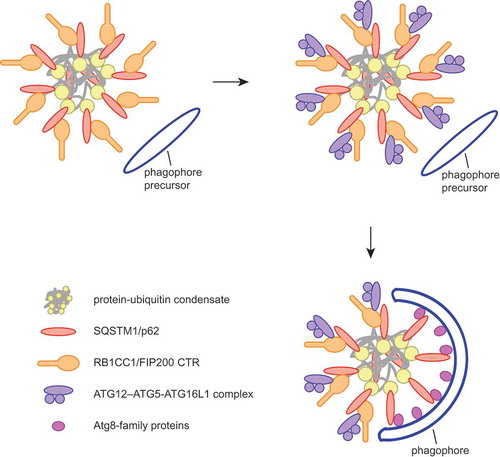
We have recently found that SQSTM1 directly interacts with the C-terminal region (CTR) of RB1CC1, which is a subunit of the ULK1 kinase complex. Because the ULK1 kinase complex acts at the early stages of autophagosome nucleation, we hypothesized that the SQSTM1-RB1CC1 interaction could promote autophagosome formation in the vicinity of the ubiquitin-positive condensates by recruiting and activating the upstream-acting autophagy machinery ().
Notably, the RB1CC1 CTR shows a high degree of sequence similarity with S. cerevisiae Atg11, a subunit of the Atg1 kinase complex, which is the yeast counterpart of the ULK1 complex. Indeed, this region of Atg11 binds to the Atg19 cargo receptor. In SQSTM1, the binding to RB1CC1 is mediated by a central region we named RB1CC1/FIP200-interacting region. The RB1CC1/FIP200-interacting region contains an LC3-interacting region (LIR), and we found that the SQSTM1 LIR is necessary and sufficient for RB1CC1 CTR binding, albeit it bound with lower affinity than the entire RB1CC1/FIP200-interacting region. Furthermore, we showed that the affinity of the RB1CC1 CTR for SQSTM1 is increased by phosphorylation of 4 serines within the SQSTM1 RB1CC1/FIP200-interacting region. Three of these serines are located in a short region showing sequence similarity with the yeast Atg19 cargo receptor, and phosphorylation of Atg19 in this region was previously shown to promote its binding to Atg11. This suggests an evolutionarily conserved mode of interaction between the SQSTM1 and Atg19 cargo receptors with RB1CC1 and Atg11, respectively.
To obtain further insights into the SQSTM1-RB1CC1 interaction, we determined the crystal structure of the RB1CC1 CTR, revealing a parallel dimer composed of an N-terminal helix and a C-terminal globular domain. The dimerization is mediated by both the helix and the globular domain. Closer inspection of the globular domain revealed a shape reminiscent of a claw, therefore we named this region the claw domain. A hydrophobic pocket in the claw, surrounded by positively charged amino acids, was a candidate for the LIR motif-binding site. In agreement with this, a higher resolution structure of the isolated claw shows sulfate ions coordinated to amino acids in proximity to the hydrophobic pocket, perhaps mimicking phosphorylated serine residues within the SQSTM1 RB1CC1/FIP200-interacting region. Mutational analysis of the RB1CC1 claw confirms that the hydrophobic pocket and some of the surrounding positively charged amino acids are indeed required for the interaction with SQSTM1 both in vitro, using recombinant proteins, as well as in cell lysates.
To test the relevance of the SQSTM1-RB1CC1 interaction for the autophagic degradation of SQSTM1-ubiquitin condensates, we deleted the claw domain of the endogenous RB1CC1 in cultured cells using CRISPR-Cas9. The truncated protein is less efficiently recruited to the condensates, resulting in their accumulation in the cytoplasm. Consistent with decreased autophagosome formation at the condensates, GABARAP is less efficiently recruited to ubiquitin puncta in cells when the RB1CC1 claw domain is deleted. Interestingly, we found that, in cells where RB1CC1 has been knocked down, the recruitment of the ULK1 kinase to the condensates is not significantly impaired. In contrast, the recruitment of ATG16L1, a component of the ATG12–ATG5-ATG16L1 complex involved in the conjugation of Atg8-family proteins to the phagophore membrane, is completely abolished upon loss of RB1CC1.
Our biochemical experiments described above showed that the interaction of SQSTM1 and RB1CC1 depends on the SQSTM1 LIR motif. The LIR motif also binds Atg8-family proteins including LC3B and GABARAP, suggesting that the interaction of SQSTM1 with RB1CC1 and Atg8-family proteins might be mutually exclusive. This in turn implies that during phagophore elongation these proteins may displace RB1CC1 from the SQSTM1-ubiquitin condensates upon their conjugation to the phagophore. In line with this hypothesis, we found that in an in vitro reconstituted system increasing concentrations of LC3B gradually displace RB1CC1 CTR from SQSTM1. In a cellular context, RB1CC1 is largely absent from SQSTM1-containing mature autophagosomes that accumulate in bafilomycin A1-treated cells. In contrast, RB1CC1 accumulates at SQSTM1-ubiquitin condensates upon deletion of Atg7, which is required for the lipidation of Atg8-family proteins. Taken together, these experiments suggest an inbuilt directionality in the autophagosome formation process, where the lipidation of Atg8-family proteins leads to the displacement of the upstream-acting autophagy machinery from the cargo, confining it to regions of the cargo where the phagophore has not yet formed ().
In summary, by revealing a direct interaction between SQSTM1 and RB1CC1, our results provide mechanistic insights into how the recognition of the cargo is coupled to the induction of autophagosome formation in mammalian cells.
Disclosure statement
J.H.H. is a co-founder of Casma Therapeutics. S.M. is member of the scientific advisory board of Casma Therapeutics
