ABSTRACT
Macroautophagy/autophagy is a conserved degradative pathway that host cells use to deal with invading pathogens. Despite significant overlap with starvation-induced autophagy, the early signaling that potentiates anti-bacterial autophagy is still unclear. Here we report AMPK, an upstream kinase regulating starvation-mediated autophagy induction, is activated in response to bacterial infection. AMPK inhibits MTORC1, an autophagy repressor, and activates autophagic ULK1 and PIK3C3/VPS34 complexes. Although AMPK-mediated inhibition of MTORC1 is not accompanied by the induction of bulk autophagy, AMPK regulation is critical for selectively targeting the bacteria for degradation. Moreover, AMPK signaling is triggered by the detection of bacteria-derived outer membrane vesicles and does not require bacterial invasion. Together, these data characterize and highlight the significance of AMPK signaling in priming the autophagic response to bacterial infection.
Abbreviations
AMPK: AMP-activated protein kinase; MTORC1: MTOR complex 1; ULK1: Unc-51 like kinase 1; PIK3C3/VPS34: Phosphatidylinositol 3-kinase catalytic subunit type 3.
As part of the innate immune response macroautophagy (hereafter referred to as autophagy) is capable of targeting internalized pathogenic bacteria for autophagic degradation. Several recent studies have begun to reveal extensive crosstalk between the pathways regulating starvation- and pathogen-induced autophagy. The reason for this close relationship may be tied to damage caused to the plasma membrane by bacterial invasion, which may result in nutrient leakage and activation of the starvation response. Nutrient deprivation is capable of inhibiting MTOR complex 1 (MTORC1) activity, a key repressor of mammalian autophagy. In concert, reductions in cellular energy result in activation of AMP-activated protein kinase (AMPK), which has been described to initiate autophagy through inhibition of MTORC1 as well as the direct activation of autophagy enzymes. In addition to crosstalk with starvation-induced autophagy, specific pathways have been described to promote anti-bacterial autophagy (hereafter referred to as xenophagy). These include mechanisms to recognize bacteria-containing vacuoles and pathogen-induced membrane damage. However, it remains unclear if mammalian cells have mechanisms to deal with pathogenic bacteria that do not require membrane damage or loss of nutrients.
Previous literature has shown that autophagy is activated via AMPK signalling and the downregulation of MTORC1 target phosphorylation in response to nutrient withdrawal. However, in response to bacterial stress the regulation of MTORC1 may be system dependent. Indeed, treatment of bone marrow-derived macrophages with Salmonella enterica serovar Typhimurium (hereafter referred to as Salmonella) results in activation of MTORC1, whereas Salmonella stress results in a potent downregulation of MTORC1 in all other cell types tested. Conversely, AMPK signaling is increased in all cell types, indicating that AMPK may be an important part of the anti-bacterial response [Citation1].
AMPK is also activated in response to other Gram-negative pathogenic bacteria including Shigella flexneri or adherent invasive E. coli. When mouse embryonic fibroblasts (MEF) cells lacking the 2 catalytic isoforms of AMPK (PRKAA1/α1 and PRKAA2/α2) are infected, the MTORC1 activity is not significantly repressed, indicating that AMPK activity contributes to the inhibition of MTORC1 in response to Salmonella. Purification of the MTORC1 kinase complex following infection reveals a potent upregulation in AMPK-mediated phosphorylation of the RPTOR/raptor subunit on serine 792, which inhibits MTORC1 activity. Together, these data show a role for AMPK signaling in the inactivation of MTORC1, a potent repressor of autophagy initiation, in response to Gram-negative bacteria.
Salmonella treatment also results in AMPK-mediated phosphorylation of the autophagy kinase complexes ULK1 (unc-51 like kinase 1) and PIK3C3/VPS34 (phosphatidylinositol 3-kinase catalytic subunit type 3). AMPK-meditated phosphorylation of ULK1 and the BECN1/beclin-1 subunit of the PIK3C3/VPS34 complex are sufficient to induce kinase activity. Indeed, in vitro kinase assays show that both the ULK1 and PIK3C3/VPS34 kinase complexes have increased activity upon infection with Salmonella. More importantly, AMPK (prkaa1/α1 and prkaa2/α2) double-knockout cells have impaired xenophagy as determined by increased intracellular Salmonella at 4 h post infection and reduced SQSTM1/p62- and LC3B-positive bacteria by immunofluorescence, showing an important contribution for AMPK in xenophagy.
Several aspects of the Salmonella life cycle, including host cell membrane damage or intracellular detection, have been linked to autophagy induction. However, mutants of Salmonella that are defective for internalization or virulence also activate AMPK, indicating this pathway is distinct from known pathways leading to xenophagy induction. However, AMPK activation does require treatment with live bacteria, indicating that this signaling is not due to binding of cellular receptors to bacterial ligands. Testing of various bacterial ligands and centrifugation fractions led to the discovery that internalized outer-membrane vesicles (OMV) from Gram-negative bacteria are responsible for priming xenophagy. Importantly, OMV production is independent of Salmonella invasion and virulence and requires live bacteria, which is consistent with previous observations on the mechanism of AMPK activation. Treatment of cells with purified OMV results in activation of AMPK and inhibition of MTORC1, and pre-treatment of OMV before infection can enhance xenophagy. These experiments show that OMV-detection triggers a priming response, pre-activating the autophagy kinases in case of bacterial invasion ().
Figure 1. A diagram of AMPK signaling through ULK1 and PIK3C3/VPS34 complexes in response to Salmonella infection. (a) Upon detection of bacteria-secreted outer membrane vesicles, activated AMPK promotes upregulation of pro-autophagic kinases without inducing bulk autophagy. (b) Internalized bacteria are captured and targeted for xenophagic degradation.
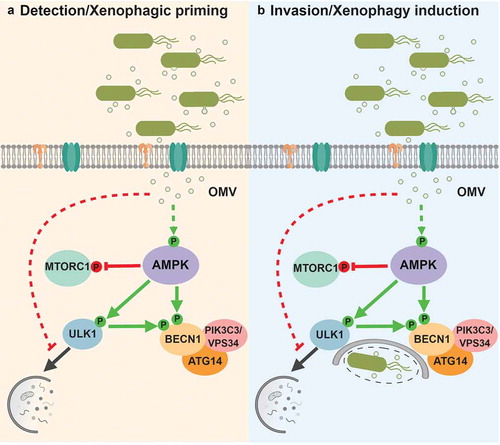
Curiously, despite the priming of autophagy kinases and inhibition of MTORC1, bulk autophagy is not increased upon infection. Analysis of LC3B and SQSTM1/p62 by western blot and immunofluorescence shows that autophagosome formation in Salmonella-treated cells is largely limited to bacteria. MTORC1 inhibition is widely known to be sufficient to induce bulk autophagy, raising the possibility that an inhibitory signal in response to Salmonella detection is responsible for the block in bulk autophagy. Accordingly, cells treated with the MTOR inhibitor Torin-1 in the presence of Salmonella do not induce autophagy, confirming the presence of a potent autophagy-inhibiting signal in response to bacterial detection. Together, these studies highlight a role for OMV-mediated AMPK activation as an early warning system that triggers autophagic priming prior to bacterial invasion. At the same time a blockage of indiscriminate degradation of cytoplasm by bulk autophagy is put in place, thereby preventing an energetically wasteful and potentially ineffective response to the detection of extracellular pathogen. Several questions are raised by this study including the pathways: A) linking OMV detection to AMPK activation, and B) responsible for inhibiting bulk autophagy. Further study of xenophagy regulation will undoubtedly reveal answers to these questions, leading to a more complete picture of the increasingly complex and interconnected processes of stress-induced autophagy regulation.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) Project Grants awarded to RCR (#PJT153034). TTL is supported by an Ontario Graduate Scholarship.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Losier, T.T., Akuma, M., McKee-Muir, O. C., et al. AMPK promotes xenophagy through priming of autophagic kinases upon detection of bacterial outer membrane vesicles. Cell Rep. 2019;26:2150–2165 e2155.
