ABSTRACT
ENDOG (endonuclease G), a mitochondrial endonuclease, is known to participate in apoptosis and paternal mitochondria elimination. However, the role and underlying mechanism of ENDOG in regulating macroautophagy remain unclear. We recently reported that ENDOG released from mitochondria promotes autophagy during starvation, which we demonstrated is evolutionarily conserved across species by performing experiments in human cell lines, mice, Drosophila, and C. elegans. This study demonstrates that ENDOG can be phosphorylated by GSK3B, which enhances the interaction between ENDOG with YWHAG and leads to the release of TSC2 and PIK3C3 from YWHAG, followed by MTOR pathway suppression and autophagy initiation. Additionally, the endonuclease activity of ENDOG is essential for activating the DNA damage response and thus inducing autophagy. Consequently, this study uncovered an exciting new role for ENDOG as a crucial regulator of autophagy.
ENDOG, a nuclear-encoded endonuclease that is located in mitochondrial intermembrane space, is a well-known apoptosis inducer that acts through cleaving chromatin DNA into nucleosomal fragments independently of caspases. Our previous study showed that during fertilization in C. elegans, paternal ENDOG relocates from the mitochondrial intermembrane space to the matrix, where it digests the paternal mtDNA and promotes paternal mitochondrial elimination through the maternal autophagy machinery. Interestingly, we observed that the ENDOG protein accumulated with an increase in autophagy markers during starvation. It has been shown that autophagy and apoptosis often occur in the same cell, and autophagy usually manifests well before apoptosis. These data motivated us to investigate whether ENDOG also regulates autophagy. Thus, we performed gain- and loss-of-function experiments to examine the role of ENDOG in autophagy [Citation1]. Upon starvation, depletion of ENDOG significantly decreases the accumulation of autophagosomes in human hepatic cell lines, liver of mice, fat body cells of Drosophila, and seam cells and pharyngeal muscle of C. elegans. These data suggest that ENDOG-promoted autophagy is conserved across species.
To explore the mechanism responsible for the induction of autophagy by ENDOG, we examined the involvement of the MTOR signaling pathway. We found that ENDOG promotes autophagy partially by suppressing the MTOR signaling. By co-IP and mass spectrometry analysis, we identified the interaction between ENDOG and YWHAG, which weakened the interaction of TSC2-PIK3C3 with YWHAG, eventually resulting in MTOR pathway suppression and autophagy initiation. YWHA family proteins are known to act mainly as phosphorylation readers by binding to the consensus sequence RXXp-S/TXP of target proteins. Then, we demonstrated that dual phosphorylation of ENDOG at threonine 128 and serine 288 is necessary for its interaction with YWHAG and the induction of autophagy. Next, we determined that GSK3B is a likely kinase for the phosphorylation of ENDOG, which enhances the interaction of ENDOG with YWHAG. However, further studies are needed to identify other kinases that could phosphorylate ENDOG.
Given the fact that suppression of the MTOR pathway could only partially restore ENDOG-induced autophagy, we reasoned that an additional pathway might exist in ENDOG-promoted autophagy. The DNA damage response is reported as an early event during starvation and plays an important role in regulating autophagy. As an endonuclease, ENDOG cleaves DNA at the GC-rich region and causes a DNA damage response. Hence, we assume that ENDOG promotes autophagy by activating the DNA damage response. Indeed, loss of ENDOG represses the DNA damage even at early time points of starvation, and blocking the DNA damage response pathway can sufficiently abolish ENDOG-induced autophagy. Furthermore, a mutation affecting ENDOG endonuclease activity and the use of an ENDOG-specific inhibitor (PNR-3-80) showed that the endonuclease activity is essential for ENDOG-induced DNA damage response and autophagy. Consistent with the previous study, we found that CASP8 (caspase 8)-BID contributes to the ENDOG release from mitochondria, which may further promote autophagy. Our results implied that under stress stimuli, ENDOG may first induce autophagy to adapt to and cope with stress; however, if the stress is unsolved or continuous, ENDOG may activate apoptosis to dismantle cells. Further study is needed to address the interplay of ENDOG-mediated autophagy and apoptosis.
In summary, we revealed the role and mechanism of ENDOG in promoting autophagy. Under starvation, CASP8-BID mediates the release of ENDOG from mitochondria. In the cytosol, ENDOG is phosphorylated at T128 and S288 by GSK3B, which enhances the interaction between ENDOG and YWHAG, dissociates TSC2 and PIK3C3 from YWHAG, and eventually suppresses the MTOR pathway and promotes autophagy initiation. In parallel, ENDOG can also translocate to the nucleus, activate the DNA damage response pathway and enhance autophagy via its endonuclease activity ().
Figure 1. Model depicting how ENDOG promotes autophagy through its phosphorylation-mediated interaction with YWHAG and its endonuclease activity-mediated DNA damage response
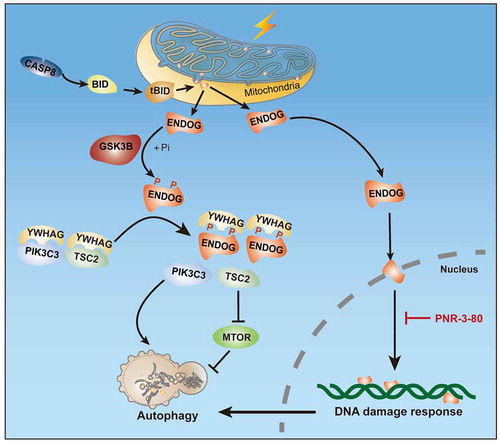
Future work will be needed to determine how ENDOG is translocated into the nucleus, as it lacks a nuclear localization sequence. Interestingly, we found that ENDOG without a mitochondrial targeting sequence (MTS, aa 1–48) failed to induce a DNA damage response, but it could still promote autophagy. We did not observe the nuclear localization of ENDOG without the MTS; instead, it concentrated around the nucleus. Thus, we propose that the maturation of ENDOG by being imported into mitochondria is essential for its translocation into the nucleus. Alternatively, do other proteins bind to YWHAG and regulate MTORC1 activity? Does ENDOG regulate the metabolite concentration in the cell or the lysosome? Further studies are needed to address these questions.
Disclosure statement
No potential conflicts of interest were disclosed.
Additional information
Funding
Reference
- Wang W, Li J, Tan J, et al. Endonuclease G promotes autophagy by suppressing mTOR signaling and activating the DNA damage response. Nat Commun. 2021;12:476.
