ABSTRACT
The YAP1-WWTR1/TAZ transcription co-factors are key determinants of cell growth that are perturbed in many cancers. Previous studies have reported divergent responses in YAP1-WWTR1/TAZ activities after autophagy perturbations in different contexts. Recently, we identified that α-catenin levels determine whether YAP1-WWTR1/TAZ signaling will be increased or decreased after macroautophagy/autophagy inhibition/induction. CTNNA1/α-catenin can act as a switch in this pathway, as it is an autophagy substrate and a negative regulator of YAP1-WWTR1/TAZ. However, YAP1-WWTR1/TAZ are also directly degraded by autophagy and there is a feedback loop where YAP1-WWTR1/TAZ positively regulate autophagy. These features were integrated into a mathematical numerical model based on a set of differential equations in order to clarify the integrated output on YAP1-WWTR1/TAZ activity at different time-points after autophagy perturbation in cells with distinct initial levels of α-catenins (CTNNA1 and CTNNA3). Our theoretical and experimental data allow an understanding of cell-type specific and time-dependent responses to autophagy manipulations that may be relevant in many contexts, including different types of cancer.
(Macro)autophagy is a highly conserved degradation process where cytoplasmic contents are sequestered in double-membraned organelles, called autophagosomes. Autophagosomes ultimately fuse with lysosomes, where their contents are degraded. As a clearance pathway, autophagy maintains cellular homeostasis and buffers starvation, and hence, perturbations cause pathologies, including cancers and neurodegeneration. The roles of autophagy in cancer evolution, from malignant cell transformation toward cell migration and metastasis, are controversial and complex, as contradictory results are reported for each distinct step in this process, and also in different cancer types. Thus, it is unclear whether autophagy inhibition is beneficial or harmful to different cancers, and much of the controversy may arise from the general expectation that all cancers should react in the same direction, possibly to various extents, when autophagy is perturbed.
Many cancers are associated with dysregulation of YAP and WWTR1/TAZ. These co-transcriptional regulators encoded by paralogous genes are critical effectors of the Hippo pathway and positively regulate cell growth. Autophagy perturbations in different contexts have been reported to trigger contrasting YAP1-WWTR1/TAZ-dependent responses. In a series of cell lines characterized by high basal levels of α-catenins (MCF10A, HEK293T, primary mammary epithelial cells and embryonic fibroblasts), we found [Citation1] that YAP1-WWTR1/TAZ are inhibited when autophagy is genetically or chemically downregulated (silencing of key autophagy genes: ATG7, ATG10 or ATG16L1, and bafilomycin A1 treatment), and that YAP1-WWTR1/TAZ are activated when autophagy is upregulated (starvation, Tat-beclin 1 peptide, trehalose, SMER28 exposure). However, in cell lines with low basal α-catenin levels (HepG2, THLE2, A549 cells), autophagy modulation provoked the opposite effect on YAP1-WWTR1/TAZ activity: YAP1-WWTR1/TAZ were stimulated by autophagy downregulation, while they were repressed by autophagy upregulation.
Next, we identified α-catenins (specifically CTNNA1 and CTNNA3), which are known to suppress YAP1-WWTR1/TAZ activities, as autophagy substrates, and showed that their degradation is mediated by two LC3-interacting regions (LIRs). This provided a mechanism for autophagy to activate YAP1-WWTR1/TAZ by promoting the degradation of α-catenins. As the link between autophagy and YAP1-WWTR1/TAZ is via α-catenins, we have called this the “indirect” effect, to contrast it with the previous observation that YAP1-WWTR1/TAZ are direct autophagy substrates themselves. Thus, we concluded that autophagy simultaneously produces contrasting effects on YAP1-WWTR1/TAZ in cells: indirect activation (via α-catenins), but also direct degradation/inactivation YAP1-WWTR1/TAZ. These effects, together with our earlier data showing that YAP1-WWTR1/TAZ positively inflect autophagy (from autophagosome biogenesis to increasing autophagic flux), led us to explore the possibility of an autophagy-YAP1-WWTR1/TAZ-autophagy feedback loop, with the initial levels of α-catenin as a key variable explaining the output differences in YAP activity among various cell types.
As most of the perturbations (external or internal) that impact the autophagy pathway induce gradual, and not instant changes, the autophagy-YAP1-WWTR1/TAZ system is dynamic and integrates, at any time point, the three mechanisms mentioned above: (i) autophagy directly degrades YAP1-WWTR1/TAZ, (ii) autophagy indirectly (by suppressing α-catenins) stimulates YAP1-WWTR1/TAZ, and (iii) YAP1-WWTR1/TAZ boost autophagy (). Consequently, we also expected distinct output effects on YAP1-WWTR1/TAZ activities at various times after triggering the autophagy perturbation in certain cell types.
Figure 1. Autophagy-YAP1-WWTR1/TAZ -autophagy loop. (A) The three mechanisms that control the autophagy-YAP system: (left, in green) autophagy indirectly (by suppressing α-catenin via LIR-dependent degradation) stimulates YAP1-WWTR1/TAZ activity; (right, in red) autophagy directly represses YAP1-WWTR1/TAZ (autophagy directly targets YAP1-WWTR1/TAZ for degradation); (middle, in gray) the feedback generated by YAP1-WWTR1/TAZ activity boosting autophagy. (B) Mathematical modeling for cells with high α-catenin (CTNNA1 and CTNNA3) onset levels: the indirect effect of autophagy inhibition on YAP1-WWTR1/TAZ activity (green) overcomes the direct effect (red) for the entire duration of autophagy perturbation. (C) Mathematical modeling for cells with low α-catenin onset levels: the indirect effect of autophagy on YAP1-WWTR1/TAZ activity (green) is initially dominated by the direct effect (red), but the balance is reversed over time after autophagy inhibition. Yi represents the initial YAP activity, and t represents time
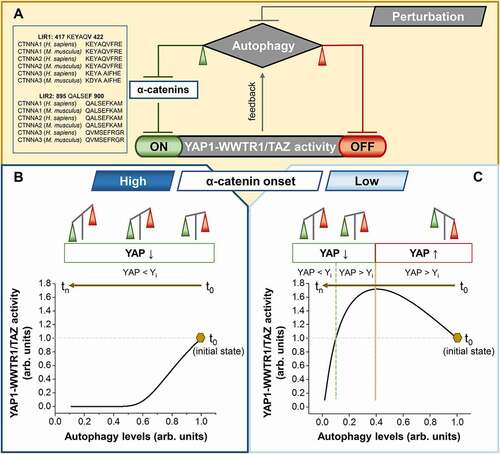
To explore this postulated time-dependency of the autophagy-YAP1-WWTR1/TAZ loop after autophagy perturbation, we developed a numerical mathematical model based on three coupled differential equations that follow the temporal evolution of the three inter-linked variables: autophagy, YAP activity, and levels of α-catenins. As this is a dynamic process, the values of the variables at a given time-point depend on previous steps, while the evolution rates of our variables depend on their values at a given time-point. Our mathematical simulations enabled us to decipher the differences in the temporal evolution of YAP1-WWTR1/TAZ activity when we explored scenarios characterized by high or low starting levels of α-catenins, as seen in different cell types. Briefly, when autophagy is compromised in cells with high basal levels of α-catenins, the initial tendency for YAP activity is to decrease (as the indirect effect of autophagy on YAP is stronger than the direct effect), which is further amplified, as the strength of the indirect effect rises with the continuous accumulation of α-catenins. For cells with low basal levels of α-catenins, the YAP1-WWTR1/TAZ activity will initially increase (as the direct effect exerted by autophagy dominates the indirect one), but as α-catenins accumulate, the strength of the indirect effect rises, up to a time-point when it actually dominates the system, and therefore, YAP activity start to decrease from this step onwards.
This study, focusing on the YAP1-WWTR1/TAZ activity as output, reveals how autophagy modulates cell behavior in a time-dependent manner, causing completely opposite effects in cells with different starting levels of an intermediary protein (α-catenin). It will be interesting to apply the principles underlying the mathematical model used to explain these cellular controversies to other signaling pathways, where autophagy perturbations appear to cause contrasting reactions in distinct tissues, organs or individuals. Our new perspective also highlights the important issue that cellular responses to perturbations often change over time, and that better understanding of such dynamic effects may be important for our appreciation of the roles of autophagy and related processes in health and disease.
Acknowledgments
We are grateful for funding from the UK Dementia Research Institute (funded by MRC, Alzheimer’s Research UK and the Alzheimer’s Society) and The Roger de Spoelberch Foundation (D.C.R.), Wellcome Trust [095317/Z/11/Z, 100140/Z/12/Z], Romanian Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-III-P1-1.1-PD-2019-0733, within PNCDI-III (M.P.).
Disclosure statement
D.C.R is a consultant for Aladdin Healthcare Technologies SE and Nido Biosciences. All other authors declare no competing interests.
Additional information
Funding
Reference
- Pavel M, Park SJ, Frake RA, et al. α-Catenin levels determine direction of YAP/TAZ response to autophagy perturbation. Nat Commun. 2021;12(1):1703.
