ABSTRACT
Target of rapamycin complex 1 (TORC1) promotes cellular anabolism and suppresses macroautophagy/autophagy. In mammalian cells starved of amino acid, the GATOR1 complex, a negative regulator of TORC1, is released from its inhibitor GATOR2 and inactivates TORC1. We have recently identified the evolutionarily conserved GATOR2 components in fission yeast including Sea3, an ortholog of mammalian WDR59, but, unexpectedly, Sea3 acts as a part of GATOR1 to suppress TORC1. Moreover, fission yeast GATOR1 is not required for the amino-acid starvation-induced TORC1 attenuation, which is instead mediated by the Gcn2 pathway. Conversely, absence of a nitrogen source suppresses TORC1 in a manner dependent on GATOR1 as well as the Tsc1-Tsc2 complex, whose mammalian equivalent functions as a growth-factor sensitive TORC1 inhibitor. Thus, the evolutionarily conserved signaling modules are utilized differently between fission yeast and mammals to control TORC1 activity and autophagy.
In response to nutrients and growth factors, TORC1 phosphorylates its substrates to promote biosynthesis of macromolecules and inhibit autophagic degradation. Mammalian TORC1 is regulated by the two types of small GTPases on the lysosomal surface, RHEB and RRAG, both of which are under the control of the protein complexes with GTPase-activating protein (GAP) activity. Growth factors inhibit the RHEB GAP activity of the TSC complex, so that the GTP-loaded form of RHEB activates TORC1. Amino acid availability stimulates TORC1 via the RRAG GTPase heterodimer constituted by RRAGA/RRAGB and RRAGC/RRAGD, with RRAGA/RRAGB bound to GTP. This activated form of the RRAG heterodimer recruits TORC1 to lysosomes and facilitates TORC1 activation by RHEB. The GATOR1 complex carries the GAP activity toward RRAGA/RRAGB and thus, suppresses the lysosomal recruitment and activation of TORC1. GATOR1 interacts with another protein complex called GATOR2, together forming the GATOR holocomplex. GATOR2 is a negative regulator of GATOR1; under amino-acid rich conditions, GATOR2 inhibits the GATOR1-dependent suppression of TORC1. Once cells are starved, the amino acid sensor proteins, such as SESN (sestrin) and CASTOR, bind GATOR2 and prevent it from interfering with GATOR1, leading to TORC1 inactivation.
The fission yeast Schizosaccharomyces pombe serves as an excellent model system to study TORC1 signaling. As in mammals, S. pombe TORC1 is activated by the RHEB GTPase called Rhb1, which is under the negative regulation of the TSC complex. The RRAG heterodimer Gtr1-Gtr2 and the GATOR1 complex are also conserved in fission yeast. SESN and CASTOR orthologs are not present in the organism, implying that amino acid availability regulates TORC1 by a mechanism different from the SESN/CASTOR–GATOR2 pathway.
Recently, we have successfully identified the fission yeast GATOR2 components by biochemically isolating GATOR1-binding proteins [Citation1]. Unexpectedly, among the identified components, gene disruption of only sea3, a mammalian WDR59 ortholog, brings about phenotypes similar to those caused by the GATOR1 defect, suggesting that Sea3 plays an important role in the GATOR1 function as a GAP for Gtr1 (). Indeed, Sea3 binds to GATOR1 even in the absence of the other GATOR2 components and promotes the interaction of GATOR1 with its target, the Gtr1 GTPase. Although its mammalian ortholog WDR59 was reported as a GATOR2 component, Sea3 appears to serve as a fourth subunit of the GATOR1 complex.
Figure 1. TORC1 regulation by GATOR1, the TSC complex, and Gcn2 in fission yeast. (A) The Gtr1-Gtr2 heterodimer is anchored to the vacuole by the Ragulator complex. Sea3 functions as part of GATOR1 to promote the GAP activity of GATOR1 toward the Gtr1 GTPase. Sea3 also mediates the association between GATOR1 and GATOR2 in the assembly of the GATOR holocomplex. (B) In response to amino acid starvation, Gcn2 suppresses TORC1 activity, whereas nitrogen starvation does not induce robust Gcn2 activation (a thinner arrow). Upon nitrogen starvation, the Sea3-GATOR1 complex primarily attenuates TORC1, with less contribution of the TSC complex (Tsc1-Tsc2), inducing autophagy that supplies enough amino acids to restrain Gcn2 activation. Cells deficient in GATOR1 and the TSC complex fail to induce autophagy, resulting in a cellular amino acid shortage that triggers the Gcn2-mediated TORC1 suppression
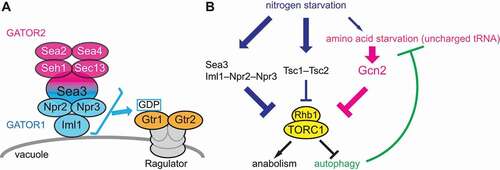
In fission yeast, either nitrogen starvation or amino acid starvation swiftly inactivates TORC1, thereby inducing autophagy. We have found that, unlike in mammals, the GATOR complex is dispensable for the suppression of TORC1 upon amino acid starvation in fission yeast. Instead, Gcn2, an EIF2S1/eIF2α (fission yeast Tif211) kinase activated by uncharged tRNAs during amino acid starvation, predominantly acts as a negative regulator of TORC1. EIF2S1/eIF2α phosphorylation by Gcn2 downregulates general translation initiation but stimulates translation of the specific transcriptional activators, such as mammalian ATF4 and fission yeast Fil1. Indeed, the Tif211 phosphorylation and Fil1 accumulation are involved in the TORC1 suppression. Therefore, in fission yeast cells starved of amino acids, uncharged tRNAs stimulate the Gcn2-Tif211-Fil1 pathway and suppress TORC1. Though the exact mechanism by which the Gcn2 pathway suppresses TORC1 remains elusive, the target gene(s) of the Fil1 transcription factor may modulate TORC1 activity.
In contrast to amino acid starvation, absence of a nitrogen source such as ammonium attenuates fission yeast TORC1 independently of Gcn2. Consistently, nitrogen starvation does not induce strong activation of Gcn2, implying that removal of the nitrogen source does not immediately result in a severe shortage of cellular amino acids. Conversely, TORC1 attenuation in response to nitrogen starvation is significantly delayed in cells lacking GATOR1 and the TSC complex, suggesting their major roles in TORC1 suppression upon nitrogen starvation. In those mutant cells, robust Gcn2 activation is detectable, probably due to reduced cellular amino acid levels. Furthermore, the loss of Gcn2 aggravates the defective TORC1 suppression in the mutant without GATOR1 and the TSC complex under nitrogen starvation conditions. Thus, only in the absence of GATOR1 and the TSC complex, Gcn2 is activated and undertakes the negative regulation of TORC1. We speculate that, in wild-type cells starved of nitrogen, GATOR1 and the TSC complex mediate TORC1 suppression to induce autophagy, which supplies enough amino acids to prevent Gcn2 activation (). As expected, the mutants defective in autophagy exhibit robust Gcn2 activation upon nitrogen starvation, corroborating the contribution of autophagy to Gcn2 repression in nitrogen-starved cells.
In addition to the GATOR and TSC complexes, the Gcn2 pathway is also evolutionarily conserved, but the roles assigned to these signaling components in TORC1 regulation appear to be different from species to species. Fission yeast might have evolved multiple nitrogen-sensing mechanisms because lack of nitrogen nutrition is a key trigger for sexual reproduction. By contrast, mammalian cells need to monitor individual amino acids because of their contributions in amino acid biosynthesis. Currently, the physiological role of GATOR2 remains elusive in fission yeast, and further studies may uncover a novel and conserved function of GATOR2. It would also be of interest to elucidate the mechanism by which the GATOR1 and TSC complexes monitor the nitrogen source in fission yeast; possibly, ammonium-derived metabolites regulate these complexes directly or indirectly. Such signaling mechanisms might be conserved also in more complex eukaryotes as part of TORC1 regulation.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Fukuda T, Sofyantoro F, Tai YT, et al. Tripartite suppression of fission yeast TORC1 signaling by the GATOR1-Sea3 complex, the TSC complex, and Gcn2 kinase. eLife. 2021;10:e60969. PMID: 33534698.
