ABSTRACT
During macroautophagy/autophagy, autophagosomes fuse with lysosomes to form autolysosomes. After fusion, the autophagosome inner membrane and enclosed substrates are degraded and transported out of lysosomes for recycling. The lysosomal membrane components are recycled by autophagic lysosome reformation (ALR) to generate new lysosomes. However, the fate of autophagosome outer membrane components on autolysosomes remains unknown. Our recent work discovered that autophagosome outer membrane components are not degraded but are recycled through an unidentified process which we named autophagosomal components recycling (ACR). Further investigation revealed the recycler complex (SNX4-SNX5-SNX17) responsible for ACR. The discovery of ACR not only fills a missing part in autophagy, but also reveals a new recycling pathway on autolysosomes.
Autophagy is a common mechanism to remove misfolded proteins and damaged organelles for maintaining cellular homeostasis. In response to external or internal stress, phagophore membranes begin to assemble and extend around the substrates for degradation. During phagophore expansion, this structure receives membranes from the endoplasmic reticulum, mitochondria, plasma membrane, Golgi apparatus, endosomes, and ERGIC. When phagophore membranes seal to form completed autophagosomes, they move toward lysosomes with enclosed substrates and membrane sources from multiple organelles. Once autophagosomes fuse with lysosomes, the inner membrane and enclosed substrates are degraded inside lysosomes and the lysosomal components are recycled via ALR. However, the fate of the autophagosome outer membrane remains completely unknown. Our recent work discovered that autophagosome outer membrane components are recycled via ACR by the recycler complex [Citation1].
To investigate the fate of autophagosome outer membrane components, we first selected STX17 as a cargo for this study. STX17, an autophagosomal SNARE protein, is recruited to complete autophagosomes and assembles into a ternary SNARE complex with the other two SNARE proteins, SNAP29 and VAMP8, for mediating autophagosome-lysosome fusion. After fusion, STX17 translocates to autolysosomes from autophagosomes. We studied the autophagosome outer membrane fate by tracking STX17. Interestingly, STX17 on the autolysosome does not undergo degradation inside autolysosomes but concentrates on some regions of the autolysosomal membrane and is sorted out of autolysosomes. In addition, ATG9A has been shown on endosomes, phagophores, lysosomes, autolysosomes and has limited localization on autophagosomes in mammalian cells. Live cell images show some ATG9A on autophagosomes, which translocates to lysosomes through autophagosome-lysosome fusion, and ATG9A on autolysosomes is also recycled. Because two cargos, STX17 and ATG9A, are recycled from autolysosomes, we thus coined this recycling process as autophagosomal components recycling (ACR). Interestingly, it was recently found that ATG9A follows a retrograde trafficking route from lysosomes during starvation.
Further investigation identified the recycler complex composed of SNX4, SNX5 and SNX17 which is required for ACR. Elimination of SNX4, SNX5 or SNX17 blocks the recycling of STX17 and ATG9A. SNX4 and SNX5, two BAR domain-containing proteins, are gradually recruited to STX17-positive autolysosomes, rather than STX17-positive autophagosomes. The STX17-concentrated vesicles bud off from autolysosomes with SNX4 and SNX5, and then SNX4 and SNX5 disassemble from the STX17 vesicles. SNX4 and SNX5 recognize STX17 and ATG9A via their BAR domains. Two stretches of amino acids in the C-terminal tail of STX17 are critical for this recognition. A similar cargo recognition mode by BAR domains is also found in the ESCPE-1 complex. In addition, SNX4 and SNX5 form a heterodimer to remodel the autolysosomal membrane likely via their BAR domains for transporting carrier generation.
SNX17 functions together with retriever on endosomes for recycling plasma membrane cargos. We found that SNX17 also localizes to lysosomes. The lysosomal SNX17 translocates to autolysosomes via autophagosome-lysosome fusion where SNX4 and SNX5 coexist, which provides an opportunity for SNX4-SNX5-SNX17 complex formation. SNX17 interacts with the BAR domains of SNX4 and SNX5, and with STX17 via its FERM domain. In addition, SNX17 also interacts with the dynactin subunit DCTN1 and several dynein subunits. We proposed that SNX17 likely functions as a linker between SNX4-SNX5-STX17 and dynactin-dynein to facilitate the carriers’ generation and transport.
Snx4/Atg24 functions with Snx41 and Atg20/Snx42 to retrieve Atg27 on yeast vacuoles. SNX5 and SNX17 also function in the retromer and retriever, respectively. However, we found that their roles in ACR are independent of their previously described functions. In addition, ACR and ALR both occur on autolysosomes, but ACR occurs prior to ALR. Further, the ALR essential genes are dispensable for ACR. All these lines of evidence support an unidentified recycling process on autolysosomes by the recycler complex identified here.
Triple depletion of SNX4, SNX5 and SNX17 does not result in further inhibition of ACR compared to single deletion of these three genes, suggesting SNX4, SNX5 and SNX17 function in the same pathway. Biochemical experiments demonstrated that SNX4, SNX5 and SNX17 interact with each other directly. Overexpression of any one of these three proteins promotes the interactions between the other two subunits. In the recycler complex model, SNX4 and SNX5 interact with each other via their BAR domains to form a heterodimer; SNX17 binds the BAR domains of SNX4 and SNX5 via the F1 and F3 regions of the FERM domain, respectively; SNX17 connects SNX4-SNX5 to dynactin-dynein likely as a linker to facilitate ACR ().
Figure 1. The model for autophagosomal components recycling from autolysosomes.
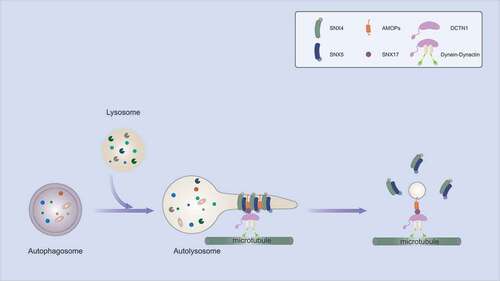
Deficient ACR caused by loss of function of recycler inhibits autophagic substrate degradation without effect on lysosome activity. This defect is likely caused by the impaired autophagosome-lysosome fusion based on the measurement of autophagic flux. Although STX17 and ATG9A have been reported to function at the autophagosome formation stage, our speculation is that the STX17 involved in autophagosome formation does not participate in autophagosome-lysosome fusion and ACR; ATG9A on other organelles, such as the Golgi apparatus and endosomes, may complement its function in autophagosome formation in ACR-deficient cells. The effects of ACR deficiency on autophagy may vary due to ATG9A mobilization efficiency from endosomes and lysosomes in different types of cells or upon different autophagy stimuli.
In spite of the discovery of ACR and identification of recycler, many details remain to be answered: 1. The final destination of the budding vesicles is not clear due to their rapid movement in the three-dimensional space inside cells. This issue may be solved by advanced high-speed microscopy which can acquire three-dimensional images; 2. To date, only two cargos are shown to be recycled via ACR, the further identification of ACR cargos is essential for a comprehensive understanding of the fate of autophagosomal membrane proteins on autolysosomes; 3. How ACR is initiated and regulated remains largely unknown. It will depend on the systematic identification of the players in ACR; 4. The physiological and pathological roles of ACR remain to be further investigated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Zhou C, Wu Z, Du W, et al. Recycling of autophagosomal components from autolysosomes by the recycler complex. Nat Cell Biol. 2022;24:497–512.
