ABSTRACT
The body shape of plants varied in proportion to the logarithm of the magnitude of gravity in the range from microgravity to hypergravity to resist the gravitational force. Here we discuss the roles of cortical microtubule and 65 kDa microtubule-associated protein-1 (MAP65-1) in gravity-induced modification of growth anisotropy. Microgravity stimulated elongation growth and suppressed lateral expansion in shoot organs, such as hypocotyls and epicotyls. On the other hand, hypergravity inhibited elongation growth and promoted lateral expansion in shoot organs. The number of cells with transverse microtubules was increased by microgravity, but decreased by hypergravity. Furthermore, the levels of MAP65-1, which is involved in the maintenance of the transverse microtubule orientation, were increased by microgravity, but decreased by hypergravity. Therefore, the regulation of orientation of cortical microtubules via changes in the levels of MAP65-1 may contribute to the modification of the body shape of plants to resist the gravitational force.
Resistance to the gravitational force is a critical response for terrestrial plants to survive under 1 g conditions. We have termed this response ‘gravity resistance’Citation1 and have analyzed the nature and mechanisms of gravity resistance using hypergravity conditions produced by centrifugation and microgravity conditions in space.Citation2,Citation3 Hypergravity inhibited elongation growth and promoted lateral expansion in shoot organs, such as pea epicotyls,Citation4 radish and cucumber hypocotyls,Citation5 garden cress hypocotyls,Citation6 azuki bean epicotyls,Citation7 maize coleoptiles and mesocotyls,Citation8 Arabidopsis hypocotylsCitation9 and inflorescence stems,Citation10 and wheat coleoptiles.Citation11 Namely, plants developed a short and thick body under hypergravity conditions. On the other hand, microgravity promoted elongation growth and inhibited lateral expansion in shoot organs, such as rice coleoptiles,Citation12,Citation13 Arabidopsis hypocotylsCitation9,Citation14,Citation15 and inflorescence stems,Citation16 and azuki bean epicotyls,Citation17 i.e., the plant body became longer and thinner under microgravity conditions in space. The body shape of Arabidopsis hypocotyls varied in proportion to the logarithm of the magnitude of gravity in the range from microgravity to hypergravity ().Citation9 Taken together, these results indicated that the development of a short and thick body is the main mechanism that enables plants to grow against the gravitational force.
Figure 1. The summary of the results obtained by space and hypergravity experiments. The body shape varied in proportion to the logarithm of magnitude of gravity in the range from microgravity to hypergravity. The body shape of plants was changed by regulating the orientation of cortical microtubules via changes in the levels of MAP65-1.
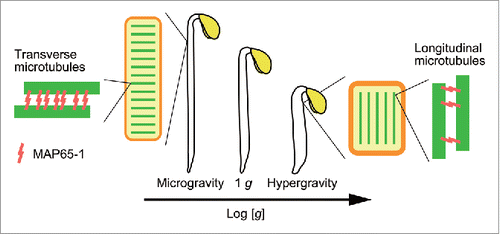
Cortical microtubules are essential for modification of the body shape because they regulate the direction of cell expansion. Hypergravity induced reorientation of cortical microtubules from transverse to longitudinal directions in azuki bean epicotylsCitation18 and Arabidopsis hypocotyls.Citation19 We carried out a space experiment, denoted as Aniso Tubule, to examine the effects of microgravity on the cortical microtubule dynamics in Arabidopsis hypocotyls, using lines in which microtubules are visualized by labelling tubulin or microtubule-associated proteins (MAPs) with green fluorescent protein (GFP). The dynamics of cortical microtubules in hypocotyls were analyzed using a fluorescence microscope, which was controlled from ground by commanding, in the Kibo Module in the International Space Station. Under microgravity conditions, cells having transverse microtubules were predominant, as observed in 1 g samples. However, the percentage of cells having transverse microtubules in seedlings grown under microgravity conditions was higher than that at 1g.Citation15 We analyzed the average angle of the cortical microtubules, and showed that the angle was decreased by microgravity. These results indicate that cortical microtubules orient more in transverse directions in microgravity conditions in space. Thus, these findings suggest that the reorientation of cortical microtubules is involved in the regulation of growth anisotropy by gravity in plant cells ().
How is the orientation of cortical microtubules regulated by gravity? MAPs bind to microtubules and regulate their dynamics, stability, and organization.Citation20 The 65 kDa MAP (MAP65) family proteins form cross-bridges between adjacent microtubules and are required for the bundling of microtubules and the maintenance of transverse microtubule orientation.Citation20 The transcript levels of MAP65-1 in azuki bean epicotyls was down-regulated by hypergravity.Citation21 We also determined the protein levels of MAP65-1, expressed by the native promoter, by analyzing GFP fluorescence in Arabidopsis hypocotyls of GFP-MAP65-1 line. The protein levels of MAP65-1 in Arabidopsis hypocotyls were decreased by hypergravity.Citation22 On the other hand, microgravity increased the protein levels of MAP65-1 in Arabidopsis hypocotyls.Citation15 These results suggest that changes in the protein levels of MAP65-1 via modification of the expression of its gene may be one of the mechanisms for the regulation of orientation of cortical microtubules by gravity (). The levels of other MAPs, such as γ-tubulin complex and katanin, which are required for the nucleation and the severing of microtubules, respectively, also changed during reorientation of cortical microtubules by hypergravity.Citation23-25 These findings suggest that changes in the levels of particular types of MAPs are involved in the modification of the dynamics of the cortical microtubules by gravity.
Conclusions
summarizes the results obtained by space and hypergravity experiments. The body shape varied in proportion to the logarithm of magnitude of gravity in the range from microgravity to hypergravity. The regulation of orientation of cortical microtubules via changes in the levels of MAP65-1 may contribute to the modification of the body shape of plants by gravity. Thus, the regulation of the body shape by modification of cortical microtubule dynamics may be required for terrestrial plants to survive at 1 g gravity on the earth.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank astronauts K. Wakata, S. Cristoforetti, K. Nyberg, T.W. Virts, A. Gerst, S. Swanson, and B. Wilmore for their in-orbit operations of the Aniso Tubule space experiment. We also thank JAXA, NASA, JSF, AES, JAMSS, and CHIYODA personnel for their preparations and ground operations of the space experiment.
References
- Hoson T, Soga K. New aspects of gravity responses in plant cells. Int Rev Cytol. 2003;229:209–44. doi:10.1016/S0074-7696(03)29005-7.
- Soga K. Gravity resistance in plants. Biol Sci Space. 2010;24:129–34. doi:10.2187/bss.24.129.
- Soga K. Resistance of plants to gravitational force. J Plant Res. 2013;126:589–96. doi:10.1007/s10265-013-0572-4.
- Waldron KW, Brett CT. Effects of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. J Exp Bot. 1990;41:71–7. doi:10.1093/jxb/41.1.71.
- Kasahara H, Shiwa M, Takeuchi Y, Yamada M. Effects of hypergravity on elongation growth in radish and cucumber hypocotyls. J Plant Res. 1995;108:59–64. doi:10.1007/BF02344306.
- Hoson T, Nishitani K, Miyamoto K, Ueda J, Kamisaka S, Yamamoto R, Masuda Y. Effects of hypergravity on growth and cell wall properties of cress hypocotyls. J Exp Bot. 1996;47:513–7. doi:10.1093/jxb/47.4.513.
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. Hypergravity increases the molecular size of xyloglucans by decreasing xyloglucan-degrading activity in azuki bean epicotyls. Plant Cell Physiol. 1999;40:581–5. doi:10.1093/oxfordjournals.pcp.a029580.
- Soga K, Harada K, Wakabayashi K, Hoson T, Kamisaka S. Increased molecular mass of hemicellulosic polysaccharides is involved in growth inhibition of maize coleoptiles and mesocotyls under hypergravity conditions. J Plant Res. 1999;112:273–8. doi:10.1007/PL00013881.
- Soga K, Wakabayashi K, Hoson T, Kamisaka S. Gravitational force regulates elongation growth of Arabidopsis hypocotyls by modifying xyloglucan metabolism. Adv Space Res. 2001;27:1011–6. doi:10.1016/S0273-1177(01)00176-4.
- Nakabayashi I, Karahara I, Tamaoki D, Masuda K, Wakasugi T, Yamada K, Soga K, Hoson T, Kamisaka S. Hypergravity stimulus enhances primary xylem development and decreases mechanical properties of secondary cell walls in inflorescence stems of Arabidopsis thaliana. Ann Bot. 2006;97:1083–90. doi:10.1093/aob/mcl055.
- Wakabayashi K, Soga K, Kamisaka S, Hoson T. Increase in the level of arabinoxylan-hydroxycinnamate network in cell walls of wheat coleoptiles grown under continuous hypergravity conditions. Physiol Plant. 2005;125:127–34. doi:10.1111/j.1399-3054.2005.00544.x.
- Hoson T, Soga K, Mori R, Saiki M, Wakabayashi K, Kamisaka S, Kamigaichi S, Aizawa S, Yoshizaki I, Mukai C, et al. Morphogenesis of rice and Arabidopsis seedlings in space. J Plant Res. 1999;112:477–86. doi:10.1007/PL00013903.
- Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, Kamisaka S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol. 2002;43:1067–71. doi:10.1093/pcp/pcf126.
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta. 2002;215:1040–6. doi:10.1007/s00425-002-0838-x.
- Soga K, Yamazaki C, Kamada M, Tanigawa N, Kasahara H, Yano S, Kojo KH, Kutsuna N, Kato T, Hashimoto T, et al. Modification of growth anisotropy and cortical microtubule dynamics in Arabidopsis hypocotyls grown under microgravity conditions in space. Physiol Plant. 2018;162:135–44. doi:10.1111/ppl.12640.
- Hoson T, Soga K, Wakabayashi K, Hashimoto T, Karahara I, Yano S, Tanigaki F, Shimazu T, Kasahara H, Masuda D, et al. Growth stimulation in inflorescences of an Arabidopsis tubulin mutant under microgravity conditions in space. Plant Biol. 2014;16(S1):91–6. doi:10.1111/plb.12099.
- Soga K, Biology Club, Kurita A, Yano S, Ichikawa T, Kamada M, Takaoki M. Growth and morphogenesis of azuki bean seedlings in space during SSAF2013 program. Biol Sci Space. 2014;28:6–11. doi:10.2187/bss.28.6.
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta. 2006;224:1485–94. doi:10.1007/s00425-006-0319-8.
- Matsumoto S, Kumasaki S, Soga K, Wakabayashi K, Hashimoto T, Hoson T. Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants. Plant Physiol. 2010;152:918–26. doi:10.1104/pp.109.147330.
- Hamada T. Microtubule organization and microtubule-associated proteins in plant cells. Int Rev Cell Mol Biol. 2014;312:1–52. doi:10.1016/B978-0-12-800178-3.00001-4.
- Soga K, Kotake T, Wakabayashi K, Hoson T. Changes in the transcript levels of microtubule-associated protein MAP65-1 during reorientation of cortical microtubules in azuki bean epicotyls. Acta Physiol Plant. 2012;4:533–40. doi:10.1007/s11738-011-0850-5.
- Murakami M, Soga K, Kotake T, Kato T, Hashimoto T, Wakabayashi K, Hoson T. Roles of MAP65-1 and BPP1 in gravity resistance of Arabidopsis hypocotyls. Biol Sci Space. 2016;30:1–7. doi: 10.2187/bss.30.1.
- Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T. Transient increase in the transcript levels of γ-tubulin complex genes during reorientation of cortical microtubules by gravity in azuki bean (Vigna angularis) epicotyls. J Plant Res. 2008;121:493–8. doi:10.1007/s10265-008-0179-3.
- Soga K, Kotake T, Wakabayashi K, Kamisaka S, Hoson T. The transcript level of katanin gene is increased transiently in response to changes in gravitational conditions in azuki bean epicotyls. Biol Sci Space. 2009;23:23–8. doi:10.2187/bss.23.23.
- Soga K, Yamaguchi A, Kotake T, Wakabayashi K, Kamisaka S, Hoson T. Transient increase in the levels of γ-tubulin complex and katanin are responsible for reorientation by ethylene and hypergravity of cortical microtubules. Plant Signal Behav. 2010;5:1480–2. doi:10.4161/psb.5.11.13561.
