 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sophora davidii is a cross-pollinated plant with important ecological protection and medicinal value in China, but its seed yield is low due to backward and nonstandard production technology. Therefore, we divide the flowering period of Sophora davidii into initial, full and final flowering period, measuring the floral morphology, pollen viability, stigma receptivity, nectar volume and nectar concentration, foraging behavior of pollinators, fertilization physiology, seed yield and quality through field observation and indoor testing to explore whether the flowering period affects the floral traits, pollinator behavior and seed production of plants. Our results revealed that the nectar volume, nectar concentration, pollen viability and stigma receptivity at full flowering period were the highest. The single visit time and visit time per flower of Chinese honey bees were the longest in the full flowering period, while the number of transfer, visit frequency and number of touching stigma were the least. The visiting number of the bees was the most and the most active in the full flowering period. The bees pollination not only improved the pollen amount, germination rate, pollen tube length and the ovule number of S. davidii, but also their effect was the most obvious in full flowering period. The principal component analysis showed that pollination by Chinese honey bees during the whole flowering period of S. davidii was the best way to produce seeds. We can conclude that flowering period affects flower traits, foraging behavior of pollinators, seed yield and quality of S. davidii.
Introduction
The David’s mountain laurel plant Sophora davidii, belongs to the Fabaceae family, is a spiny, deciduous, perennial shrub native to southwestern China, and typically grows from 2 to 4 m in length and width. It is not only an important pioneer plant for soil and water conservation and rocky desertification control in karst area of China, especially in the Guizhou province, but also a high-quality feed source for herbivorous livestock and one of the main nectar plants in spring.Citation1,Citation2 S. davidii is also used in the medical field. Its seeds and flowers contain a variety of alkaloids, flavonoids and other medicinal components, which can be used as effective components of therapeutic drugs for Alzheimer’s disease.Citation3 Hence, its extract has certain inhibitory effects on Ebola virus and Lassa virus, and has various pharmacological activities such as anticancer, antitumor, antiviral, anti-inflammatory and anti-allergic.Citation4–6
S. davidii is widely cultivated for its high ecological protection, forage grass and medicinal values, but its seed yield is low due to backward and unstandardized production technology.Citation7 In addition, the successful fusion of male and female gametes in the process of seed formation depends on the factors such as pollinators, flower characteristics and various environmental conditions.Citation8–10
S. davidii is a hermaphroditic plant that is cross-pollinated.Citation11 Hence, in the absence of pollinators, the number of seeds per pod is small and even abortion exists.Citation12 This means that the development and formation of the seeds of S. davidii is still dependent on the pollinators. However, studies on improving seed yield of S. davidii have focused mainly on cultivation and management, while studies on the pollination have been rarely reported.
Studies showed that about 76% of the world’s 115 major crops depended on bee pollination to form seeds and fruits.Citation13 As pollinators, bees are able to pollinate in time, effectively, fully, recognized and safely, creating the necessary conditions for plant fertilization. Through bee pollination, more pollen cannot only fall on the stigma to germinate, making the group effect of pollen germination and pollen tube growth stronger, but also it can accelerate the physiological effect after fertilization and promote the development of fruits and seeds, thus achieving the effect of increasing production and improving quality.Citation14 Among them, Chinese honey bees Apis cerana cerana Fabricius, as a native bee species in China, has the characteristics of diligent collection, strong resistance and adaptability, and is good at collecting sporadic nectar sources, which has been widely used in pollination of field crops and agricultural facility crops in China.
Pollination is a crucial step in the fertilization process of angiosperms, which means that active and mature pollen grains needs to be transported to the appropriate stigma by wind or pollinators such as bees.Citation15 In the process of bee visiting flowers, the floral traits, flower reward and environmental factors will all affect their visiting behavior, and then affect their pollination effect. The success of pollination directly influences the success rate of plant reproduction.Citation16 Because of this, it is necessary for us to observe the flower characteristics of plants and study pollination media in order to better understand the pollination methods of plants and lay a scientific foundation for choosing pollination methods.
However, the flower traits of plants vary with the growth cycle, and different floral traits also have certain effects on seed yield and quality.Citation17 And the pollen viability and stigma receptivity of plants at different flowering periods are also different.Citation18 It is evident that studying the flower characteristics of plants at different flowering periods is helpful to further understand the pollination and fertilization mechanism of plants, especially angiosperms.
Therefore, in order to explore the floral traits at different flowering periods and their effects on pollinator behavior and seed production, in this study, we measured the floral morphology, pollen viability, stigma receptivity, nectar volume and nectar concentration of S. davidii at different flowering periods, and in addition, we also investigated the foraging behavior of A. cerana cerana at different flowering periods and the effects of pollination treatment at different flowering periods on fertilization physiology, seed yield and quality of S. davidii. After collecting all the data, we will try to draw the correlation between flower traits and pollinator behavior, and determine the best pollination period of S. davidii through production performance, which will provide more meaningful reference value for rational pollination of insects, efficient production of seeds and honey production.
Materials and methods
Study site
The study area belongs to the subtropical monsoon climate and is located in the Guizhou Grassland Technology Test Extension Station (N25°04′~25°21′, E107°41′~107°55′), Dushan County, Guizhou Province, China. The lowest and highest temperature in the area was 2°C and 32°C, respectively, the annual precipitation about 1150 mm, and the annual sunshine hours about 1300 h.Citation19
Study materials
The main study was conducted from April to August 2021. The test material were 3-year-old potted S. davidii plants, which were transplanted to the test area upon reaching the flowering conditions. The pollinator was A. cerana cerana Fabricius, which is the locally dominant bee species in this study area.
Experimental method
Observation and determination of floral traits
The division of flowering periods was done according to the population flowering criteria as follows: (1) Initial is when 5% of the sample plants are flowering as the initial flowering period; (2) Full is when over 30% of the sample plants are flowering as the full flowering period; and (3) Final is when over 95% of the sample plants have been flowered as the final flowering period.Citation20
We randomly marked 30 inflorescences from 30 plants of S. davidii, and observed their opening days from the first flower to the last flower. Another 30 flowers from different plants of S. davidii were randomly collected in the initial, full and final flowering periods, respectively. Each flowering periods was done with three repetitions, and each repetition had 10 flowers. The collected flower samples were placed in a centrifuge tube containing FAA fixative, marked and brought back to the laboratory for testing. The length of calyx, filament and pistil, and the length and width of corolla, anther, stigma, vexillum, wing and keel were measured with Vernier caliper with an accuracy of 0.02 mm.
Nectar volume and nectar concentration
We randomly selected 10 inflorescences from 9 plants of S. davidii which isolated by net room at the initial, full and final flowering periods, respectively. The total nectar on each inflorescence was extracted by a 25-µL syringe, and the average nectar volume of each inflorescence was calculated. The nectar concentration was measured by a portable refractometer.
Pollen viability and stigma receptivity
Another 30 flowers from 9 plants were randomly collected in three different periods as described above. The collected flower samples were placed in a centrifuge tube containing FAA fixative, marked and brought back to the laboratory, and then their pollens were dispersed by an oven at 45°C and stored in a refrigerator at 4°C for testing.
The pollen at the initial, full and final flowering periods of S. davidii were taken for histochemical evaluation. The pollen grains was distributed on slides and stained with I2-KI solution. We randomly selected three visual fields to observe the pollen staining. Blue is a well-developed and vigorous pollen grain, while yellow-brown is a poorly developed pollen grain. And the number of pollen grains in each visual field was not less than 100.Citation21 The stigma receptivity was determined by the benzidine-hydrogen peroxide methodCitation22 with composition ratio 1% benzidine: 3% hydrogen peroxide: distilled water = 4:11:22.
Foraging behavior of A. cerana cerana
Twenty seven neighboring S. davidii plants were randomly divided into nine plots (three plants per plot). At the initial flowering stage of S. davidii, three plots were randomly selected to observe the foraging behavior of A. cerana cerana, while the other six plots were covered with nylon nets (100 mesh of size). During the full flowering period, three of the remaining six plots were randomly selected as observation the foraging behavior of A. cerana cerana, while the remaining three plots and three plots at the initial flowering period were covered with the nylon nets. The foraging behavior of A. cerana cerana was observed in the remaining three plots at the final flowering period, while other plots were covered with nylon nets. The indices of foraging behavior was determined by visual inspection, stopwatch timing and camera shooting. All measurements were carried out on sunny and breezy days, and each flowering periods was repeated for 3 d as three repetitions. The definitions of indicators are as follows:
Single visit time: The time it takes for bees to collect nectar and pollen from flowers and leave the same flower.
Visit time per flower: The time it takes for bees to fall on flowers to collect nectar and pollen and temporarily leave flowers to comb pollen.
Whole visiting time: The time from the time when bees enter the pollination plot to collect nectar and pollen and leave the plot.
Number of transfer per minute: The transfer times of bees on flowers in the process of visiting flowers for 1 min.
Visit frequency: The number of flowers visited by bees within 1 min.
Number of touching stigma: The number of times that bees contact the style during the 1 min collection.
Number of visiting bee: The number of bees visiting a flower within 1 min in the pollination community.
Pollination test
Five pollination treatments were set up, that is, bee pollination at initial flowering period (Initial pollination), bee pollination at full flowering period (Full pollination), bee pollination at final flowering period (Final pollination), bee pollination at whole flowering period (Whole pollination) and spontaneous self-pollination at whole flowering period (Self-pollination). Bee pollination treatment was to add A. cerana cerana colonies around the study area and let bees pollinate freely. However, spontaneous self-pollination in the whole flowering period uses the nylon net to cover the plants to avoid the influence of pollinators. Random block arrangement was used to carry out three replicates for each treatment, and three neighboring S. davidii plants were selected for each replication.
(1) Determination the indexes of fertilization physiology: 10 flowers pollinated by A. cerana cerana and 10 flowers pollinated without pollinators were randomly collected at the initial, full and final flowering period, respectively, and put into a centrifugal tube filled with FAA stationary solution and marked. After fixation for 24 h, it was washed with distilled water, and the pistil and ovary were taken out.
The pistils were softened with 8 mol/L NaOH solution and stained with 0.1% aniline blue solution to make slides. The indexes of fertilization physiology which included the amounts of pollen and germinated pollen on the stigma, the numbers of ovules in the ovary and the lengths of pollen tubes, were measured with use of an optical or fluorescence microscope.
(2) Determination of seed yield: The seed yield of S. davidii was evaluated by pod setting rate, seed setting rate and 1000-grain-weight. After pollination, 10 inflorescences were randomly marked in each treatment, and the pod setting situation was counted in the pod setting period. After pods matured, 10 pods were randomly collected in each treatment, and the seed setting situation of each pod was counted. 100 seeds were randomly selected from the seeds obtained after pollination, then weighed and converted into 1000-grain-weight. The counting unit was grams. Each treatment was repeated three times.
(3) Determination of seed quality: Seed quality was evaluated by seed germination test. Fifty seeds with full grains were selected for each treatment. The seeds were evenly placed in a Petri dish covered with filter paper, and placed in a constant temperature (25℃) and humidity (80%) incubator to observe and record the germination of seeds, the length of root and seedlings. Each treatment was repeated three times. The calculation formulas of seed germination rate, germination potential, germination index and total activity were as follows:
Germination rate (%) = (Number of seeds germinated during two weeks/Total number of seeds tested) × 100%
Germination potential (%) = (Number of germinated seeds when the seeds germinate to the peak of the day/Total number of tested seeds) × 100%
Germination index (GI) = ∑ Gt/Dt, (Gt is the number of seeds germinated in t days, and Dt is the corresponding number of seeds germinated)
Vigor index (VI) = GI × S (‘GI’ is the germination index, and ‘S’ is the sum of the finally measured root length and seedling length)
Statistical analysis
The software used for the collation, analysis and drawing of test data were Excel 2016, GraphPad Prism 9 and SPSS 27.0. Most of the data obeyed a normal distribution, while percentage data and nectar volume were transformed by and
, respectively. One-way analysis of variance was used to compare the differences in floral morphology, pollen viability, stigma receptivity, nectar volume and nectar concentration at different flowering periods as well as to compare the effects of different pollination treatments on the seed yield and quality of S. davidii, and the two PC values were extracted by principal component analysis (PCA) to calculate the scores of different pollination treatments and to derive the optimal pollination method. The independent samples t-test was used to compare the changes of indexes of fertilization physiology in S. davidii after bee pollination and spontaneous self-pollination at different flowering periods.
Results
Floral traits
The phenological periods of S. davidii was 9.30 ± 0.24 d in the Guizhou Grassland Technology Extension Station of Dushan County, Guizhou Province, China. The initial, full and final flowering periods were 3.00 ± 0.14 d, 3.43 ± 0.13 d and 2.93 ± 0.16 d, respectively.
As shown in , there was no significant difference in calyx length, anther length, anther width, stigma length and stigma width during the three flowering periods (p > .05). But, the indices of stamen length, corolla length, corolla width, wing length, wing width and keel length at full flowering period were significantly lower than those at initial flowering period and final flowering period (p < .05). Among the three flowering periods, the pistil length at the full flowering period was only significantly longer than the length at the initial flowering period (p < .05). The vexillum length only showed that the length at full flowering period was significantly longer than the length at initial flowering period and the final flowering period (p < .05). The order of vexillum widths were as follows: initial flowering period > final flowering period > full flowering period (p < .05). The keel width at the initial flowering period, was only significantly wider than the width at the full flowering period (p < .05).
Table 1. The floral morphology of S. davidii at different flowering periods.
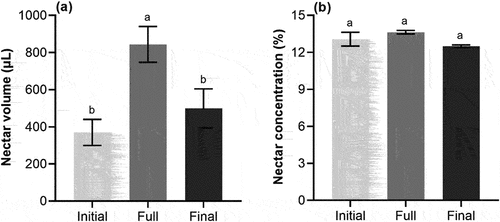
There were significant differences in nectar volume in different flowering periods (p < .05; ), but no significant difference in nectar concentration (p >.05; ). Among them, the maximum volume of nectar is 844 ± 11 μL at full flowering period, followed by 499 ± 2 μL at final flowering period and 370 ± 6 μL at initial flowering period. Although there was no difference in nectar concentration, the highest nectar concentration was 13.6 ± 0.2% at full period.
Figure 1. Mean (±SE) nectar volume (a) and nectar concentration (b) from S. davidii at different flowering periods. Based on three repetitions and each repetition consists of 30 inflorescences per flowering period. Different letters indicate a significant difference in different flowering periods (p < .05).
There were significant differences in pollen viability of S. davidii in different flowering periods (). The results of the I2-KI staining showed that the pollen viability at full flowering period was significantly higher than that at initial and final flowering period. Although the pollen viability at initial flowering period was higher than that at final flowering period, the difference was not significant (p > .05).
Table 2. Pollen viability (mean ± SE) and stigma receptivity of S. davidii at different flowering periods, initial, full and final.
The stigma of S. davidii had a receptivity at each flowering period, but the strongest was at full flowering period (p < .05). It was thus clear that S. davidii had high pollen viability and stigma receptivity at full flowering period.
Foraging behavior
The nectar foraging behavior and pollen foraging behavior of A. cerana cerana on S. davidii are shown in . Because the head and beak of A. cerana cerana cannot suck nectar from the flower base, it is observed that they will bite an opening near the flower base 2/5 (), and this nectar robbing behavior happens in most flowers.
Figure 2. Foraging behavior of A. cerana cerana on S. davidii. (a) Pollen foraging behavior, (b) nectar foraging behavior, (c) flowers that have not been foraged, (d) flowers after foraging. (white arrow: undamaged. Black arrow: damaged.).

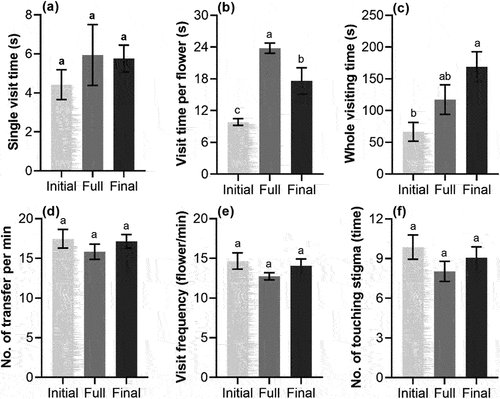
The foraging behavior of A. cerana cerana at different flowering periods of S. davidii is shown in , in which the visit time per flower at full flowering period was significantly more than that at initial flowering period and final flowering period (p < .05), and the time at final flowering period was also significantly more than that at initial flowering period (p < .05). The whole visiting time at the final flowering period was significantly more than that at the initial flowering period (p < .05). However, there was no significant difference in the single visit time, the number of transfer per minute, the visit frequency and the number of touching stigma at different flower periods (p > .05).
Figure 3. Foraging behavior (mean ± SE) of A. cerana cerana at different flowering periods of S. davidii, initial, full and final. (a) Single visit time, (b) visit time per flower, (c) whole visiting time, (d) number of transfer per minute, (e) visit frequency, (f) number of touching stigma. Different letters indicate a significant difference in different flowering periods (p < .05).
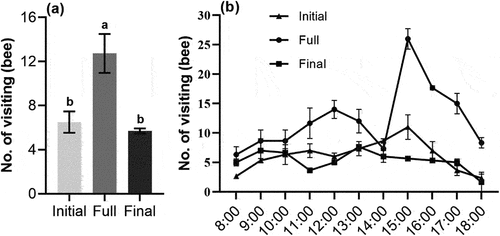
The number of bees visiting flowers in the full flowering period of S. davidii was significantly higher than that at the initial flowering period and the final flowering period (p < .05) (). Although the dynamic changes of the number of bees at different flowering periods were different, they all showed a “double peak” change. Such as the initial flowering period (11:00 and 13:00), the full flowering period (12:00 and 15:00), and the final flowering period (9:00 and 13:00), as well as the number of bees increased sharply at 15:00 in the full flowering period.
Figure 4. The number and dynamics of visiting bees (mean ± SE) at different flowering stages of S. davidii, initial, full and final. (a) Number of visiting bees, (b) the dynamic of visiting bees. Different letters indicate a significant difference in different flowering periods (p < .05).
According to the results of correlation analysis (), there was no significant correlation between the single visit time and total visit time of Chinese honey bees and the floral traits of S. davidii (p > .05), but the visit time per flower, transfer times, visit frequency, times of touching stigma and number of visits were significantly (p < .05) or extremely significantly (p < .01) related to the flower traits.
Table 3. Correlation between floral traits of S. davidii and foraging behavior.
Fertilization physiology
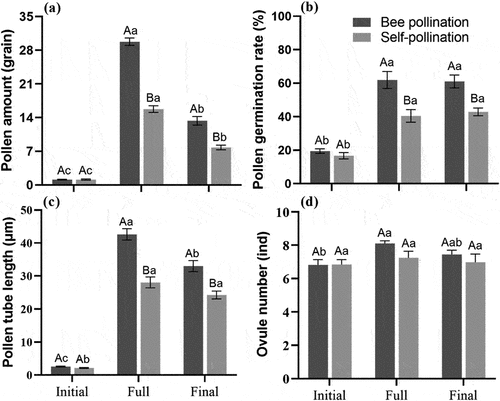
There were significant differences in pollen amount on, pollen germination rate and pollen tube length during different flowering periods under the two pollination methods (; p < .05), and also some differences under different pollination methods at the same flowering period. In terms of stigma pollen amount (), the significance of bee pollination and self-pollination during the three flowering periods was as follows: full flowering period > final flowering period > initial flowering period (p < 0.05). Bee pollination scored significantly higher than self-pollination at full flowering period (F = 0.083, t = 13.587, p < 0.05) and final flowering period (F = 1.225, t = 5.522, p < .05). In terms of pollen germination rate (), both bee pollination and self-pollination showed that the full and final flowering period were significantly higher than the initial flowering period (p < .05), and bee pollination was significantly higher than self-pollination at the full (F = 2.838, t = 3.909, p < .05) and final flowering period (F = 1.643, t = 4.460, p < .05). In terms of pollen tube length (), the significance of bee pollination during the three flowering periods was as follows: full flowering period > final flowering period > initial flowering period (p < .05). While self-pollination showed that the full and final flowering period were significantly greater than the initial flowering period (p < .05), but there was no significant difference between full flowering period and final flowering period (p > .05). Bee pollination was significantly greater than self-pollination at full flowering period (F = 0.001, t = 6.149, p < .05) and final flowering period (F = 0.256, t = 4.235, p < .05). On the number of ovules (), the full flowering period was significantly greater than the initial flowering period only in the presence of the Asian honey bees that were pollinating (p < .05).
Figure 5. Mean (± SE) fertilization physiology indices at different flowering periods in S. davidii after pollination. (a) pollen amount, (b) pollen germination rate, (c) pollen tube length, (d) ovule number. Different lowercase letters indicate a significant differences in different flowering periods (p < .05), and different uppercase letters indicate a significant differences between different pollination methods in the same flowering period (p < .05).
Seed yield
Different pollination treatments have significant effects on pod setting rate, seed setting rate and thousand-grain weight of S. davidii (). Among them, the pod setting rate at full flowering period was the highest, the rate of which was significantly higher than other pollination treatments (p < .05). The seed setting rate in full flowering period, was also the highest, which was significantly higher than other pollination treatments except full flowering period (p < .05). However, the highest thousand-grain weight of seeds was the whole pollination treatment, which was significantly heavier than both treatments of the final and self-pollination (p < .05).
Table 4. Effects of different pollination treatments on seed yield of S. davidii.
Seed quality
The results in show that different pollination treatments had significant effects on the seed quality of S. davidii. Among them, the germination rate of whole flowering pollination was the highest, followed by full flowering pollination, and the final flowering pollination was the lowest. The germination rates of whole flowering pollination and full flowering pollination were significantly higher than other pollination treatments (p < .05). The highest germination potential was whole flowering pollination which was significantly higher than that of other treatments except full flowering pollination (p < .05). And the highest germination index was full flowering pollination which was significantly higher than other pollination treatments (p < .05). However, the highest index of vigor was full flowering pollination, followed by initial flowering pollination, but only the vigor index of full flowering pollination and initial flowering pollination were significantly higher than that of self-pollination (p < .05).
Table 5. Effects of different pollination treatments on seed quality of S. davidii.
Principal component analysis (PCA)
PCA shows that () there were two PC values greater than 1, and their cumulative contribution rate reached 91.36% (76.94% and 14.42%, respectively), which could be used as a comprehensive evaluation index of S. davidii seed yield and quality under different pollination treatments. The principal components, extracted in , were converted into normalized feature vectors. And the score calculation model and comprehensive evaluation model of the two principal components obtained as follows:
Table 6. Principal component extraction of seed yield and quality of S. davidii after different pollination treatments.
Y1 = 0.393X1 +0.420X2 +0.357X3 +0.331X4 +0.386X5 +0.371X6 +0.381 X7;
Y2 = −0.285 X1 +0.134 X2 +0.324X3 +0.505X4 +0.322X5 −0.485X6 −0.450X7;
Y = 0.7694Y1 +0.1442Y2。
In the formula, X1=Pod setting rate,X2=Seed setting rate,X3=Thousand-Grain Weight,X4=Germination rate,X5=Germination potential,X6=Germination index,X7=Vigor index.
The higher the comprehensive score, the more favorable pollination treatment was to increase the yield and improve the quality of S. davidii seeds. It could be seen from that the comprehensive score of pollination at full flowering stage was the highest (2.83), followed by pollination at full flowering stage (0.99), and self-pollination was the lowest (−2.14).
Table 7. Principal component factor scores of seed yield and quality of S. davidii after different pollination treatments.
Discussion
Floral traits and flowering phenology of plants are important for studying the reproductive ecology of plants. Among them, it is accepted that the flowering time and duration, flower structure characteristics and nectar volume have a significant effect on the plant reproductive success and pollinator behavior.Citation10,Citation23 The flowering periods of S. davidii population are happening between March and May, and the flowering periods of a single flower take about 9 d. The times of initial, full and final flowering periods were all a duration of about 3 d The flowering period of a single flower is consistent with the description of the Apicultural Science Association of China.Citation24
The pistil of S. davidii is wrapped in the stamens. The pistil is short and the stamens are long at the initial and final flowering period, while the full flowering period is the opposite. This is similar to some of the interactive dimorphic stamens in family Rubiaceae, which is beneficial for insects to achieve accurate pollination and improve mating efficiency.Citation25 In addition, nectar volume of S. davidii was abundant, and the sugar concentration of nectar was higher (about 13%). The secretion of nectar began at the initial flowering period, while the most vigorous was at the full flowering period, and there was still a high amount of nectar volume at the final flowering period. The nectar volume at the full flowering period was significantly greater than that at the initial and final flowering periods, which could provide a rich nectar reward for pollination insects throughout the phenological period of S. davidii. The result was similar to the nectar secretion rhythm of the new branches of Lycium barbarum, also known as Chinese wolfberry in China or Duke of Argyll’s tea tree in the UK, that is an important commercial shrub crop in northern China.Citation26 But, S. davidii has a peak honey production period of about 20 d, and throughout all the flowering periods it can brew between 20 and 25 kg of first-class honey per colony of bees,Citation24 which is reflecting that S. davidii is an ideal plant of nectar source and honey production.
Pollen, as the male gametophyte of plants, plays an important role in sexual reproduction, and active pollen can germinate on the stigma to complete fertilization.Citation27 But, the pollen viability of S. davidii was changing over the different flowering periods and was as follows: full > initial > final flowering period (), and we believe this may be caused by plant genetic genes or environmental conditions as was also recently reported by Karim et al.Citation28
The period of stigma receptivity is the reproductive period in the process of flower maturation, which can affect to a large extent the self-pollination rate and the pollination rate at different periods after flowering. For example, the stigma receptivity of G. rigescens is weak at the end of the bud, strong at the full flowering period, and strongest at the late of the flowering period, but there is no receptivity at the wilting period. This is in agreement with previous work that the stigma receptivity of flowering plants is different at different opening periods of flowers.Citation29,Citation30 The results of this study showed that the stigma of S. davidii had a strong receptivity at full flowering period, and some stigmas had a receptivity at the initial and final flowering period (), indicating that S. davidii was suitable for pollination during the whole phenophase, but its pollen viability and stigma receptivity were the best at full flowering period.
The collection behavior of bees on pollinating plants is usually used as an evaluation index of bee pollination ability and effectiveness, which is generally influenced by plant resources, environmental factors and their own physiological factors.Citation31,Citation32 It is a mutually beneficial process for bees to visit flowering plants. Plants can provide some rewards for bees, such as nectar and pollen, which are stored in the hive as the energy source for their reproduction. At the same time, the collection behavior of bees increases the chances of flower pollination and improves the cross-pollination rate.Citation25,Citation33 However, due to the limited resources and the change of flower morphology, there are some differences in the behavior of bees in different collection periods. In this study, the visit time (such as single visit time, visit time per flower and whole visiting time) of bees in the initial flowering period was less than that in the full flowering period and the final flowering period, so bees needed to move and visit between different flowers frequently, which led to higher transfer times, visit times and times of touching the stigma (). However, there were many flowers and available resources in the full flowering period, so more time was spent on a flower, which led to the least number of transfer per minute, visit frequency and number of touching stigma in the full flowering periods, but it was not statistically significant among the three flowering periods (). Some researchers believe that the most effective pollinators should be large in number and move quickly between flowers.Citation34 In this study, the bees in full flowering period were the most active, indicating that the full flowering period was an effective period for Chinese honey bees to pollinate S. davidii.
Bee pollination can bring more pollen to the plant stigma, promote pollen germination on the stigma and provide a strong guarantee for the germination of more pollen tubes and double fertilization on the stigma.Citation35 The results of this study demonstrated that the pollen amount and germination rate of S. davidii pollinated by the local bees (A. cerana cerana) was higher than those of self-pollination (). It might be affected by bee pollination, which increases the probability of cross-pollination of S. davidii and promotes pollen germination.Citation36,Citation37 When comparing bee pollination and self-pollination (), the amount of pollen at the initial flowering period were the same, which may be related to the volatile substances of flowers and the frequency of bee visits.Citation38,Citation39
Similar to the pump pollination mechanism of family Papilionidae, the self-pollination efficiency of S. davidii is low, and it depends on strong and specialized pollinators.Citation40–42 In this study, compared with self-pollination at whole flowering period, the pod setting rate, seed setting rate, thousand-grain weight, germination rate, germination potential, germination index and vigor index of bee pollination at whole flowering period increased by 30.54%, 27.66%, 0.37 g, 18.66%, 25.77%, 4.15 and 121.16, respectively ( and ). This increase could be due to frequent foraging activities caused by the introduction of bees which reduces the pollination failure and less number of flowers left without proper pollination.Citation43 From the perspective of pollinator’s body shape, A. cerana cerana may not be the best pollinator of S. davidii, but can significantly improve the seed yield and quality of S. davidii. And pollination by A. cerana cerana during the whole flowering period may be the best way to promote its seed production.
Acknowledgments
We thank the Guizhou Grassland Technology Test Extension Station for providing experimental sites.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhao X, Sun XF, Zhao LL, Huang LJ, Wang PC. Morphological, transcriptomic and metabolomic analyses of Sophora davidii mutants for plant height. BMC Plant Biol. 2022;22(1):144. doi:10.1186/S12870-022-03503-1.
- Chen Q, Huang LC. Sophora davidii——rocky desertification governance and development native shrubs (eds), the fourth Yunnan ecological civilization and ecological economy academic conference papers. 2015.
- Qiu TW, Zhang DD, Shi YX, Long MZ, Li JW, Wang YH, Yang XF, Yi P. Chemical constituents from Sophora davidii flower buds and their butyrylcholinesterase inhibitory activities. Nat Prod Res And Dev. 2020;32(6):980–11. doi:10.16333/j.1001-6880.2020.6.010.
- Wang CW, Chen C, Lin BB, Wu SQ. Research and application progress of alkaloids in Sophora vicifolia. Jiangsu Agr Sci. 2019;47(4):16–21. doi:10.15889/j.issn.1002-1302.2019.04.004.
- Li ZH. Inhibition of lassa virus entry by Sophora davidii extract and its mechanism. Yunan: Dali University; 2024. doi:10.27811/d.cnki.gdixy.2023.000230.
- He Z. Study on the inhibition of Ebola virus entry and its mechanism by extracts of Geranium strictipes R. Knuth and Sophora davidii (Franch.) Skeels. Yunan: Dali University; 2024. doi:10.27811/d.cnki.gdixy.2023.000206.
- Zhao LL, Sun XF, Huang LJ, Wang LT, Zhao X. High-yield cultivation and management techniques of Sophora davidii seeds. Mod Agr Sci Tech. 2020;6:205–206.
- Speroni G, Izaguirre P, Bernardello G, Franco J. Intrafloral phenology of Trifolium polymorphum Poir. (leguminosae) aerial flowers and reproductive implications. Acta Bot Bras. 2009;23(3):881–888. doi:10.1590/s0102-33062009000300029.
- Ceuppens B, Ameye M, Van Langenhove H, Roldan-Ruiz I, Smagghe G. Characterization of volatiles in strawberry varieties ‘Elsanta’ and ‘Sonata’ and their effect on bumblebee flower visiting. Arthropod-Plant Inte. 2015;9(3):281–287. doi:10.1007/s11829-015-9375-y.
- Vleugels T, Ceuppens B, Cnops G, Lootens P, Van Parijs FRD, Smagghe G, Roldan-Ruiz I. Models with only two predictor variables can accurately predict seed yield in diploid and tetraploid red clover. Euphytica. 2016;209(2):507–523. doi:10.1007/s10681-016-1679-1.
- Long ZF, Hou FJ, Zhang JB. Genetic diversity of wild sophora davidii germplasm in Guizhou province based on AFLP markers. Chin J Grassl. 2017;39(2):105–110. doi:10.16742/j.zgcdxb.2017-02-16.
- Tang M, Chen GT, Zheng SW, Mu CL, Xie TZ, Zhong Y, Li YQ. Study on seed rain and natural regeneration of Sophora davidii (Franch.) population in arid valley of the Minjiang river. J Sichuan For Sci Technol. 2020;41(6):29–34. doi:10.12172/202006160001.
- Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. P Biol Sci. 2007;274(1608):303–313. doi:10.1098/rspb.2006.3721.
- Lu YG. Mechanism of bee pollination increasing yield and quality. Gansu Anim Husb Vet Med. 2017;47(7):103–105. doi:10.15979/j.cnki.cn62-1064/s.2017.07.026.
- Ji WY, He M, Wei WJ, Ye WB, Song ZQ, She XY, Yang Y. Preliminary study on the biological characteristics and breeding system of four species of syringa. J Northeast For Univ. 2020;48(12):23–26. doi:10.13759/j.cnki.dlxb.2020.12.005.
- Liu YX, Zhang JJ, Yang SB, Tang J, Wang YY. Flowering biological characteristics of 6 Prunusdomestica - armeniaca varieties. J Northwest A & F Univ (Nat Sci Ed). 2021;50(3):84–90. doi:10.13207/j.cnki.jnwafu.2022.03.011.
- Pauldasan A, Vipin P, Durai A, Mayavel A, Anand GV, Nicodemus A. Floral biology, pollen viability and stigma receptivity in three species of Casuarina. S Afr J Bot. 2023;152:182–191. doi:10.1016/j.sajb.2022.11.044.
- Yang JH, Wu T, Zhu J, Yang SH, Zhao X, Ge H, Wu RH, Jia RD. Studies on flowering characteristics and breeding system of Paphiopedilum dianthum. Acta Hortic Sin. 2021;48(5):1002–1012. doi:10.16420/j.issn.0513-353x.2020-0809.
- Zhu X, Chen GY, Li LX, Wang JH, Luo YJ, Zhao LL. Comparison of production performance and correlation analysis of main characters of silage corn in Guizhou Province. Jiangsu Agr Sci. 2020;48(16):212–216. doi:10.15889/j.issn.1002-1302.2020.16.042.
- Yang PZ, Xie Y, Cao YQ, Xu ZY, Cun MH, Ren HD, Duan EX. Flowering phenophase of Camellia reticulata. For Inventory Plan. 2020;45(5):19–25. doi:10.3969/j.issn.1671-3168.2020.05.004.
- Fu QC, Wang J, Zhang XY, Liu C, Liu F. Study on detection methods for pollen viability of Sinojackia sacocarpa L.Q Luo. Mol Plant Breed. 2015;13(5):1146–1150. doi:10.13271/j.mpb.013.001146.
- Ma YZ, Cui ZJ, Cheng CY, Li R, Wu HX, Jin L, Ma Y, Wang ZH. Flowering characteristics and mating system of Fritillaria cirrhosa (Liliaceae), an endangered plant in China. Braz J Bot. 2022;45(4):1307–1318. doi:10.1007/S40415-022-00844-X.
- Mclntosh ME. Flowering phenology and reproductive output in two sister species of Ferocactus (Cactaceae). Plant Ecol. 2002;159(1):1–13. doi:10.1023/A:1015589002987.
- Apicultural Science Association of China, CAAS Institute of Apicultural Research, Heilongjiang Mudanjiang Institute of Agricultural Sciences. The nectar and pollen plants of China and application. Beijing: Agricultural Press; 1993.
- Consolaro H, Silva SC, Oliveira PE. Breakdown of distyly and pin‐monomorphism in Psychotria carthagenensis Jacq. (Rubiaceae). Plant Spec Biol. 2011;26(1):24–32. doi:10.1111/j.1442-1984.2010.00300.x.
- Luo SD, Wang B, Chu ZQ, Wu J, Yang WS, Zhang JL. Flowering biological characteristics of Lycium barbarum in Guyuan, Ningxia Hui Autonomous Region. Acta Agri Boreali-occidentalis Sin. 2011;20(10):150–156.
- Anita CF, Roslynn D, Nicole J, Lindsay F, Jeffrey G, Matthew HK. Disentangling the components of pollen limitation in a widespread herb with gametophytic self-incompatibility. Am J Bot. 2022;110(2):e16122. doi:10.1002/AJB2.16122.
- Karim K, Awad MA, Manar A, Monia J, Karim A, Mohammed E. Effect of flowering stage and storage conditions on pollen quality of six male date palm genotypes. Saudi J Biol Sci. 2022;29(4):2564–2572. doi:10.1016/J.SJBS.2021.12.038.
- Chen XA, Deng MH, He SY. Florescence, pollen viability and stigma receptivity of Qiubei Chilli. J Yunnan Agric Univ(Nat Sci). 2010;25(3):383–387. doi:10.16211/j.issn.1004-390x(n).2010.03.023.
- Duan SF, Zhu CY, Yu CH, Liang YH. Study on the biological characteristics of pollination of Gentiana rigescens Franch. J Yunnan Agric Univ(nat Sci). 2021;36(4):660–665. doi:10.12101/j.issn.1004-390X(n).202009004.
- Vazquez DP, Morris WF, Jordano P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Lett. 2010;8(10):1088–1094. doi:10.1111/j.1461-0248.2005.00810.x.
- He CL, Zhang KY, Hou XG, Han DB, Wang SB. Foraging behavior and pollination efficiency of Apis mellifera L. on the oil tree peony ‘Feng Dan’ (Paeonia ostii T. Hong et J.X. Zhang). Insects. 2019;10(4):116. doi:10.3390/insects10040116.
- Simpson BB, Neff JL. Floral rewards: alternatives to pollen and nectar. Ann Mo Bot Gard. 1981;68(2):301–322. doi:10.2307/2398800.
- Rader R, Howlett BG, Cunningham SA, Westcott DA, Newstrom-Lloyd LE, Walker MK, Teulon DAJ, Edwards W. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J Appl Ecol. 2009;46(5):1080–1087. doi:10.1111/j.1365-2664.2009.01700.x.
- Shi YY, Guan C, Zeng ZJ, An JD, Luo SD. Yield-increasing effect and mechanism of honeybee on rape pollination. Acta Agr Univ Jiangxiensis. 2009;31(6):994–999+1005. doi:10.13836/j.jjau.2009194.
- Liang SK, Hong RX, Zhou DW, Lu QH, Sun T, Qin XQ, Qin SH, Lu YM. Study on flower visiting behavior and pollination effect of litchi honeybee in Guangxi province. Southwest China J Agr Sci. 2018;31(12):2723–2728. doi:10.16213/j.cnki.scjas.2018.12.043.
- Hong JY, Huang R, Huang CY, Wang JH, Xu YF, Li PP, Hu YY, Huang JQ, Li Y. Research progress and prospects of xenia. Plant Physiol J. 2020;56(2):151–162. doi:10.13592/j.cnki.ppj.2019.0344.
- Alitong Q, Jin XF, Ye ZM, Wang QF, Chen JM, Yang CF. Reproductive ecology research on populations of a medicinal plant (Lycium ruthenicum Murr.) from Xinjiang reveals factors affecting fruit production. Plant Sci J. 2014;32(6):570–576. doi:10.11913/PSJ.2095-0837.2014.60570.
- Guo YW, Peng YQ, Yang P, Zhang T, Hu DM, Zhao RH. Study on pollinating insects and efficiency of Amomum villosum in Jinping county of Yunnan. J Chin Med Mater. 2018;41(9):2048–2052. doi:10.13863/j.issn1001-4454.2018.09.006.
- Arroyo MTK. Breeding systems and pollination biology in leguminosae. In: Polhill RM, and Raven PH, editors. Advances in legume systematics, part 2. Kew: Royal Botanic Garden; 1981. p. 723–769.
- Etcheverry VA, Vogel S. Interactions between the asymmetrical flower of Cochliasanthus caracalla (Fabaceae: Papilionoideae) with its visitors. Flora. 2018;239:141–150. doi:10.1016/j.flora.2017.10.006.
- Alemán MM, Hoc P, Etcheverry ÁV, OrtegaBaes P, Sühring S, LópezSpahr D. Morphological traits in keel flowers of Papilionoideae (Fabaceae) and their relationships with the pollination mechanisms. Plant Syst Evol. 2022;308(6):43. doi:10.1007/s00606-022-01826-y.
- Stanley J, Sah K, Subbanna AR, Preetha G, Gupta J. How efficient is Apis cerana (Hymenoptera: Apidae) in pollinating cabbage, Brassica oleracea var. capitata? Pollination behavior, pollinator effectiveness, pollinator requirement, and impact of pollination. J Econ Entomol. 2017;110(3):826–834. doi:10.1093/jee/tox115.
