?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Imaging adult zebrafish’s cardiac organ is often hampered by the limited volume of the internal cavity. This research study aims to design, manufacture and apply a miniature illumination probe amended with an aluminum-coated polydimethylsiloxane (PDMS) concave cylindrical mirrors as well as 3D-printed casings for probe assembly in a light-sheet fluorescence microscopy to explore its applicability of visualizing adult zebrafish’s ventricle. The system was calibrated and characterized. The experimental results successfully demonstrated the tracking of the ventricular regeneration in female adult zebrafish at multiple time points and can be conducive to other heart-related research in in vivo settings.
1. Introduction
Fluorescence microscopy has become an invaluable and indispensable tool for biological or biomedical investigation owing to its merits of high signal-to-noise ratio, sharp contrast and great specificity. However inevitably, standard conventional microscopes like confocal fluorescence systems, configured in epi or inverted mode, still suffer from their inherent drawbacks such as the limited viewing field, dichroic mirror reducing signal intensity, and severe photo-bleaching and toxicity by large out-of-focus illumination.[Citation1–5] While the photo-beaching and toxicity can be largely reduced by the utilization of near-infrared (NIR) femtosecond pulses, the limited viewing field, dichroic mirror-rendered signal reduction and out of focus illumination were well addressed by an alternative scheme of fluorescence microscopy, termed selective plane of illumination microscopy (SPIM) or lightsheet fluorescence microscopy (LSFM).[Citation6]
Based on a cylindrical lens that generates a thin sheet of light for sample focusing LSFM adopts a configuration of separate illumination and detection path, and has created some advancing avenues for the demands of various sample sizes, finer optical spatial resolution and visualization of the dynamic phenomena. For instance, though initially invented to visualize the green fluorescent protein (GFP)-encoded muscle structure in the transgenic Medaka line Arnie, improvements in the spatiotemporal resolution by an interference-based optical lattice as well as a dual illumination and viewing scheme allow an elucidation into protein dynamics in drosophila melanogaster’s embryogenesis and the neural wiring in C. elegans’ developing brain, respectively.[Citation7,Citation8] Likewise, combining the orthogonal configuration of LSFM and NIR femtosecond pulses-based excitation not only increases the imaging depth into drosophila melanogaster’s embryonic gastrulation but also raises the acquisition speed up to 10 volumetric frames/sec for capturing the heart beating motion in 5-day old zebrafish larvae.[Citation9] Also, a laser raster scanner-equipped LSFM enables reconstruction of multi-viewing planes, tracking the cell position, divisions and migratory path in entire zebrafish embryos,[Citation10] and when implemented with dual opposing illumination, can acquire cell-level volumetric images on 80% of larval zebrafish’s brain tissue at 0.8 Hz.[Citation11] To further enhance contrast and spatial resolution, another integration of LSFM with structure light illumination that interrogates samples by frequency-modulated wavefront patterns have been applied to image the neural activity in 7-day old zebrafish larvae at 7 frames per sec.[Citation12] In other studies unraveled with the beating heart motion in zebrafish larvae during embryogenesis, incorporation of an electrically tunable lens or 3D/3D + time synchronization algorithms helped determine the cardiac blood cell distribution and ascertain the hemodynamic stress necessitated for the trabecular formation, correspondingly.[Citation13,Citation14]
Since most cardiovascular diseases occur in adulthood and late adulthood,[Citation15] LSFM also provides valuable insights into cardiac biology and pathology of adult zebrafish for studying toxicity of doxorubicin,[Citation16] chemotherapy-induced regeneration,[Citation17] myopathy models,[Citation18] and cryoinjury-induced regeneration.[Citation19] Nonetheless, these studies all require the removal of zebrafish heart, fixation by 4% paraformaldehyde (PFA) and dye-staining with an agent like Acid Fuchsin Orange G for optical imaging. Also, a great number of sacrificed samples were often required for the statistical analysis, and in some cases the tissue cleaning and staining agents, which may slightly alter the tissue morphology and structures, were applied to process the removed heart for 3D image analysis.[Citation20]
Up to date, most studies relevant to optically imaging zebrafish heart in live condition can only take place in the larval stage;[Citation21,Citation22] and only magnetic resonance imaging (MRI), which requires a long anesthetic duration for maintaining stability, a lengthy scan and the technical complexity,[Citation23] and lacks the real-time information on heart motion and blood flow,[Citation24] has been used for in vivo observation of adult zebrafish’s cardiac regeneration.[Citation25] Commercially, despite the meticulous elucidation provided by a LSFM unit, Zeiss Z1, into the nervous system and vasculature during larval zebrafish’s embryogenesis,[Citation26,Citation27] the unit is not capable of imaging medium-size animal model like adult zebrafish, and other LSFM systems from Leica, LaVision, Applied Scientific Instrumentation and Luxendo are only suitable for optically transparent, gelatin-mounted or coverslip-held samples like cell lines, bacteria and cleared animal organs.[Citation28–30]
In this research study, the goals are aimed at designing and constructing a fluorescence imaging tool that allows a real-time observation of adult zebrafish’s ventricle, evaluating its imaging ability for monitoring the healing process of the ventricular injury induced by a direct dissection. With such an imaging apparatus, experiments of the injury-induced ventricular regeneration in adult zebrafish can be conducted in vivo with the smallest number of samples possible and without the usage of chemicals for staining, dyeing and tissue clearing. In details, the design and construction of a light sheet fluorescence microscopy (LSFM) equipped with a miniature illumination probe, which allows a direct access to the internal cavity of adult zebrafish, are calibrated and assessed. Expressly, three adult females of the transgenic zebrafish labeled with green fluorescent protein in cardiac cells were utilized to prepare for the model of ventricular injury by dissecting about 15 ∼ 20% of its ventricle which is then imaged with the miniature LSFM for analysis.
2. Materials and methods
emulates the sample mounting in a lightsheet fluorescence microscopy (LSFM) system in that the region of the induced wound is situated in the overlapping focal volumes of the side-illuminated excitation (blue) and low numerical aperture (N.A.) detection (green) beams for image registration.
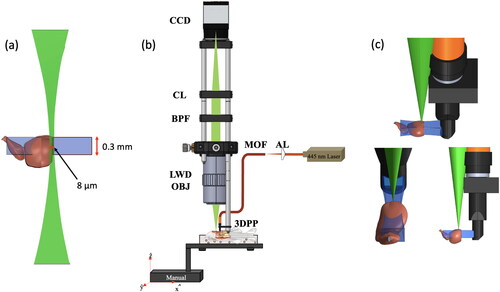
Figure 1. Lightsheet fluorescence microscopy. Orthogonal configuration of (a) the side-illumination and image detection beams relative to the ventricular wound, (b) the component-level optical setup, and (c) the side, frontal and oblique views of the system’s imaging operation are illustrated. CL, BPF, LWD-OBJ, MOF, AL, and 3DPP are abbreviations for condenser lens, bandpass filter, long-working distance objective, multi-mode optical fiber, aspherical lens, and 3D printed probe. Scale bar in (a) is 0.5 mm.
2.1. Miniature lightsheet fluorescence microscopy
Central to the LSFM’s component-level setup shown in is a continuous-wave (CW) 445 nm wavelength laser beam that irradiates an illumination probe for sample interrogation. Specifically, the optical beam is first focused by an aspherical lens (C280TMD-A, Thorlabs, USA) onto a multimode optical fiber (M72L02, Thorlabs, USA). Afterward, the divergent beam exiting from the distal end of the multimode optical fiber gets collimated by a ball lens (2.0 mm Diameter, N-BK7, f = 0.467 mm) and subsequently impinges upon a 45°-tilted home-made aluminum-coated polydimethylsiloxane (PDMS) concave cylindrical mirror that focuses the collimated beam longitudinally along the optical axis to generate a sheet of light for sample excitation. While the ventricular wound of an adult transgenic zebrafish is excited by the illumination beam, the fluorescence images are collected by a plan apo air objective lens (5X, N.A. 0.14, Mitutoyo, Japan), filtered with a 509 nm bandpass filter (25 nm in bandwidth) and focused by a condenser lens (f = 8 cm) onto the sensor surface of a CMOS camera (EO 6412-C, Edmund Optics, USA) for registration at 58.7 frames per second (fps). To acquire the LSFM images, the zebrafish was anesthetized and placed in the slot of a hydrated sponge in order to maintain stability, and the miniature illumination probe was operated with a great care to observe the ventricular wound of an adult zebrafish in vivo, tracking its healing process and reaffirming its regeneration ability. shows the imaging operation seen from the side, front and oblique views as well as the position and orientation of the wounded ventricle relative to the illumination and detection beams.
Design and fabrication of a miniature illumination probe
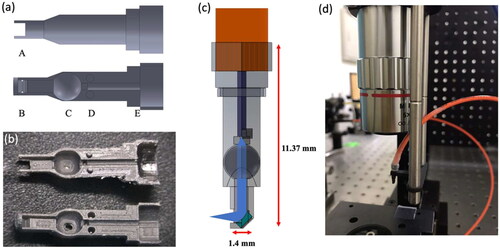
The illumination probe was designed based on the optical requirements both to deliver the excitation light and enable a maximal degree of freedom for the probe maneuver in the spatially limited internal cavity of adult zebrafish. presents the structural design and geometric ray tracing of the illumination probe as well as its implementation in the experimental setup. Principally, Solidworks software was employed to draw the structures of the probe’s upper and lower casings () and a 3D printer (Form2, Formlabs, United States) was used to fabricate these parts shown in . After uploading the 3D design file from the Solidworks software (Dassault Systèmes, 2021, France) onto the 3D printer, a focused laser beam scanned laterally on the resin material to trace out a 2D cross-section pattern according to the machine’s software instructions. The resin in the traced area was rapidly cured, and a thin layer of a component’s structure was formed. This procedure was repeated as a sample platform elevated to harden additional layers that complete the entire 3D structure. Afterward, the remaining resin was cleaned away with isopropyl alcohol for approximately 15 minutes (mins.) using the Form Wash module and the entire printed structure under a rotational motion was uniformly photo-cured by the Form Cure module. There are five main design features in the lower and upper structures labeled A, B, C, D, E. Feature A indicates an opening through-hole that allows light ray to pass through for sample excitation. Feature B’s v-groove design allows a home-made miniature concave mirror to sit in and be held tightly when the upper and lower probe casings are snapped to close. Additionally, the diameter of the probe tip is 1.4 mm which is an optimized size undergone a series of optimization experiments to avoid any potential damage to other organs of the adult zebrafish. Feature C is a circular concave with a diameter of 2.3 mm that holds a 2 mm diameter N-BK7 ball lens (#32744, Edmund Optics, USA); feature D serves as a reference extrusion for an accurate assembly of the entire miniature probe as well as an alignment mark for the multi-mode optical fiber. Likewise, Feature E has a semi-circular groove for the jacket and core of a multimode optical fiber to be accurately positioned at the center of the probe. Moreover, the distal end of a multimode optical fiber is cleaved with an optical fiber cleaver to obtain a flat and clean fiber core surface. Lastly, all components, the multi-mode optical fiber, the ball lens and the PDMS concave mirror were placed in the probe casings and fixed with a super glue, and the entire probe device was bonded using a rubber O-ring. Once the assembly was done, the probe was inserted into a 3D printed probe holder which was then mounted on a 6 mm supporting rod and incorporated in the LSFM.
2.2. Design and fabrication of concave cylindrical mirror
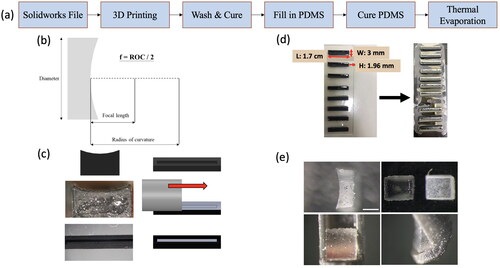
Fabrication of concave mirrors was carried out by designing and making a mold using the 3D printing method described in the previous section, and the entire procedure is shown in (a). Shown in (b) is the optical design of the mirror in that the focal length, f, is one half of the radius of curvature (ROC). In this study, a curved surface with a ROC of 4 mm was carved out, resulting in a focal length of 2 mm. This design was utilized and chosen under consideration of the ventricular size, the dimension of an adult zebrafish’s internal cavity and the distance from the cavity wall to the cardiac ventricle. After the mold was fabricated, the groove in the mold was filled with PDMS (Sylgard 184, 3 M, USA), smeared away with a stainless bar to remove the excessive PDMS outside the groove (), and thermally annealed at 100 °C for an hour. Thereafter, a glass slide sticked with an array of molds was fastened on a holding plate which is mounted in the sample chamber of a thermal evaporator for metal deposition. An electron beam was activated with an input of the electrical current that sets the deposition rate ranging from 0.8 to 1.2 Å/s. A shutter was open to initiate the pre-melting process, and the deposition of aluminum (Al) vapor continues for around 30 mins. until the thickness of 200 nm is attained. (d) illustrates the PDMS-filled molds before and after the Al deposition. Furthermore, the close-up images of a final mirror product seen from the lateral, front, and oblique views are depicted in (e). Also note that the transparent PDMS mold before and after Al-deposition stand in sharp contrast and are presented in the upper right-hand picture of (e) and the photos in the lower row were acquired after being mounted in the illumination probe.
Figure 3. Fabrication of concave mirrors. (a) a process flow of the fabrication method of the mirror mold, (b) the mirror’s optical design, (c) the PDMS filling procedure, (d) the PDMS-filled mold before and after the thermal deposition of aluminum films and (e) photos of a final product arranged in the counterclockwise fashion illustrating the lateral, front and oblique views starting in the upper left-hand corner are depicted. The picture in the upper right-hand corner of (e) is the zoomed-in of (d). A scale bar in (e) is 0.5 mm for all images.
2.3. Ventricular surgery of adult zebrafish
The zebrafish strain used in this experiment was Tg(BMP4:EGFP) purchased from the National Institute of Health, Taiwan, and the experiment was conducted in accordance to the institutional guideline of National Yang Ming Chiao Tung University. The zebrafish rearing was carried out in a fish tank conditioned at the temperature of 28.0 ± 0.5 °C, 7.0 ± 0.5 in pH value, and
< 48 mg/L, and
<0.001 mg/L alongside a light/dark cycle of 14/10 hours. The zebrafish were fed with paramecia and brine shrimp twice a day. To perform the ventricular resection surgery, an adult zebrafish was first anesthetized by gently moving it in a bath of 0.03% tricaine solution for about 60-180 seconds until its gill movement was decelerated. The anesthetized fish was then put in a groove-shaped slot on a hydrated sponge with its head pointing to the left side and its ventricular apex facing upward. Afterward, the fish-loaded sponge was placed under a stereo microscope, and the focal plane of the microscope was finely adjusted to bring the beating heart into view. By placing the tips of a tweezer on the pectoral fin muscle and gently lifting it upwards, a scissor (15000-08, Fine Science Tools, Germany) was used to carefully remove the top layer of the skin that covers the zebrafish heart, thereby exposing the ventricle for resection. A mechanical pressure was then applied to the abdomen using a tweezer and a curved scissor until 30–50% of the ventricle is visible, and the heart beating motion was reduced by compressing the ventricle. Once the ventricular movement slowed down, the scissor was cautiously slid towards the ventricle, and resected 15–20% of the ventricular apex. Immediately after the ventricular resection, a twisted tissue paper was used to blot and stop the bleeding, and the injured zebrafish was gently transferred to a water-filled recovery tank with a plastic spoon, and a plastic pipette was used to slowly and continuously inject water into the gills until the fish was capable of swimming independently. Also, the flow of the tank’s water circulation is set to a low speed for the next 24 hours, and an attentive monitoring of the water quality was necessitated to avoid any contaminants or metabolic waste.
2.4. Quantitative analysis of ventricular recuperation
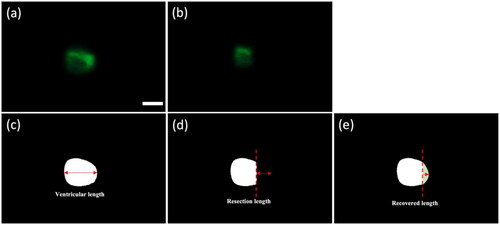
The LSFM images depicting the ventricular recuperation were displayed through uEye cockpit software, which is developed by IDS Imaging Development Systems, providing a comprehensive and user-friendly interface for managing and configuring the CCD camera. To quantitatively evaluate the extent of the ventricular recovery, the ImageJ software was then utilized to analyze the tissue before and after the resection (). A threshold-color function was used to present and accentuate the fluorescent region in the images with a specific brightness level as shown in . The region of the ventricular apex under the full relaxation and contraction was examined at the selected observation points, 0th day post-injury (DPI), 1st DPI, 7th DPI, 14th DPI, 21st DPI and 30th DPI. The analytic method in the following provides numerical estimations of the resection length and the percentage of recovery. The resection length (indicated by a two-way arrow in ) from the 1st DPI was determined by comparing the ventricular length on 1st DPI with that of the pre-surgery length (0th DPI). On the 7th DPI, the resection length with respect to the pre-surgical length was obtained and compared with the measurement taken on the 1st DPI, which helps compute the recovery length (indicated in ) on the 7th DPI by subtracting the resection length on the 1st DPI from that of the 7th DPI. The same procedure was iterated on the 14th DPI, 21st DPI and 30th DPI, and can be expressed as
(1)
(1)
Figure 4. Analysis of the ventricular recuperation. The LSFM images of the ventricle (a) before and (b) after the resection surgery, their corresponding images after the threshold-color processing in (c, d), and (e) a hypothetical recovered length are illustrated. Scale bar in (a) is 0.5 mm, applicable to all other images.
Likewise, the percentage of recovery can then be estimated by dividing the recovered length over the resection length measured from the previous DPI. By analyzing the results obtained from the weekly based observation, the functionality of the miniature illumination probe is demonstrated, and the ventricular recuperating process can be quantitatively evaluated.
3. Results
The knife-edge method was utilized to characterize the dimensions of the focal volumes created by the Al-coated concave mirror in the miniature illumination probe as well as the detection objective lens. Expressly, point-spread functions (PSF) of the illumination and detection focal volumes along the axial (optical) and lateral axes were measured using a single fluorescin-coated 60 nm CsWO3 NP (fNPs) embedded in a PDMS membrane ((d)), which is made by a mixture with the fNPs at low concentration (0.01 mg/mL), shown in (a). Likewise, two thin PDMS membranes infused with the fNPs of 0.1 mg/mL ((b)) and 0.3 mg/mL ((c)) were produced to calibrate the orthogonality of the illumination and detection beams, yielding 26 µm in the depth of field. shows the axial and lateral PSFs of the illumination and detection beams’ focal volumes, yielding 500 µm and 300 µm, and 11 μm and 8 μm, respectively. As can be seen from the estimated figures, the axial and lateral lengths of the illumination beam’s focal volume are 45 and 37 times those of the detection beam, which explains the design scheme of the miniature LSFM in that an appropriate size of the focused illumination beam is necessitated for visualization and quantification of the ventricular wound.
Figure 5. Fluorescence images of PDMS membranes infused with (a) a single fluorescein-coated CsWO3 NP and fluorescin-coated NPs of (b) 0.1 mg/mL and (c) 0.3 mg/mL in concentration, and (d) a brightfield image of the membrane are presented. Scale bars in (a), which is applicable for all fluorescence images, and (d) are 100 µm and 1 cm, respectively.
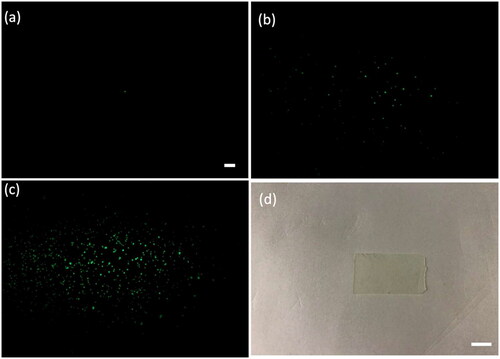
Figure 6. Point-spread functions of the (a) illumination and (b) detection beams’ focal volumes along the lateral and axial axes are illustrated. The illumination optical power at the focal plane was 2.5 mW.
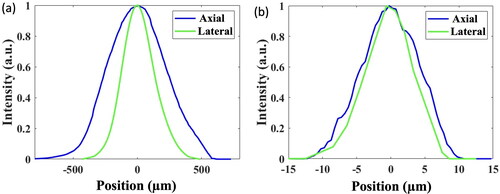
Afterward, the miniature LSFM was applied to acquire fluorescence images of adult zebrafish’s ventricle, assessing applicability of the illumination probe to real-time imaging the resection-induced ventricular wound and to monitoring the entire recuperation process weekly for a month. Since the image acquisition was constrained by the damage threshold of the Al film coated on the concave mirror at around 5 mW, beyond which the Al film may be peeled off by the excessive optical power, the raw images shown in Figs. S1 and S2 in supplementary file were brightness-adjusted using ImageJ software before analysis. and depict the enhanced LSFM images of the adult zebrafish’s ventricles under a full relaxation and a full contraction acquired at 0th (Pre-surgery) DPI (Day-Post-Injury), 1st DPI, 7th DPI, 14th DPI, 21st DPI and 30th DPI, correspondingly.
Figure 7. Enhanced fluorescence images of the adult zebrafish’s ventricle under a full relaxation acquired before surgery (0th DPI) and after 1st DPI, 7th DPI, 14th DPI, 21st DPI and 30th DPI. Three adult zebrafish (N = 3) under examination were denoted as A, B, and C. DPI stands for day-post-injury. The illumination optical power at the focal plane is 2.5 mW. The scale bar is 0.5 mm, applicable to all images.
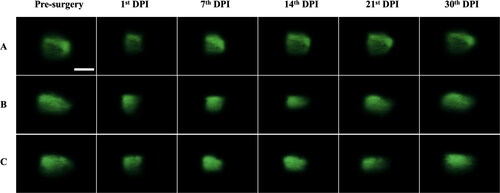
Figure 8. Enhanced fluorescence images of the adult zebrafish’s ventricle under a full contraction acquired before surgery (0th DPI) and after 1st DPI, 7th DPI, 14th DPI, 21st DPI and 30th DPI. Three adult zebrafish (N = 3) under examination were denoted as A, B, and C; DPI stands for day-post-injury. The illumination optical power at the focal plane is 2.5 mW. The scale bar is 0.5 mm, applicable to all images.
4. Discussion
Imaging adult zebrafish heart in vivo on a routine basis has long been impeded by a handful of technical issues like the spatial constrain of the internal cavity, unavailability of a probing device that allows a direct access to the organ, and lack of a surgical procedure that can keep the fish sample alive at multiple observation points. In this study, a lightsheet fluorescence microscopy (LSFM) equipped with a miniature illumination probe and a wide detection scope was constructed, calibrated and successfully applied to assessing its ability in extracting fluorescence images of the surgically resected ventricle inside the cardiac chamber of a female adult zebrafish. The entire recuperating process was monitored at the designated 0th DPI, 1st DPI, 7th DPI, 14th DPI, 21st DPI and 30th DPI when a fully recovered ventricle can be observed.
As presented in and the fluorescence images of 1st DPI indicate a reduction in the fluorescent region when compared with the pre-surgery (0th DPI) images, exhibiting a partial removal of the ventricular tissue. As the recovery progressed, a gradual regrowth of the ventricular tissue surrounding the wound can be seen from the lengthened ventricle. The fluorescence images taken on 7th, 14th, and 21st DPI display a progressively recovering trend by the inching of the fluorescent region, which suggests a continuous wound repair and regeneration by the newly formed cardiac cells that repopulate the damaged region. By the 30th DPI, one can observe that the resected wound narrows toward a complete recuperation over a one-month period, resembling the pre-surgical ventricle apex.
Using the fluorescence images in videos recorded by the CCD camera, the ventricular length in the viewing scope under the full contraction and relaxation can be measured and analyzed quantitatively. depicts the recuperating trends of the wound including the ventricular length, recovered length and recovery percentage.
Figure 9. Analysis of the ventricular recuperation. The measurement of (a) the ventricular length, and the computational results of (b) the recovered length and (c) the recovery percentage are depicted. N = 3 for the statistical analysis. PS stands for pre-surgery.
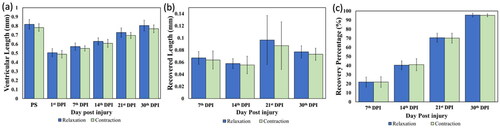
When compared with the pre-surgery ventricle, the wounded ventricle underwent the recovery process since the 1st DPI until a complete recuperation was observed on the 30th DPI. Note that the recovered ventricular length () stays at around 0.05 to 0.06 mm for the first 2 weeks of the recuperating period and undergoes the greatest extent of recovery on the 21st DPI when the recovered ventricular length tops 0.096 ± 0.04 mm and 0.087 ± 0.039 mm at the full relaxation and contraction, correspondingly, and settles down to 0.077 mm and 0.073 mm on the 30th DPI. Likewise, the recovery percentage also exhibits a monotonical growth from the 7th DPI to the 30th DPI, which further substantiates the recuperating trend of the wound over a span of one month; a complete detail of the data measurements, computation and statistical analysis of the ventricular length under the full relaxation and contraction are respectively shown in the supplementary file’s tables 1 and 2.
In summary, this study presents a miniature illumination probe-equipped LSFM that generates a thin sheet of excitation light with the beam dimension designed for the ventricular size of adult zebrafish and extracts the fluorescence images of the ventricular wound using a long working distance detection objective lens. Despite a lack of the structural details and cellular morphology in fluorescence images when compared to those acquired with the heart slicing and staining,[Citation31,Citation32] the system developed in this study is capable of long-term tracking and the real-time image recording of adult zebrafish’s ventricular wound at the selected time points without animal sacrifice and cardiac removal. Furthermore, this imaging technique can evade the usage of chemicals applied in the histological staining for tissue visualization and increase the authenticity of the experimental results. In the future, this imaging technique can also be applied to investigate some cardiac parameters like the rhythmic pulsation, ventricular ejection volume and blood volume flow rate.
5. Conclusion
This research designed, manufactured a miniature illumination probe, and implemented it in a light-sheet fluorescent microscopy (LSFM) to gain an access to the internal cavity of adult zebrafish for in vivo imaging the resected ventricle and monitoring its recuperation at the selected time points.
Empirically, the system was characterized and calibrated for the axial and spatial resolution of the illumination and detection beams, depth of field, and dimension of the viewing scope, and successfully tracked the recuperating progression of adult zebrafish’s resected ventricles. Three adult zebrafish were used for the statistical analysis. The LSFM images illustrated a noticeable ventricular recovery each week, and by the 30th DPI, an average of 95% recovery was observed, demonstrating the LSFM’s capability of real-time observation at the slated time points. Lastly, the research findings of this work indicate that the use of a miniature illumination probe in LSFM allows in vivo monitoring and tracking of the recuperation process in adult zebrafish’s internal cavity and potentially offers a new strategy and approach for investigating the zebrafish models of cardiac diseases in in vivo settings.
Supplemental Material
Download PDF (341.4 KB)Acknowledgements
Ho-Ching Hsiao likes to thank Prof. Chih-Ming Wang for helping deposit aluminum thin films on the concave mirrors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- So, P. T. C.; Dong, C. Y.; Masters, B. R.; Berland, K. M. Two-Photon Excitation Fluorescence Microscopy. Annu. Rev. Biomed. Eng. 2000, 2, 399–429. DOI: 10.1146/annurev.bioeng.2.1.399.
- Ragazzi, M.; Piana, S.; Longo, C.; Castagnetti, F.; Foroni, M.; Ferrari, G.; Gardini, G.; Pellacani, G. Fluorscence Confocal Microscopy for Pathologists. Mod. Pathol. 2014, 27, 460–471. DOI: 10.1038/modpathol.2013.158.
- Hu, P. S.; Hsueh, C. M.; Su, P. J.; Chen, W. L.; Hovhannisyan, V. A.; Chen, S. J.; Tsai, T.-H.; Dong, C.-Y. The Use of Second-Order Susceptibility as Contrast Mechanism for Label-Free Imaging of Biological Tissue. IEEE J. Select. Topics Quantum Electron. 2012, 18, 1326–1334. DOI: 10.1109/JSTQE.2011.2174619.
- Helmchen, F.; Denk, W. Deep Tissue Two-Photon Microscopy. Nat. Methods. 2005, 2, 932–940. DOI: 10.1038/nmeth818.
- Svoboda, K.; Yasuda, R. Principles of Two-Photon Excitation Microscopy and Its Applications to Neuroscience. Neuron. 2006, 50, 823–839. DOI: 10.1016/j.neuron.2006.05.019.
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E. H. K. Optical Sectioning Deep inside Live Embryos by Selective Plane Illumination Microscopy. Science. 2004, 305, 1007–1009., DOI: 10.1126/science.1100035.
- Chen, B.-C.; Legant, W. R.; Wang, K.; Shao, L.; Milkie, D. E.; Davidson, M. W.; Janetopoulos, C.; Wu, X. S.; Hammer, J. A.; Liu, Z.; et al. Lattice Light-Sheet Microscopy: imaging Molecules to Embryos at High Spatiotemporal Resolution. Science. 2014, 346, 1257998. DOI: 10.1126/science.1257998.
- Wu, Y.; Wawrzusin, P.; Senseney, J.; Fischer, R. S.; Christensen, R.; Santella, A.; York, A. G.; Winter, P. W.; Waterman, C. M.; Bao, Z.; et al. Spatially Isotropic Four-Dimensional Imaging with Dual-View Plane Illumination Microscopy. Nat. Biotechnol. 2013, 31, 1032–1038. DOI: 10.1038/nbt.2713.
- Truong, T. V.; Supatto, W.; Koos, D. S.; Choi, J. M.; Fraser, S. E. Deep and Fast Live Imaging with Two-Photon Scanned Light-Sheet Microscopy. Nat. Methods. 2011, 8, 757–760. DOI: 10.1038/nmeth.1652.
- Keller, P. J.; Schmidt, A. D.; Wittbrodt, J.; Stelzer, E. H. Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy. Science. 2008, 322, 1065–1069. DOI: 10.1126/science.1162493.
- Ahrens, M. B.; Orger, M. B.; Robson, D. N.; Li, J. M.; Keller, P. J. Whole-Brain Functional Imaging at Cellular Resolution Using Light-Sheet Microscopy. Nat. Methods. 2013, 10, 413–420. DOI: 10.1038/nmeth.2434.
- Liu, Y.; Dale, S.; Ball, R.; VanLeuven, A. J.; Sornborger, A.; Lauderdale, J. D.; Kner, P. Imaging Neural Events in Zebrafish Larvae with Linear Structured Illumination Light Sheet Fluorescence Microscopy. Neurophotonics. 2019, 6, 015009. DOI: 10.1117/1.NPh.6.1.015009.
- Lee, J.; Fei, P.; Packard, R. R. S.; Kang, H.; Xu, H.; Baek, K. I.; Jen, N.; Chen, J.; Yen, H.; Kuo, C.-C. J.; et al. 4-Dimensional Light-Sheet Microscopy to Elucidate Shear Stress Modulation of Cardiac Trabeculation. J. Clin. Invest. 2016, 126, 1679–1690. DOI: 10.1172/JCI83496.
- Fahrbach, F. O..; Voigt, F. F.; Schmid, B. ; Helmchen, F. ; Huisken, J. et al. Rapid 3D light-sheet microscopy with a tunable lens. Opt. Express 2013, 21, 21010–21026. DOI: 10.1364/OE.21.021010.
- Rodgers, J. L.; Jones, J.; Bolleddu, S. I.; Vanthenapalli, S.; Rodgers, L. E.; Shah, K.; Karia, K.; Panguluri, S. K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. DOI: 10.3390/jcdd6020019.
- Packard, R. R. S.; Baek, K. I.; Beebe, T.; Jen, N.; Ding, Y.; Shi, F.; Fei, P.; Kang, B. J.; Chen, P.-H.; Gau, J.; et al. Automated Segmentation of Light-Sheet Fluorescent Imaging to Characterize Experimental Doxorubicin-Induced Cardiac Injury and Repair. Sci. Rep. 2017, 7, 8603. DOI: 10.1038/s41598-017-09152-x.
- Baek, K. I.; Ding, Y.; Chang, C.-C.; Chang, M.; Sevag Packard, R. R.; Hsu, J. J.; Fei, P.; Hsiai, T. K. Advanced Microscopy to Elucidate Cardiovascular Injury and Regeneration: 4D Light-Sheet Imaging. Prog. Biophys. Mol. Biol. 2018, 138, 105–115. DOI: 10.1016/j.pbiomolbio.2018.05.003.
- Dvornikov, A. V.; de Tombe, P. P.; Xu, X. Phenotyping Cardiomyopathy in Adult Zebrafish. Prog. Biophys. Mol. Biol. 2018, 138, 116–125. DOI: 10.1016/j.pbiomolbio.2018.05.013.
- Marques, I. J.; Sanz-Morejon, A.; Mercader, N. Ventricular Cryoinjury as a Model to Study Heart Regeneration in Zebrafish. Methods Mol. Biol. 2021, 2158, 51–62.
- Ellman, D. G.; Slaiman, I. M.; Mathiesen, S. B.; Andersen, K. S.; Hofmeister, W.; Ober, E. A.; Andersen, D. C. Apex Resection in Zebrafish (Danio rerio) as a Model of Heart Regeneration: A Video-Assisted Guide. Int. J. Mol. Sci. 2021, 22, 5865. DOI: 10.3390/ijms22115865.
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.-F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D. Y. R.; et al. In Vivo Cardiac Reprogramming Contribute to Zebrafish Heart Regeneration. Nature. 2013, 498, 497–501. DOI: 10.1038/nature12322.
- Kaveh, A.; Bruton, F. A.; Buckley, C.; Oremek, M. E. M.; Tucker, C. S.; Mullins, J. J.; Taylor, J. M.; Rossi, A. G.; Denvir, M. A. Live Imaging of Heart Injury in Larval Zebrafish Reveals a Multi-Stage Model of Neutrophil and Macrophage Migration. Front. Cell Dev. Biol. 2020, 8, 579943. DOI: 10.3389/fcell.2020.579943.
- Hamilton, J.; Franson, D.; Seiberlich, N. Recent Advances in Parallel Imaging for MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 71–95. DOI: 10.1016/j.pnmrs.2017.04.002.
- Saeed, M.; Van, T. A.; Krug, R.; Hetts, S. W.; Wilson, M. W. Cardiac MR Imaging: current Status and Future Direction. Cardiovasc. Diagn. Ther. 2015, 5, 290–310.
- Koth, J.; Maguire, M. L.; McClymont, D.; Diffley, L.; Thornton, V. L.; Beech, J.; Patient, R. K.; Riley, P. R.; Schneider, J. E. High-Resolution Magnetic Resonance Imaging of the Regenerating Adult Zebrafish Heart. Sci. Rep. 2017, 7, 2917. DOI: 10.1038/s41598-017-03050-y.
- Kugler, E. C.; Rampun, A.; Chico, T. J. A.; Armitage, P. A. Segmentation of the Zebrafish Brain Vasculature from Light Sheet Fluorescence Microscopy Datasets. BioRxiv. 2020, 213843.
- Park, O. K.; Kwak, J.; Jung, Y. J.; Kim, Y. H.; Hong, H. S.; Hwang, B. J.; Kwon, S. H.; Kee, Y. 3D Light-Sheet Fluorescence Microscopy of Cranial Neurons and Vasculature during Zebrafish Embryogenesis. Mol. Cells. 2015, 38, 975–981.
- Venkataraju, K. U.; Gornet, J.; Murugaiyan, G.; Wu, Z.; Osten, P. Development of Brain Templates for Whole Brain Atlases. Proc. SPIE 10865, Neural Imag. Sens. 2019, 2019, 1086511.
- Parthasarathy, R. Monitoring Microbial Communities Using Light Sheet Fluorescence Microscopy. Curr. Opin. Microbiol. 2018, 43, 31–37. DOI: 10.1016/j.mib.2017.11.008.
- Diosdi, A.; Hirling, D.; Kovacs, M.; Toth, T.; Harmati, M.; Koos, K.; Buzas, K.; Piccinini, F.; Horvath, P. Cell Lines and Clearing Approaches: A Single-Cell Level 3D Light-Sheet Fluorescence Microscopy Dataset of Multicellular Spheroids. Data Brief. 2021, 36, 107090. DOI: 10.1016/j.dib.2021.107090.
- Kim, J.; Wu, Q.; Zhang, Y.; Wiens, K. M.; Huang, Y.; Rubin, N.; Shimada, H.; Handin, R. I.; Chao, M. Y.; Tuan, T. L.; et al. PDGF Signaling is Required for Epicardial Function and Blood Vessel Formation in Regenerating Zebrafish Hearts. Proc. Natl. Acad. Sci. USA. 2010, 107, 17206–17210. DOI: 10.1073/pnas.0915016107.
- Lai, S.-L.; Marín-Juez, R.; Moura, P. L.; Kuenne, C.; Lai, J. K. H.; Tsedeke, A. T.; Guenther, S.; Looso, M.; Stainier, D. Y. Reciprocal Analyses in Zebrafish and Medaka Reveal That Harnessing the Immune Response Promotes Cardiac Regeneration. Elife. 2017, 6, e25605. DOI: 10.7554/eLife.25605.