?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose
The prognostic role of TET2 and/or ASXL1 mutations which are common gene mutations in chronic myelomonocytic leukemia (CMML) remains controversial. Therefore, we conducted this meta-analysis to evaluate the prognostic efficacy of ASXL1 and TET2 mutations in CMML population.
Methods
PubMed, Cochrane and Embase for relevant research were employed to identify 16 studies. Overall survival rate (OS) with hazard ratios (HRs) was used for analysis, and each individual HR was applied to calculate the combined HR.
Results
The total HR of OS was 0.74, 95% CI = 0.61 - 0.91, P = 0.005, compared with CMML patients without TET2 mutations (TET2MT), and the total HR of OS was 1.56, 95% CI = 1.34 - 1.80, P = 0.000, compared with CMML patients without ASXL1 mutation (ASXL1WT), indicating that TET2MT and ASXL1WT were favorable for prognosis of CMML. According to whether the gene is mutated or not, the acute transformation rate of disease and mortality rate were further considered for assessment. Compared with the CMML patients with TET2MT and ASXL1WT, the HR of patients with in both TET2MT and ASXL1MT was 1.51 (95% CI = 1.14 - 1.99; P = 0.004), the HR of patients with neither TET2MT nor ASXL1MT was 1.49 (95%CI = 1.12 - 1.98; P = 0.007), and the HR of TET2WT and ASXL1MT patients was 1.88 (95%CI = 1.21 - 2.94; P = 0.005).
Conclusion
Presence of TET2MT and ASXL1WT genotype was the most beneficial for the survival of CMML patients.
1. Introduction
Chronic myelomonocytic leukemia (CMML) is a disease with overlapping features of myelodysplastic syndromes (MDS) and myeloproliferative neoplasm (MPN), which average median survival time for patients is about 30 months. The inherent risk of leukemia transformation is about 15-20% within 3–5 years [Citation1]. In the guideline of World Health Organization (WHO) for myeloid neoplasms classification revised in 2016, CMML is defined as a clonal hematopoietic stem cell disease characterized by the presence of sustained (>3 months) peripheral blood (PB) mononucleosis (>1×109/L, with monocytes≥10% of the white blood cell count) and myelodysplastic (BM) [Citation2]. According to diverse clinicopathology, it is divided into 2 types: CMML type 1 (CMML-1) with less than 10% medullary and less than 5% peripheral blasts, and CMML type 2 (CMML-2) with 10% to 19% medullary and/or 5% to 19% peripheral blasts [Citation3]. Hypomethylating agents (HMA) and therapeutic allogeneic blood or bone marrow transplantation (BMT) are still the most available standard treatment regimens. However, BMT is a rarely feasible treatment option because of the age and/or the complications in CMML patients [Citation4, Citation5]. And also therapeutic drugs such as HMA cannot significantly change the natural history of CMML, therefore, effective and available treatments or strategies need to be upgraded [Citation6].
Previous studies have shown that there are gene mutations in more than 90% of CMML cases [Citation7]. In recent years, with the development of next-generation sequencing technology, new somatic mutations might play an important role in the pathogenesis and clinical prognosis of CMML. Epigenetic regulators (TET2∼60%), modulators of chromatin (ASXL1∼40%) are often involved in CMML [Citation8]. TET2 (ten-eleven translocation (TET) oncogene family member 2—chromosome 4q24), a member of the TET protein family (TET1-TET3), is the only gene frequently mutated in the TET family in medullary malignancies, especially in CMML. TET2MT (TET2 mutation) inhibits the hydroxymethyl catalytic activity of epigenetic enzymes and can lead to low levels of 5-hmc in genomic DNA, which will disrupt hematopoietic differentiation [Citation8–11]. Some studies reported there was no significant relationship between TET2 mutation and overall survival (OS) in overall CMML population [Citation12–15]. However, a few studies also discovered that the OS of CMML-1 patients with TET2MT was shortened, while the OS of TET2MT patients was significantly better than that of TET2WT (TET2 wild type) patients [Citation12]. Therefore, the prognostic effect of TET2 mutation in CMML still remains controversial.
The additional sex combs like 1 (ASXL1) gene involved in modulators of chromatin is located on human chromosome 20q11 and encodes a highly conserved protein [Citation13, Citation16]. Similarly, the ASXL1 gene is also one of the most common mutated genes in malignant myeloid diseases. It was reported that myeloid tumors with the highest frequency of ASXL1 gene mutations were also CMML in MDS/MPN [Citation14]. ASXL1 mutation is also related to the acute transformation of CMML. Patients with ASXL1MT (ASXL1 mutation) are prone to transform into acute myeloid leukemia (AML) [Citation15, Citation17]. And this AML caused by CMML is a high-risk subtype, usually associated with poor prognosis and the abnormal changes related to myeloproliferation [Citation18]. There were studies reporting that ASXL1 gene mutation was associated with a lower survival rate and decrease in CMML [Citation19, Citation20]. It has been reported that 9 patients treated with hypo methylation medication during follow-up period, the prognosis of ASXL1MT was poor among 79 CMML patients [Citation21]. However, data reported by MM Patnaik et al. showed that ASXL1MT had no effect on OS or leukemia-free survival in patients with CMML [Citation22]. But there were a few studies indicating that ASXL1 has no relationship with prognosis. So, ASXL1, as an independent prognostic factor of CMML, was to be suspected.
Then, how about TET2 and ASXL1 combinative efficacy? The studies have combined two more representative genes in CMML disease and found that there was no obvious interaction between TET2 and ASXL1 status [Citation21]. However, there were some studies supporting that CMML patients with both ASXL1WT (ASXL1 wild type) and TET2MT has a better prognosis. [Citation23–26]. Therefore, we evaluated the efficacies of TET2 and/or ASXL1 mutations for prognosis prediction of CMML, and also accessed whether the potential interaction between TET2 and ASXL1 played as beneficial predictor in CMML. In this study, we conducted the meta-analysis focused on fully understand the optimal strategies for the clinical prognosis of CMML.
2. Methodology
2.1. Study selection
The study was carried out based on the PRISMA statement of systematic reviews and meta-analysis [Citation27]. Before searching the literature, all members had confirmed and approved the research protocol. Until March 18, 2021, we have searched electronic databases including PubMed, Cochrane (Cochrane database of systematic reviews) and Embase for relevant research. We used the following MeSH words and free words which were combined with Boolean operations: (‘TET2’ and/or ‘ASXL1’) and (‘CMML’ or ‘Chronic myelomonocytic leukemia’). The search was limited to human research and there were no language restrictions. Search the qualified articles from the above-mentioned databases and obtain additional articles by manually searching references of related studies.
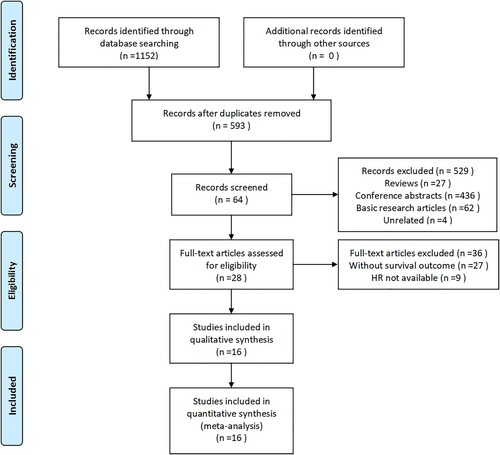
The search strategy received 1152 literature. Literature was included in this meta-analysis if they met the following criteria: (1) Provide detailed information of patients with ASXL1 and/or TET2 mutations. (2) According to ASXL1 and/or TET2 gene mutations, patients in the original study were clearly grouped. (3) Reported survival results or hazard ratio (HR) of clinicopathological data were based on the expressions of mutated and unmutated ASXL1 and/or TET2. (4) The presented survival curve or other sufficient data could be used to calculate 95% CI of HR. Literature was excluded if one of the following criteria existed: (1) In vitro or laboratory studies; (2) Overlapping studies; (3) Review or case reports; (4) Letters and comments to editor; (5) Studies with unclear groupings; (6) Studies with no obvious prognostic indicators. Two reviewers had screened by reviewing the title and abstract first, then reviewing the full text, and independently evaluated the full text of the selected references according to the inclusion or exclusion criteria. Disagreements were resolved through discussion, or by a third reviewer when needed. 16 articles were eventually included in the study. The specific process is shown in .
2.2. Date extraction
Two reviewers used standardized abstract forms to independently extract data from each included study. Data extraction was discussed. If there were disagreements between the observers, the other two reviewers would record the decision. All disagreements were resolved through discussion and consensus (). The extracted information included: first Author’s name, year of publication, study design, Journal, region, total patients, mean age, gender distribution of patients, mutated gene, number of mutations, criteria for classification of CMML, therapeutic method and outcome (OS).
Table 1. Summary of the data extracted from the 16 studies included.
For the extraction of survival data, HR and 95% CI were used directly from the second priority of multivariate survival analysis or univariate analysis. Otherwise, if the study only provides Kaplan-Meier curves, we would use Engauge Digitizer version 10.7 for extraction. Simultaneously, we also tried to contact the corresponding author to make up for the missing data.
2.3. Quality assessment
The study used Newcastle-Ottawa quality assessment (NOS) to assess the quality of non-randomized studies in the meta-analysis (Ottawa, Canada: Department of Epidemiology and Community Medicine, University of Ottawa). It also independently extracted the necessary information and verified the extracted information. In the NOS case–control study standard, there are 8 items in total, which were divided into three main categories: selection (4 items), comparability (1 item) and exposure (3 items). Among the 10 criteria evaluated, 10 stars can be marked. If these studies meet a standard, they will get a star (one point), otherwise they will not. The total score is 10, and studies with a total score greater than 6 are considered as high quality. The above was all independently evaluated by four reviewers, and any disagreement will be resolved by final consensus. The results are shown in .
2.4. Statistical analysis
Stata version 14.0 (StataCorp, University Town, Texas, USA) was used for data analysis. Compared HRs and 95% CIs evaluation with wild type, the effects of ASXL1 and/or TET2 mutations on prognosis of CMML patients could be shown. We also performed a subgroup analysis of CMML patients, using I2 to evaluate the statistical heterogeneity of the efficacy (The degree of heterogeneity: I2 = 0-25%, no heterogeneity. I2 = 25-50%, moderate heterogeneity. I2 = 50-75%, high heterogeneity. I2 = 75-100%, extremely poor heterogeneity). Generally, when the heterogeneity was moderate or below, the fixed effects model would be used to calculate the data. Otherwise, the random effects model would be used. But comprehensive consideration should be done in combination with clinical heterogeneity and methodological heterogeneity. When substantial heterogeneity was detected, our assessment was only based on random effects models. Funnel plots, Begg’s and Mazumdar’s and Egger's s-tests were used to assess possible publication bias. The statistical significance was set at P < 0.05. The reported results conformed to the AMSTAR (the Assessment of Multiple Systematic Reviews) guidelines.
For prognostic-related indicators that cannot be assessed with forest plots directly, the two rates of each result can be combined. The two rates are P1i = E1i/n1i, P2 = E2i/n2i (E: events, the number of people who had the event, n: the total number of people in the group). The combined rate is Pi = (E1i+E2i) / (n1i+n2i), di = P1i-P2i. The mean and estimated variance of the mean are:
(1)
(1)
(2)
(2)
(3)
(3)
According to Cochrane's definition [Citation34], the weights in formulas (1) and (2) are:
3. Results
3.1. The effects of TET2 mutation on prognosis in CMML patients
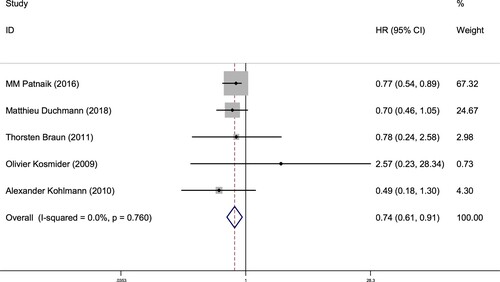
A total of 5 studies involving TET2 gene mutation status reported OS (From random allocation to death due to any cause), 643 patients was included. The results of OS subgroup analysis were shown as below: for CMML patients with TET2MT, HR = 0.74, 95% CI = 0.61 - 0.91, P = 0.005 (), suggesting the better prognosis was found in the CMML patients with TET2MT, compared with TET2WT. The heterogeneity test results were P = 0.760, I2 = 0%, suggesting that there was no heterogeneity in the result of this part. Therefore, it was speculated that TET2 mutation indicated a better prognosis in CMML patients.
Figure 2. Forest plot of the risk ratio (HR) and 95% confidence interval of the overall survival (OS) of patients with TET2 mutation and non-mutation CMML. The size of the square or square represents the weight, and the length of the line represents the width of the 95% CI.
Sample size and analysis method were also considered in the further subgroup analysis. As shown in , TET2 mutation did not indicate a correlation with prognosis analyzed with multivariate analysis in the subgroup further study. However, it might be biased for the further analysis due to the insufficient sample size.
Table 2. Subgroup analysis of pooled HRs for OS with or without TET2 mutation.
Among studies involving TET2 mutation or not, there were three studies respectively reported the relationship between the acute conversion rate and mortality of the disease and the gene status (). We performed the combination of the difference between the two rates (Incidence of events with or without mutation) () and the consistency test. For the acute conversion rate, the estimated variance S2d is 0.000367, and the calculated weighted mean d is 0.0845 (95% CI = 0.0654 - 0.104). For mortality, the estimated variance S2d is 0.000659, and the calculated weighted mean is 0.133 (95% CI = 0.108 - 0.159). The above suggests that TET2 mutation had advantages.
Table 3. The effect of TET2 mutation status on the acute transformation and death rate of CMML patients.
Table 4. Combining to calculate the difference between the two rates (Incidence of events with or without mutation) in different events.
3.2. The effect of ASXL1 mutation on prognosis of CMML patients is different from TET2
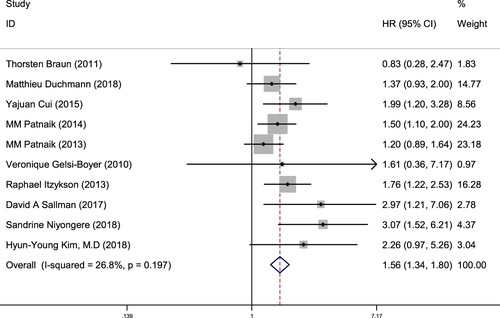
10 studies were included by ASXL1 gene status reported OS, 1726 patients were included in the study. The OS subgroup analysis revealed CMML patients with ASXL1 mutation HR = 1.56, 95% CI = 1.34 - 1.80, P = 0.000 (), suggesting that the ASXL1 mutation in CMML patients was unfavorable for the prognosis compared with ASXL1 without mutation. The heterogeneity test results were P = 0.197, I2 = 26.8%, indicating that there was no heterogeneity among the studies. Therefore, it was suggested that ASXL1 non-mutation could be used as a prognostic indicator in CMML, which had a positive significance for the prognosis.
Figure 3. Forest plot of the risk ratio (HR) and 95% confidence interval of the overall survival (OS) of patients with ASXL1 mutation and non-mutation CMML. The size of the square or square represents the weight, and the length of the line represents the width of the 95% CI.
Next, we conducted subgroup analyses based on gene type, sample size and different analysis methods. As shown in , among ten studies involving ASXL1 above, the difference in sample size and analysis method did not affect the final results.
Table 5. Results of subgroup analyses of pooled HRs for OS with or without ASXL1 mutation.
Among studies involving the gene status of ASXL1, there are four and three respectively reports the relationship between the acute conversion rate and mortality of the disease and the gene status (). We performed the combination of the difference between the two rates () and the consistency test on the data. For the acute conversion rate, the estimated variance S2d is 0.000773, and the calculated weighted mean is −0.0595, (95% CI = −0.0873 – −0.0317). For mortality of the disease, the estimated variance S2d is 0.00135, and the calculated weighted mean is −0.146, (95% CI = −0.183 – −0.109). The above suggests that ASXL1 mutation had no advantage. Although TET2 and ASXL1 had higher mutation rates in CMML, the prognostic results of mutations were opposite.
Table 6. The effect of ASXL1 mutation status on the acute transformation and death rate of CMML patients.
Table 7. Combining to calculate the difference between the two rates (Incidence of events with or without mutation) in different events.
3.3. Publication bias and sensitivity analysis
A publication bias study was conducted on the included studies. In a study on the effect of TET2 mutation status on CMML patients, the Begg's test result was P = 0.624, P >0.05, indicating that no publication bias was observed in the HR assessment (A), which was verified by Egger's test result—— P = 0.841, P >0.05. And in a study on the effect of ASXL1 mutation status on CMML patients, the Begg's test result indicated P = 0.421, P >0.05, indicating that no publication bias was observed in the HR assessment (B), which was verified by the result of Egger's test P = 0.209, P >0.05.
Figure 4. Funnel plot analysis of publication bias in research on different genes (A) TET2 gene (B) ASXL1 gene.
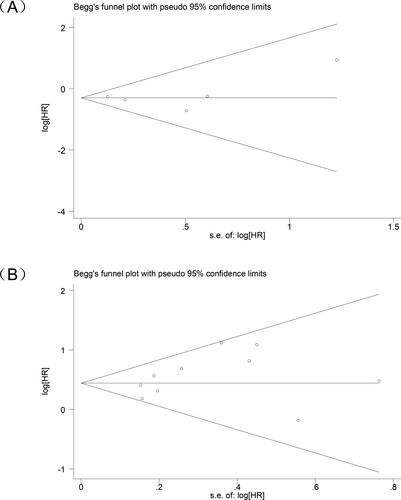
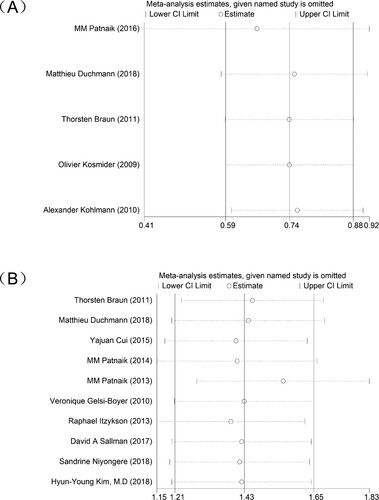
In addition, we conducted a sensitivity analysis of the included studies to confirm the stability of the results of the meta-analysis of TET2 or ASXL1 on OS in CMML patients ().
3.4. The effect of the interaction between TET2 and ASXL1 mutation status on the prognosis of CMML patients
According to the results of single gene analysis, TET2MT or ASXL1WT is beneficial for the prognosis. A total of 3 studies combined the two genes for analysis, including 1397 patients involving both TET2 and ASXL1 mutation status. In the OS subgroup analysis, with TET2MT and ASXL1WT as controls, the HR of both TET2 and ASXL1 mutations (TET2MT+ASXL1MT) in CMML patients, was 1.51, 95% CI = 1.14 - 1.99, P = 0.004 (A). The heterogeneity test results were P = 0.613, I2 = 0%, suggesting that both TET2 and ASXL1 mutations in CMML patients are not good for the prognosis of CMML patients. The HR of neither TET2 nor ASXL1 mutations (TET2WT+ASXL1WT) in CMML patients was 1.49, 95% CI = 1.12 - 1.98, P = 0.007 (B). The heterogeneity test results were P = 0.414, I2 = 0.0%, It is suggested that the mutation status of TET2 and ASXL1 not mutated in CMML patients is not conducive to the prognosis. The HR of TET2 without mutation and ASXL1 mutation (TET2WT+ASXL1MT) In CMML patients was 1.88, 95% CI = 1.21 - 2.94, P = 0.005 (C). The heterogeneity test results were P = 0.086, I2 = 59.2%, suggesting that the genetic status of TET2WT and ASXL1MT in CMML patients is not conducive to the prognosis.
Figure 6. Forest plot of the risk ratio (HR) and 95% confidence interval of the overall survival (OS). The size of the square or square represents the weight, and the length of the line represents the width of the 95% CI. (A) Patients with TET2 mutation ASXL1 without mutation and TET2&ASXL1 mutations (B) Patients with TET2 mutation ASXL1 without mutation and TET2&ASXL1 without mutation (C) Patients with TET2 mutation ASXL1 without mutation and TET2 without mutation ASXL1 mutation.
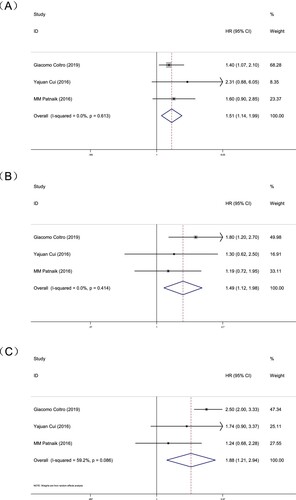
Therefore, for CMML patients, having both TET2MT and ASXL1WT are more beneficial for the prognosis than TET2MT or ASXL1WT alone, and is a better prognostic indicator of CMML.
4. Discussion
CMML is a rare type of bone marrow tumor in the elderly. There is limited prospective data on the natural history of CMML. And about 25% of patients will progress to AML. However, these secondary AMLs are notoriously difficult to treat [Citation7, Citation36]. The prognosis of CMML disease is of great significance to the quality of life of patients. In recent years, with the rapid development of next-generation sequencing technology, targeted therapies to identify new somatic mutations may have a potential impact on myeloid malignancies.
Mutations in TET2 and ASXL1 are widespread in a variety of myeloid tumors. Mutations in ASXL1 in medullary carcinoma usually coincide with mutations in the TET2 gene [Citation37]. The mechanism of their occurrence and development in the disease is not yet fully understood. As for their roles in the prognostic evaluation of CMML, there is still no consensus. Our study included 16 studies and conducted a meta-analysis of the prognosis of TET2 and ASXL1 mutations on CMML. In this meta-analysis, single gene studies or two gene combination studies are single-center non-random trials or small sample sizes or lack of high-quality evidence. Therefore, we conducted this meta-analysis to estimate the prognostic value of ASXL1 and TET2 in CMML more accurately.
Our meta-analysis combined the results of CMML patients from 16 independent studies, and showed that TET2MT had a survival advantage in CMML (HR = 0.74, 95% CI = 0.61 - 0.91, P = 0.005), and ASXL1MT had a significant negative impact on the prognosis of CMML (HR = 1.56, 95% CI = 1.34 - 1.80, P = 0.000). According to the results of single gene analysis, TET2MT or ASXL1WT is beneficial for the prognosis. And the subgroup analyses showed that the sample size of the study and the difference of the analysis method will not affect the final results ( and ). We combine the two genes for further analysis. Our results showed that compared with patients with TET2MT and ASXL1WT, the HR of patients with in both TET2MT and ASXL1MT was 1.51 (95% CI = 1.14 - 1.99; P = 0.004), the HR of patients with neither TET2MT nor ASXL1MT was 1.49 (95%CI = 1.12 - 1.98; P = 0.007) and the HR of TET2WT and ASXL1MT patients was 1.88 (95%CI = 1.21 - 2.94; P = 0.005). All showed a poor prognosis, suggesting that the genotype of TET2MT and ASXL1WT is the most beneficial for the survival of CMML patients. It is speculated that ASXL1 and TET2 might have interaction and regulatory mechanisms in CMML.
ASXL1 can regulate chromatin by interacting with multiple sets of inhibitory complex proteins (PRC1 and PRC2). Monoubiquitination of lysine 119 on histone H2A (H2AK119Ub) is a histone modification related to the inhibition of chromatin state. H2AK119Ub and histone 3 lysine 27 trimethyl (H327Kme3) play a synergistic role in PRC-mediated gene suppression [Citation38]. And ASXL1MT causes PRC2 to mediate H3K27 trimethylation [Citation39]. Studies have shown in vitro [Citation40], ASXL1MT can enhance the activity of the ASXL1-BAP1 (BRCA-related protein 1) complex in bone marrow precursors. This complex leads to the complete elimination of H2AK119Ub and depletion of H327Kme3, thereby promoting transcription disorders [Citation39]. And TET2 is essentially an epigenetic enzyme that can convert DNA 5-methylcytosine (5mc) to 5-hydroxymethylcytosine (5hmc). TET2MT can damage the hydroxymethyl catalytic activity of epigenetic enzymes and may result in a decrease in 5-hmc levels in genomic DNA [Citation41]. This is one of the mechanisms of CMML pathogenesis caused by TET2MT. By producing bone marrow chimera, the overactive ASXL1-BAP1 complex and the loss of TET2 function synergistically promote the targeted transformation of hematopoietic cells to the myeloid lineage [Citation42]. Therefore, ASXL1 can interact with H2A ubiquitination and TET2-mediated DNA modification. From this perspective, the role of TET2 in the progression of CMML depends on the presence of mutations in ASXL1.
Although we included the previous research as fully as possible into our analysis, the results still have their limitations. First of all, due to lack of data, we cannot evaluate other clinical parameters except OS. There are only three studies on the two genes of TET2 and ASXL1. Some other statistical methods can only play a supporting role. It causes certain limitations to our analysis. Secondly, all selected studies are observational studies, not prospective randomized controlled studies. Finally, the number of included studies is relatively small, especially for TET2 studies. When the number of studies included in the meta-analysis is less than 10, the Egger’s and Begg’s test are relatively low. So, Egger’s and Begg’s test may not be able to detect publication bias. Therefore, more epidemiological data, many in vitro experimental studies and prospective randomized controlled studies, are still needed for more subgroup analysis and further analysis to verify our conclusions.
In addition, for specific mutation types of genes, only one of the studies involved showed that the ASXL1 frameshift mutation (FS) had the greatest impact on OS compared with other mutation types (HR = 5.87, 95% CI = 1.98 - 7.4, P = 0.001) [Citation20]. We cannot reveal the significance of mutation types on the prognosis of CMML through meta-analysis. Therefore, in the future, further research can be done on the specific types of gene mutations.
This study is the first meta-analysis to compare the effects of two genes (TET2, ASXL1) on patient prognosis. Our analysis observed that TET2 mutation and ASXL1 non-mutation contribute to the prognosis of CMML patients. We provide a basis for evaluating the prognosis of CMML patients, exploring potential therapeutic targets, and predicting the efficacy of treatment. It is possible to create a more accurate new prognostic scoring system for CMML including TET2 and ASXL1 mutations in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management. Am J Hematol 2018;93(6):824–840. doi:10.1002/ajh.25104.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the world Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544.
- Kohlmann A, Grossmann V, Klein HU, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL. RAS, and RUNX1. J Clin Oncol. 2010; 28(24):3858–3865. doi:10.1200/JCO.2009.27.1361.
- Geissler K, Jager E, Barna A, et al. Correlation of RAS-pathway mutations and spontaneous myeloid colony growth with progression and transformation in chronic myelomonocytic leukemia—A retrospective analysis in 337 patients. Int J Mol Sci. 2020;21:3025, doi:10.3390/ijms21083025.
- Elmariah H, DeZern AE. Chronic myelomonocytic leukemia: 2018 update to prognosis and treatment. Curr Hematol Malig Rep. 2019;14(3):154–163. doi:10.1007/s11899-019-00509-9.
- Mora E, Sanz GF. Current management of patients with chronic myelomonocytic leukemia. Curr Opin Oncol. 2018;30(6):409–417. doi:10.1097/CCO.0000000000000486.
- Harada Y, Harada H. [Chronic myelomonocytic leukemia (CMML): recent advances in molecular pathogenesis and treatment]. Rinsho Ketsueki. 2016;57(2):147–155. doi:10.11406/rinketsu.57.147.
- Patnaik MM, Tefferi A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e393, doi:10.1038/bcj.2016.5.
- Feng Y, Li X, Cassady K, et al. Tet2 function in hematopoietic malignancies, immune regulation, and DNA repair. Front Oncol. 2019;9:210, doi:10.3389/fonc.2019.00210.
- Yamazaki J, Taby R, Vasanthakumar A, et al. Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics. 2012;7(2):201–207. doi:10.4161/epi.7.2.19015.
- Abdel-Wahab O, Mullally A, Hedvat C, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. doi:10.1182/blood-2009-03-210039.
- Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824–3831. doi:10.1182/blood-2011-05-352039.
- Fisher CL, Berger J, Randazzo F, et al. A human homolog of additional sex combs. ADDITIONAL SEX COMBS-LIKE 1, maps to chromosome 20q11. Gene. 2003; 306:115–126. doi:10.1016/s0378-1119(03)00430-x.
- Sugimoto Y, Muramatsu H, Makishima H, et al. Spectrum of molecular defects in juvenile myelomonocytic leukaemia includes ASXL1 mutations. Br J Haematol 2010;150(1):83–87. doi:10.1111/j.1365-2141.2010.08196.x.
- Asada S, Fujino T, Goyama S, et al. The role of ASXL1 in hematopoiesis and myeloid malignancies. Cell Mol Life Sci. 2019;76(13):2511–2523. doi:10.1007/s00018-019-03084-7.
- Gelsi-Boyer V, Brecqueville M, Devillier R, et al. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J Hematol Oncol. 2012;5:12, doi:10.1186/1756-8722-5-12.
- Gelsi-Boyer V, Trouplin V, Roquain J, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol 2010;151(4):365–375. doi:10.1111/j.1365-2141.2010.08381.x.
- Courville EL, Wu Y, Kourda J, et al. Clinicopathologic analysis of acute myeloid leukemia arising from chronic myelomonocytic leukemia. Mod Pathol. 2013;26(6):751–761. doi:10.1038/modpathol.2012.218.
- Patnaik MM, Itzykson R, Lasho TL, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia. 2014;28(11):2206–2212. doi:10.1038/leu.2014.125.
- Sallman DA, Komrokji R, Cluzeau T, et al. ASXL1 frameshift mutations drive inferior outcomes in CMML without negative impact in MDS. Blood Cancer J. 2017;7(12):633, doi:10.1038/s41408-017-0004-0.
- Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–2436. doi:10.1200/JCO.2012.47.3314.
- Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27(7):1504–1510. doi:10.1038/leu.2013.88.
- Patnaik MM, Lasho TL, Vijayvargiya P, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e385, doi:10.1038/bcj.2015.113.
- Palomo L, Garcia O, Arnan M, et al. Targeted deep sequencing improves outcome stratification in chronic myelomonocytic leukemia with low risk cytogenetic features. Oncotarget. 2016;7(35):57021–57035. doi:10.18632/oncotarget.10937.
- Duchmann M, Yalniz FF, Sanna A, et al. Prognostic role of gene mutations in chronic myelomonocytic leukemia patients treated With hypomethylating agents. EBioMedicine. 2018;31:174–181. doi:10.1016/j.ebiom.2018.04.018.
- Coltro G, Mangaonkar AA, Lasho TL, et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)—a study of 1084 patients. Leukemia. 2020;34(5):1407–1421. doi:10.1038/s41375-019-0690-7.
- McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies. JAMA. 2018;319(4):388–396. doi:10.1001/jama.2017.19163.
- Cui Y, Tong H, Du X, et al. Impact of TET2. SRSF2, ASXL1 and SETBP1 mutations on survival of patients with chronic myelomonocytic leukemia. Exp Hematol Oncol. 2015;4:14, doi:10.1186/s40164-015-0009-y.
- Niyongere S, Lucas N, Zhou JM, et al. Heterogeneous expression of cytokines accounts for clinical diversity and refines prognostication in CMML. Leukemia. 2019;33(1):205–216. doi:10.1038/s41375-018-0203-0.
- Kim HY, Lee KO, Park S, et al. Poor prognostic implication of ASXL1 mutations in Korean patients With chronic myelomonocytic leukemia. Ann Lab Med. 2018;38(6):495–502. doi:10.3343/alm.2018.38.6.495.
- Patnaik MM, Zahid MF, Lasho TL, et al. Number and type of TET2 mutations in chronic myelomonocytic leukemia and their clinical relevance. Blood Cancer J. 2016;6(9):e472, doi:10.1038/bcj.2016.82.
- Kosmider O, Gelsi-Boyer V, Ciudad M, et al. Tet2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica. 2009;94(12):1676–1681. doi:10.3324/haematol.2009.011205.
- Cui Y, Tong H, Du X, et al. Tet2 mutations were predictive of inferior prognosis in the presence of ASXL1 mutations in patients with chronic myelomonocytic leukemia. Stem Cell Invest. 2016;3:50, doi:10.21037/sci.2016.09.04.
- Armitage P, Berry G, Matthews JNS. Statistical methods in Medical research. Malden, MA: Blackwell Sci. Ltd. 2002: 583–588.
- Smith AE, Mohamedali AM, Kulasekararaj A, et al. Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value. Blood. 2010;116(19):3923–3932. doi:10.1182/blood-2010-03-274704.
- Itzykson R, Duchmann M, Lucas N, et al. Cmml: clinical and molecular aspects. Int J Hematol 2017;105(6):711–719. doi:10.1007/s12185-017-2243-z.
- Ko M, Rao A. Tet2: epigenetic safeguard for HSC. Blood. 2011;118(17):4501–4503. doi:10.1182/blood-2011-08-373357.
- Abdel-Wahab O, Pardanani A, Patel J, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25(7):1200–1202. doi:10.1038/leu.2011.58.
- Abdel-Wahab O, Adli M, LaFave LM, et al. Asxl1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22(2):180–193. doi:10.1016/j.ccr.2012.06.032.
- Balasubramani A, Larjo A, Bassein JA, et al. Cancer-associated ASXL1 mutations may act as gain-of-function mutations of the ASXL1-BAP1 complex. Nat Commun. 2015;6:7307, doi:10.1038/ncomms8307.
- Langemeijer SM, Kuiper RP, Berends M, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 2009;41(7):838–842. doi:10.1038/ng.391.
- Cimmino L, Abdel-Wahab O, Levine RL, et al. Tet family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9(3):193–204. doi:10.1016/j.stem.2011.08.007.