ABSTRACT
Objectives
No clear consensus has been reached about the clinical features in hepatitis B virus (HBV)-associated non-Hodgkin’s lymphoma (NHL) patients. We performed a systematic review and meta-analysis to explore the clinical characteristics and prognosis of NHL patients with chronic HBV infection (HBsAg+).
Methods
Seven electronic databases were searched for relevant studies up to 31 January 2021. Hazard ratio (HR) or odds ratio (OR) corresponding to 95% confidence interval (CI) were calculated to estimate the outcomes. The primary outcome was survival outcome, including overall survival (OS) and progression-free survival (PFS). Subgroup analysis was performed in diffuse large B-cell lymphoma (DLBCL) patients.
Results
Twenty-three retrospective studies, comprising of 1202 HBsAg+ NHL patients and 4448 HBsAg− NHL patients, were included. Twenty-two studies were conducted on Chinese patients. Compared with HBsAg− NHL patients, significantly shorter OS (HR 1.68; 95% CI 1.48–1.91) and PFS (HR 1.80; 95% CI 1.20–2.71), lower rate of complete remission (OR 0.59, 95% CI 0.44–0.80) and higher frequency of hepatic dysfunction during chemotherapy (OR 3.46; 95% CI 2.61–4.57) were demonstrated in HBsAg+ NHL patients. Moreover, HBsAg+ patients were characterized by a younger age of disease onset, advanced disease stage, higher level of LDH and more frequent presence of B symptoms, and involvement of spleen and liver at diagnosis. Furthermore, subgroup analysis in DLBCL patients was also showed similar results.
Conclusion
Our study implicated that NHL patients, especially DLBCL, with chronic HBV infection displayed inferior prognosis, higher incidence of hepatic dysfunction during chemotherapy and distinct clinical features.
Introduction
Non-Hodgkin’s lymphoma (NHL) is a diverse group of diseases, which can be classified into B-cell, T-cell, and natural killer (NK)-cell lymphoma. Thereinto, diffuse large B-cell lymphoma (DLBCL) is the most common subtype of NHL, accounting for 30–40% of NHL worldwide [Citation1]. Increasing evidences implicated that viral infection contributed to the pathogenesis of different subtypes of lymphoma, such as Epstein–Barr virus (EBV) in Hodgkin disease or Burkitt’s lymphoma, human T-cell leukemia virus type 1 (HTLV-1) in adult T cell leukemia and lymphoma, hepatitis C virus (HCV) in marginal zone lymphoma [Citation2–4]. According to the 2016 revision of the World Health Organization classification of lymphoid neoplasms, two subtypes of NHL associated with a specific viral infection, EBV-positive DLBCL and human herpesvirus type-8 (HHV-8)-positive DLBCL, have been classified as separated subtypes of DLBCL [Citation5]. These new categories strongly suggested that NHL patients with specific infection displayed distinct clinical manifestations and prognoses.
Recently, hepatitis B virus (HBV) was found to infect peripheral blood mononuclear cells and lymphoblastoid cell lines [Citation6]. High level of HBV-DNA could be detected in tumor biopsies from B-cell lymphoma patients with HBV infection [Citation7], hence indicating that HBV was lymphotropic. A recent meta-analysis has also provided quantitative evidence that HBV infection led to a 2.46-fold increased risk of developing NHL [Citation8]. Nevertheless, the pathogenesis and clinical characteristic of HBV-associated lymphoma are still undefined.
Arising clinical attention to the association of HBV infection and the clinical outcomes of NHL patients, especially DLBCL, has been paid [Citation9–14]. However, the results were still inconsistent and somehow controversial based on existing retrospective studies. Deng et al. [Citation9] found that HBV-associated DLBCL had unique clinical features and poor outcomes. However, other studies [Citation10,Citation13] had shown that HBV infection was not associated with NHL patients’ survival. In addition, there was high heterogeneity in these retrospective studies with small sample sizes. In view of the limitations of previous studies, we hereby performed a systematic review and meta-analysis aiming to evaluate the clinical features and prognosis in NHL patients with HBV infection.
Methods
Identification of relevant studies
To identify all studies that explored the impact of HBV infection on the clinical characteristics and outcome of NHL patients, a literature search of seven electronic databases, including PubMed, Cochrane Library, Embase, the Chinese Biomedical Database (CBM), the China National Knowledge Infrastructure (CNKI), the VIP medicine information system (VMIS), and Wanfang, up to 31 January 2021, was conducted. The search strategy was based on combinations of the following keywords and search terms without language limitation: (‘lymphoma’ [MeSH] or ‘Lymphoma, Non-Hodgkin’ [MeSH] or Non-Hodgkin lymphoma or NHL) AND (‘hepatitis B’ [MeSH] or hepatitis B virus or HBV or Hep B virus or Hep B). We also screened the references of retrieved studies, meeting abstracts, relevant meta-analyses, and systematic reviews. Case reports, editorials, and review articles were excluded. When a publication overlapped with other publications of the same trial, only the article with more details or the most recent article was retained.
Selection criteria
The studies included in the meta-analysis should satisfy all the following criteria: (1) the study population was NHL patients; (2) the study consisted of two groups: HBsAg+ and HBsAg− NHL patients; (3) clinical features and survivals between two groups of patients were compared. Exclusion criteria were: (1) patients had co-infection with human immunodeficiency virus (HIV) or other hepatitis viruses (hepatitis A virus [HAV], hepatitis C virus [HCV], hepatitis D virus [HDV] and hepatitis E virus [HEV]); (2) patients with resolve HBV infection [HBsAg negative and hepatitis B core antibody (HBcAb) positive] as the case group. When the relevant data was not reported in the paper, we contacted the author to get the relevant information by e-mail or telephone.
The primary outcome was survival outcome, including overall survival (OS) and progression-free survival (PFS). The secondary outcome measured treatment response (rate of complete remission [CR]), incidence of hepatic dysfunction during chemotherapy, and clinical characteristics.
Data extraction and study quality
Two reviewers (Zhang M and Gao F) independently extracted data and outcomes using an electronic standard form. The following information from each study was summarized: (1) first author, (2) year of publication, (3) country, (4) subtypes of NHL, (5) number of patients with HBsAg seropositivity and seronegativity, (6) use of rituximab, (7) frequency of HBV reactivation, (8) prophylactic interventions for HBV reactivation, and (9) patient’s characteristics. Any discrepancies amongst the two reviewers were resolved by an additional investigator, Zhao Y.
Quality assessment
Newcastle-Ottawa Quality Assessment Scale (NOS) was adopted to assess the methodological quality of the included studies [Citation15]. The following three items were evaluated: (1) patient selection, (2) comparability of interventions and observations group, and (3) assessment of outcome.
Statistical analysis
Hazard ratio (HR) corresponding to 95% confidence interval (CI) were used to appraise OS and PFS. If HRs and 95% CIs were not obtained from the original article, Kaplan–Meier curves of the included studies were read and re-analyzed by software Engauge digitizer which HRs and 95% CIs were indirectly calculated from Kaplan–Meier curve using Tierney’s methods [Citation16]. Odds ratio (OR) corresponding to 95% CI were calculated to estimate other outcomes. In the current study, two models of meta-analysis were applied for outcomes, based on the results of heterogeneity tests among included studies. The heterogeneity between studies was evaluated in terms of the Chi-square-based Q-statistic test. The result of the Q-statistic test was considered significant in condition for I2 ≥ 50%. The random-effects model (if I2 ≥ 50%) or fixed-effects model (if I2 < 50%) was used to describe the combined HR or OR. The significance of the pooled outcomes was determined by the Z-test. A p-value of < 0.05 was considered significant. Publication biases were assessed by Egger's test, Begg's test, and funnel plots. Sensitivity analysis was performed to determine the origin of the heterogeneity and to evaluate whether individual studies substantially influenced the summary statistic by sequentially excluding individual studies. The methods of meta-analysis and publication biases tests were detailed in our previous publications [Citation17–20].
Statistical analysis was performed by software ReviewManager 5.3 (The Cochrane Collaboration, Oxford, UK) and Stata version 13 (Stata Corp, College Station, Texas, USA). All p-values were both-sided. p-value of < 0.05 was considered significant.
Results
Characteristics of studies
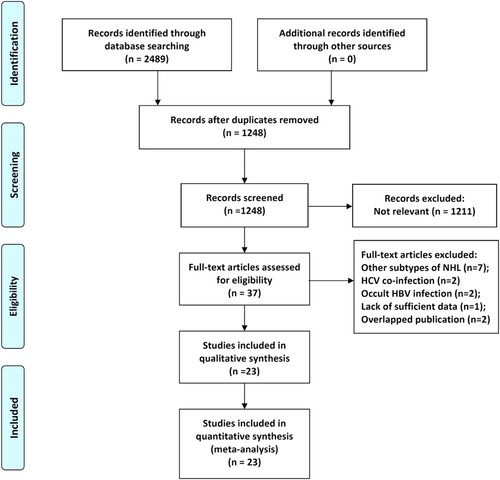
Through the comprehensive search and selection based on the criteria above, 2489 articles were identified as potentially relevant publications. Upon further assessment of the full-text, 14 publications didn’t meet the inclusion criteria and were excluded. Eventually, 23 retrospective studies [Citation9–14,Citation21–37] with a total of 5650 NHL patients, including 1202 patients with HBsAg seropositive and 4448 patients with HBsAg seronegative, were deemed suitable for the meta-analysis (). The studies were published between 2008 and 2018. The sample size was ranged from 81 to 587. The patients in 18 of 21 included studies were diagnosed as DLBCL. Except for Lim’s study [Citation27], the study population in other studies was Chinese NHL patients. Detailed characteristics of the eligible studies were outlined in . With regard to the methodological quality, all the included studies had reliable quality as indicated by NOS scores > 6 points ().
Table 1. Baseline characteristics of included studies.
HBV infection and NHL patients’ survival
A total of 19 studies, comprising of 933 NHL patients with HBsAg seropositive and 3386 NHL patients with HBsAg seronegative, were included for the assessment of OS. The result indicated that HBsAg+ patients had significantly worse OS than HBsAg− patients (HR, 1.68; 95% CI, 1.48–1.91; p < 0.00001; , ).
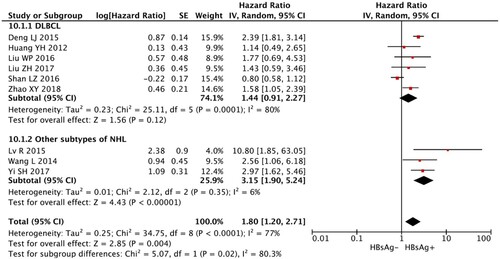
Figure 2. Meta-analysis of the association between status of HBsAg and overall survival of NHL patients.
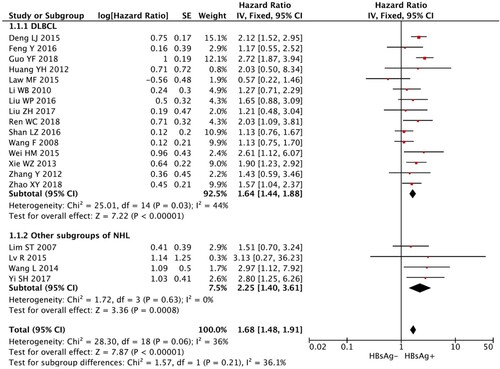
Table 2. Results of meta-analysis.
Among all 23 studies, only 9 studies (i.e. 396 DLBCL patients with HBsAg seropositivity and 1458 DLBCL patients with HBsAg seronegativity) were available for PFS analysis. The random-effects model was used to calculate the result as there was significant heterogeneity (I2 > 50%). Meta-analysis revealed that HBsAg+ patients showed significantly reduced PFS as compared with the HBsAg− patients (HR, 1.80; 95% CI, 1.20–2.71; p = 0.004; , ).
HBV infection and treatment response
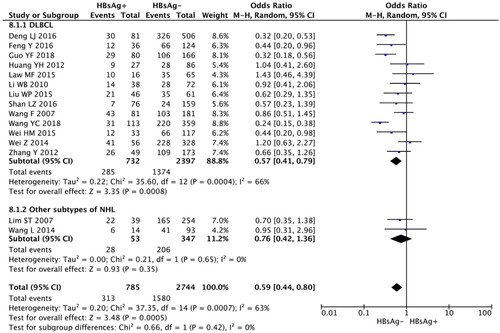
The rate of CR was analyzed by using the random-effects model as the heterogeneity tests suggested significant heterogeneity (I2 > 50%). The combined results showed that HBsAg+ NHL patients achieved a significantly inferior treatment response than HBsAg− NHL patients (OR, 0.59; 95% CI, 0.44–0.80; p = 0.0005, , ).
Hepatic dysfunction during chemotherapy
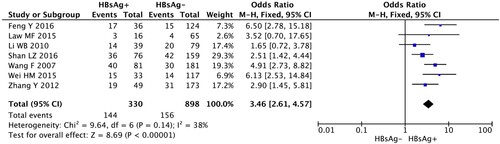
Totally, 7 studies including 330 HBsAg + and 898 HBsAg− DLBCL patients were eligible to analyze this outcome by the fixed-effects model. The result of the meta-analysis showed that the incidence of hepatic dysfunction during the chemotherapy in HBsAg+ patients significantly was significantly higher than that in HBsAg patients (OR, 3.46; 95% CI, 2.61–4.57, p < 0.00001) (, ).
HBV infection and clinical characteristics
Clinical characteristics of NHL patients, including age of onset (< 60 years old), presence of B symptoms, advanced disease stage (Ann Arbor staging III/IV), involvement of spleen, liver and bone marrow, elevated LDH level, high International Prognostic Index (IPI) score (3′–5′) at diagnosis, were also comprehensively investigated between two groups of patients. The results of the meta-analysis were summarized in Figures S1–S8 and . NHL patients with HBsAg seropositivity were associated with younger age of onset (OR, 2.57; 95% CI, 2.05–3.24, p < 0.00001, Figure S1), advanced disease stage (OR, 1.79; 95% CI, 1.55–2.07, p < 0.00001, Figure S2), higher level of LDH (OR, 1.34; 95% CI, 1.15–1.56, p = 0.0001, Figure S3), and more frequent presence of B symptoms (OR, 1.72; 95% CI, 1.22–2.43, p = 0.002, Figure S4), more frequent involvement of spleen (OR, 3.09; 95% CI, 2.39–3.99, p < 0.00001, Figure S5), and liver (OR, 2.49; 95% CI, 1.72–3.59, p < 0.00001, Figure S6). However, there was no significant difference for the outcomes of high IPI score (OR, 0.96; 95% CI, 0.67–1.39, p = 0.83, Figure S7) and incidence of involvement of bone marrow (OR, 1.19; 95% CI, 0.72–1.96, p = 0.49, Figure S8) between the two groups.
Subgroup analysis
Since NHL is a group of heterogeneous diseases, we performed subgroup analysis in DLBCL patients. Similar results could be also observed. Compared with DLBCL patients with HBsAg seronegativity, patients with HBsAg seropositivity had shorter OS (), lower CR rate (), and distinct clinical features (Figure S1–S8), including younger age of onset, advanced disease stage, higher level of LDH, more frequent presence of B symptoms, and involvement of spleen and liver. There was a trend that HBsAg+ DLBCL patients had shorter PFS than HBsAg− DLBCL patients (). The results of subgroup analysis were summarized in .
Sensitivity analysis
Sensitivity analysis was performed to find the origin of the heterogeneity and verify the sensitivity of results. The origin of the heterogeneity and overall effect after removal of origin of the heterogeneity for each outcome were summarized in . The results showed that removal of the origin of the heterogeneity didn’t affect the effect size for each outcome, suggesting the stability of the result of meta-analysis. However, for the outcome of high IPI score, the heterogeneity still existed even though studies were excluded one by one.
Table 3. Results of sensitivity analysis.
Publication bias
Begg’s test and Egger’s test were performed to assess the publication bias. As was shown in , no significant publication bias was observed for all the outcomes except for the outcome of CR.
Table 4. Results of Begg’s test and Egger’s test.
Discussion
In this study, we performed a systematic review and meta-analysis to evaluate the impact of chronic HBV infection on NHL patients. The results indicated that in comparison with HBsAg− NHL patients, HBsAg+ NHL patients, especially DLBCL, had significantly inferior survival, poorer treatment response, and distinct clinical characters, including younger age of onset, advanced disease stage, higher level of LDH, more frequent presence of B symptoms, and involvement of spleen and liver. Furthermore, patients with chronic HBV infection were more likely to undergo hepatic dysfunction when receiving chemotherapy.
Although substantial evidence has demonstrated the association between NHL and HBV infection [Citation8,Citation38–42], the pathogenic mechanism of HBV contributing to NHL was still unclear. Currently, two different models of HBV-driven DLBCL were proposed [Citation9,Citation12]. Deng et al. [Citation9] suggested HBV-associated antigen stimulation as a likely mechanism of HBV-associated DLBCL, as evidenced by long history of chronic HBV infection, frequent involvement spleen and retroperitoneal lymph nodes, strongly biased usage of both Ig heavy and light chain genes, and high homology of CDR3 sequence to specific antibodies for HBsAg. On the other hand, Ren et al. [Citation12] considered HBV-driven mutagenesis in a hit-and-run manner as an alternative mechanism of HBV-driven DLBCL as supported by a distinctive molecular profile of HBV-associated DLBCLs via whole-genome/exome and transcriptomic sequencing. They have found that HBsAg+ DLBCL patients have enhanced mutation activity. Several genes, such as BCL6, KLF2, and ZFP36L1, were preferentially mutated in HBsAg+ DLBCL patients, which mainly affected p53 signaling, FOXO signaling, and immune evasion signaling pathway.
In our study, the subgroup analysis indicated that HBsAg+ DLBCL patients showed poorer outcomes, which might be mainly due to early disease progression [Citation9]. In the era of rituximab, the treatment response and prognosis of DLBCL patients have been significantly improved [Citation43,Citation44]. However, can DLBCL patients with HBV infection benefit from rituximab treatment? Based on the existing studies, they revealed that rituximab-containing chemotherapy (R-CHOP) did not seem to overcome the inferior outcome conferred by HBV infection when compared with rituximab-absent chemotherapy (CHOP) [Citation9,Citation32], as evidenced by no significant difference of OS and PFS between HBsAg+ DLBCL patients receiving R-CHOP and CHOP chemotherapy. Therefore, new therapeutic strategies specific for these patient subgroups are of crucial demand. On the other hand, BCL6 genetic alternations were frequently observed in HBsAg+ DLBCL patients [Citation12]. BCL-6 inhibitor, FX1, has been demonstrated to inhibit the growth of DLBCL cells in vitro and in vivo [Citation45]. Therefore, targeted therapy against BCL6 might serve as adjuvant therapy to improve the clinical prognosis in HBV-infected DLBCL patients.
Recently, Rong et al. [Citation46] also conducted a meta-analysis which investigated the impact of HBV infection on DLBCL patients. The results were similar to ours. However, compared with Rong XY’s study, our study has several strengths. First, we are confident that our results were reliable supported by large sample size, moderate to high methodological quality of included studies, and low heterogeneity. Second, in the current study, all studies which investigated HBV-associated NHL, including DLBCL as well as other subtypes of NHL, have been properly assessed after an extensive literature search. However, in Rong XY’s study, only DLBCL patients were included for analysis [Citation46]. In addition, several important studies were missed in their meta-analysis. Third, the outcome of hepatic dysfunction during the chemotherapy was analyzed in the current study. To our best knowledge, this was the first study to systematically assess the influence of HBV infection on liver function of NHL patients receiving chemotherapy.
However, there were several limitations in this study. First, when evaluating OSs and PFSs, HR and 95% CI in most of the individual studies were not available from the original article; hence, they were indirectly calculated from Kaplan–Meier curve. The results calculated by this method only reflected the association between HBV infection and NHL patients’ survival. Other risk factors (e.g. Ki-67 expression, MYC/BCL2 protein co-expression, usage of rituximab) which could also predict NHL patient prognosis [Citation47–49] could not be taken into account for in-depth analysis. Second, our study implicated that hepatic dysfunction was a common complication of chemotherapy in HBsAg+ NHL patients. Hepatic dysfunction during chemotherapy may result in termination or delay of chemotherapy. But whether it could lead to an inferior prognosis in HBsAg+ NHL patients remained unknown. Third, we failed to evaluate the effect of resolve HBV infection on NHL in the current meta-analysis despite the high incidence of resolved HBV infection in B-NHL patients [Citation50]. Deng et al. [Citation9] reported that there was a trend that resolve HBV-infection DLBCL patients had inferior survivals compared with those without HBV infection. Therefore, the association of resolve HBV-infection and NHL warrants further study. Fourth, it's worth noting that there might be publication bias in the current study despite no statistical significance of Begg’s test or Egger’s test. HBV is endemic in China. Approximately 7% of the population is inflicted with HBV infection [Citation51]. Hence, most of the included studies were conducted in Chinese patients. Nonetheless, the effect of HBV infection on NHL patients’ prognosis in HBV non-endemic regions, such as European and American countries, has not been well investigated till now. Additionally, after performing comprehensive literature search, we found that all the included studies were retrospective studies. Therefore, NHL patients in our study might not represent the whole NHL population. The results of these outcomes needed to be interpreted with caution. Fifth, in the subgroup analysis of DLBCL patients, the results showed that PFS was not statistically different between DLBCL HBsAg+ and HBsAg− patients, yet OS was worse, which could be explained by a smaller sample size for PFS analysis compared with that for OS analysis as well as significant heterogeneity of included studies for PFS analysis. After removal of the origin of the heterogeneity [Citation25], PFS was statistically shorter in DLBCL HBsAg seropositivity patients than DLBCL HBsAg seronegativity patients (HR, 1.86; 95% CI, 1.42–2.43, p < 0.0001), which translated into the disadvantage of OS. Lastly, patients in most of the included studies had DLBCL. There were only few individual studies focusing on other subtypes of NHL, such as NK/T cell lymphoma and marginal zone lymphoma [Citation28,Citation33,Citation36]. Hence, subgroup analysis could not be performed in other subtypes of lymphoma patients in addition to DLBCL due to the small sample size. In view of the limitations of the current study, well-designed prospective studies with large cohorts in non-Chinese populations should be carried out in different subtypes of NHL, especially indolent lymphoma, in the future to address the issues mentioned earlier.
Conclusions
This meta-analysis provided compelling evidence about an inferior prognosis and distinct clinical characteristics in HBsAg+ NHL patients. Patients with chronic HBV infection were prone to undergoing hepatic dysfunction during chemotherapy.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this present study does not involve human subjects.
Availability of data and material
The data sets used and analysed during the meta-analysis are available from the corresponding author on reasonable request.
Supplemental Material
Download Zip (3.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32.
- de The G. Viruses and human cancers: challenges for preventive strategies. Environ Health Perspect. 1995;103(suppl 8):269–273.
- Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213–215.
- Armand M, Besson C, Hermine O, et al. Hepatitis C virus - associated marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30:41–49.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390.
- Pontisso P, Vidalino L, Quarta S, et al. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev. 2008;8:13–17.
- Wang F, Yuan S, Teng KY, et al. High hepatitis B virus infection in B-cell lymphoma tissue and its potential clinical relevance. Eur J Cancer Prev. 2012;21:261–267.
- Li M, Gan Y, Fan C, et al. Hepatitis B virus and risk of non-Hodgkin lymphoma: an updated meta-analysis of 58 studies. J Viral Hepat. 2018;25:894–903.
- Deng L, Song Y, Young KH, et al. Hepatitis B virus-associated diffuse large B-cell lymphoma: unique clinical features, poor outcome, and hepatitis B surface antigen-driven origin. Oncotarget. 2015;6:25061–25073.
- Law MF, Lai HK, Chan HN, et al. The impact of hepatitis B virus (HBV) infection on clinical outcomes of patients with diffuse large B-cell lymphoma. Eur J Cancer Care (Engl). 2015;24:117–124.
- Liu WP, Wang XP, Zheng W, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma. 2016;57:1355–1362.
- Ren W, Ye X, Su H, et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood. 2018;131:2670–2681.
- Wang F, Xu RH, Luo HY, et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer. 2008;8:115.
- Zhao X, Guo X, Xing L, et al. HBV infection potentiates resistance to S-phase arrest-inducing chemotherapeutics by inhibiting CHK2 pathway in diffuse large B-cell lymphoma. Cell Death Dis. 2018;9:61.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Zhang MY, Chen FY, Zhong H. Meta-analysis of human leukocyte antigen genetic polymorphisms and susceptibility to chronic myelogenous leukemia in Chinese population. Leuk Res. 2011;35:1564–1570.
- Zhang MY, Miao L, Li YS, et al. Meta-analysis of the methylenetetrahydrofolate reductase C677 T polymorphism and susceptibility to Alzheimer's disease. Neurosci Res. 2010;68:142–150.
- Zhang MY, Zhu GQ, Shi KQ, et al. Systematic review with network meta-analysis: comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642–30658.
- Zhang MY, Zhu GQ, Zheng JN, et al. Nucleos(t)ide analogues for preventing HBV reactivation in immunosuppressed patients with hematological malignancies: a network meta-analysis. Expert Rev Anti Infect Ther. 2017;15:503–513.
- Liu Z, Li S, Guo W, et al. MYC gene rearrangements Are closely associated with poor survival of diffuse large B cell lymphoma with hepatitis B virus infection. Biomed Res Int. 2017;2017:1967648.
- Xie W, Zhou D, Hu K, et al. Clinical analysis and prognostic significance of hepatitis B virus infections for diffuse large B-cell lymphoma with or without rituximab therapy. Exp Ther Med. 2013;6:109–114.
- Guo YF, Pan JX, Zhuang WH. Concurrent and reactivation of hepatitis B virus infection in diffuse large B-cell lymphoma: risk factors and survival outcome. Infect Agents Cancer. 2018;13:1–6, doi:10.1186/s13027-018-0215-4
- Huang YH. Clinical characters and prognostic analysis of diffuse large B-cell lymphoma patients with hepatitis B virus infection [dissertation]. Peking Union Medical College; 2012
- Shan LZ, Song T, Wang HQ, et al. Clinical features and prognosis of HBsAg-positive patients with diffuse large B-cell lymphoma. J Pract Oncol. 2016;31:163–169.
- Feng Y, Wang XW, Liu XN, et al. Relationship of HBV infection and diffuse large B-cell lymphoma. Clin J Nosocomiology. 2016;26:2887–2889.
- Lim ST, Fei G, Quek R, et al. The relationship of hepatitis B virus infection and non-Hodgkin’s lymphoma and its impact on clinical characteristics and prognosis. Eur J Haematol. 2007;79:132–137.
- Lv R. Clinical analysis of 112 cases of non-MALT marginal zone lymphoma [dissertation]. Chinese Academy of Medical Sciences & Peking Union Medical College;2015.
- Wang QX, Li JY, Ni QR, et al. Correlation study of hepatitis B virus infection and diffuse large B-cell lymphoma. Int J Virol. 2016;23:332–335.
- Wang Y, Wang H, Pan S, et al. Capable infection of hepatitis B virus in diffuse large B-cell lymphoma. J Cancer. 2018;9:1575–1581.
- Wei HM, Luo CY, Liu KP, et al. The relevance of HBV infection to clinic pathological characteristics and prognosis of diffused large B-cell lymphoma. Chin J Gerontol. 2015;9:2455–2456.
- Wei Z, Zou S, Li F, et al. HBsAg is an independent prognostic factor in diffuse large B cell lymphoma patients in rituximab era: result from a multicenter retrospective analysis in China. Med Oncol. 2014;31:845.
- Yi S, Yan Y, Xiong W, et al. Distinct clinical characteristics draw a new prognostic model for splenic marginal zone lymphoma in HBV high prevalent region. Oncotarget. 2017;8:98757–98770.
- Zhang Y. The relationship between hepatitis B virus infection and diffuse large-B celllymphoma [dissertation]. Zhejiang University; 2012.
- Zhang Y, Yang MZ. The clinical features, BCL-2, CMYC protein expression and prognosis of the hepatitis B virus-associated diffuse large B-cell lymphoma. Acta Univ Med Anhui. 2017;52:570–574.
- Wang L, Wu-Xiao ZJ, Chen XQ, et al. Hepatitis B virus infection correlates with poor prognosis of extranodal natural killer/T cell lymphoma. Leuk Lymphoma. 2015;56:936–941.
- Li WB. Clinical characteristics and prognosis of diffuse large B-cell lymphoma [dissertation]. Shantou University; 2010.
- Dalia S, Chavez J, Castillo JJ, et al. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res. 2013;37:1107–1115.
- Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11:827–834.
- Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792–1798.
- Marcucci F, Spada E, Mele A, et al. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma - a review. Am J Blood Res. 2012;2:18–28.
- Nath A, Agarwal R, Malhotra P, et al. Prevalence of hepatitis B virus infection in non-Hodgkin lymphoma: a systematic review and meta-analysis. Intern Med J. 2010;40:633–641.
- Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. Am J Clin Oncol. 2008;26:4587–4594.
- Gao G, Liang X, Jiang J, et al. A systematic review and meta-analysis of immunochemotherapy with rituximab for B-cell non-Hodgkin's lymphoma. Acta Oncol. 2010;49:3–12. doi:10.3109/02841860903150502
- Cardenas MG, Yu W, Beguelin W, et al. Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma. J Clin Invest. 2016;126:3351–3362. doi:10.1172/jci85795.
- Rong X, Wang H, Ma J, et al. Chronic hepatitis B virus infection is associated with a poorer prognosis in diffuse large B-cell lymphoma: a meta-analysis and systemic review. J Cancer. 2019;10:3450–3458.
- Park JH, Yoon DH, Kim DY, et al. The highest prognostic impact of LDH among International Prognostic indices (IPIs): an explorative study of five IPI factors among patients with DLBCL in the era of rituximab. Ann Hematol. 2014;93:1755–1764.
- He X, Chen Z, Fu T, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer. 2014;14:153.
- Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL rituximab-CHOP consortium program. Blood. 2013;121:4021–4031; quiz 4250.
- Chen MH, Hsiao LT, Chiou TJ, et al. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin's lymphoma. Ann Hematol. 2008;87:475–480.
- Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557.