ABSTRACT
Objectives
Extramedullary disease (EMD) is characterized by plasma cells outside of bone marrow in multiple myeloma (MM) patients, which results in an adverse prognosis. EMD treatment is yet challenging even in the era of novel drugs. Only limited data are available on pomalidomide-based therapy for EMD patients. The purpose of the current study was to assess the efficacy of pomalidomide-based therapy in EMD.
Methods
The current retrospective analysis of six patients assessed the utility of pomalidomide-based therapy in the treatment of EMD.
Results
The median age at diagnosis was 55 (47–70) years. A serological response was observed: 16% (n = 1) complete response (CR), 67% (n = 4) partial response (PR), and 16% (n = 1) progressive disease (PD). Extramedullary overall response rate (ORR) was 83% (n = 5), with 50% (n = 3) CR, 33% (n = 2) PR, and extramedullary progression in one patient. The median progression-free survival (PFS) was 5 months and the median overall survival (OS) was 8 months from diagnosis of EMD.
Discussion and conclusion
Pomalidomide-based therapy showed efficacy in these previously treated patients with EMD.
1. Introduction
Multiple myeloma (MM) is the second most common hematological cancer, with >160,000 patients, leading to nearly 106,000-cancer related deaths worldwide in 2018 [Citation1]. MM is a plasma cell tumor characterized by the proliferation of clonal plasma cells in the bone marrow, monoclonal immunoglobulins in serum or/and urine, and associated organ dysfunction manifestations, including hypercalcemia, renal insufficiency, anemia, and bone lesions [Citation2]. In most MM patients, the proliferation of clonal plasma cells only occurs in the bone marrow. However, a subset of MM patients relapsed with extramedullary disease (EMD), indicating that plasma cells migrate out of the bone marrow [Citation3] and invade any area of tissues and organs [Citation4]. Two types of EMD can be described: the first group comprises tumors that are extending via disruption of cortical bones into contiguous soft tissues (EM-B, bone-related), while the second results from PCs infiltration into soft tissues, with no relationship to the bone (EM-S, soft tissue-related) [Citation5,Citation6] and mainly involving liver, lymph nodes, kidney and skin [Citation4]. EM-S is often associated with shorter OS than EM-B (5 months versus 12 months) and resistance to therapy [Citation7,Citation8]. This may be due to the different biological characteristics of the two entities [Citation9]: EM-B cells are dependent on BM microenvironment and their morphology mimics plasma cells located in bone morrow [Citation10], while EM-S cells exhibit an immature plasmablastic morphology [Citation11,Citation12].
The incidence of EMD varies among previous studies because of varied definitions of EMD and the expanding use of sensitive imaging techniques [Citation13]. Mangiacavalli et al. [Citation5] recently reported that primary EMD (at the time of MM diagnosis) was detected in 14% of patients and secondary EMD (at the time of MM relapse) in 28% using the X-ray, ultrasound, magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT). A high incidence of EMD was reported in patients who underwent allogeneic stem cell transplantation treatment [Citation14]. The EMD is related to adverse prognostic factors, such as 17p deletion, high lactate dehydrogenase, high-risk gene expression, and high presentation of light-chain escape [Citation3].
To the best of our knowledge, there are no current guidelines regarding EMD treatment. In today’s generation of drugs, some targeted drugs have been used to treat EMD in the clinic recently, including proteasome inhibitors (bortezomib, carfilzomib, and ixazomib), immunomodulatory drugs (thalidomide, lenalidomide, and pomalidomide), daratumumab (an anti-CD38 monoclonal antibody), and CAR-T cells [Citation15]. According to a report by Blade, Rosinol, Beksac et al. [Citation8], a proteasome inhibitor-based regimen such as RVD (lenalidomide, bortezomib, dexamethasone) followed by autologous stem cell transplantation (ASCT) may be the best option for transplant-eligible patients with EM-B. For patients with EM-S, a lymphoma-like regimen such as VTD-PACE (bortezomib, thalidomide, dexamethasone, cisplatin, doxorubicin, cyclophosphamide and etoposide) followed by ASCT should be considered. However, the prognosis of EMD patients is poor, with the median overall survival <6 months [Citation7]. Thus, there is an urgent medical demand to identify novel therapeutic strategies for patients with EMD.
Pomalidomide, a derivative of thalidomide, is a new generation of immunomodulatory drugs (IMiDs) authorized by the US Food and Drug Administration (FDA) for the treatment of relapsed and refractory multiple myeloma (RRMM) patients, especially those who are refractory to both lenalidomide and bortezomib. However, limited data are available on pomalidomide in EMD patients. The present study aimed to evaluate the activity of pomalidomide in patients with EMD.
2. Methods
2.1. Patients
Based on the advantage of our database, we identified six EMD patients who received pomalidomide-based therapy in our department. EMD was characterized by plasma cells outside the bone marrow in MM patients based on the current consensus recommendations [Citation3]. The diagnosis of EMD was dependent on biopsy or sensitive imaging techniques, including X-ray, ultrasound, MRI, and PET/CT. We collected and analyzed the patients’ basic characteristics, type and stage of MM, and EMD-related data.
2.2. Treatment, response and statistical analysis
Pomalidomide was administered at a dose of 4 mg from days 1–21 in a 28-day cycle. In the current study, patients took pomalidomide in conjunction with at least one another drug, including dexamethasone, proteasome inhibitors, daratumumab, or chemotherapy.
We detected complete response (CR), partial response (PR), progressive disease (PD), and relapse according to the International Myeloma Working Group (IMWG) and evaluated the progression-free survival (PFS) and overall survival (OS) [Citation16].
Survival analysis was carried out using SPSS26.0. We calculated PFS and OS from EMD to disease progression or death based on the Kaplan–Meier method.
3. Results
3.1. Patients’ characteristics
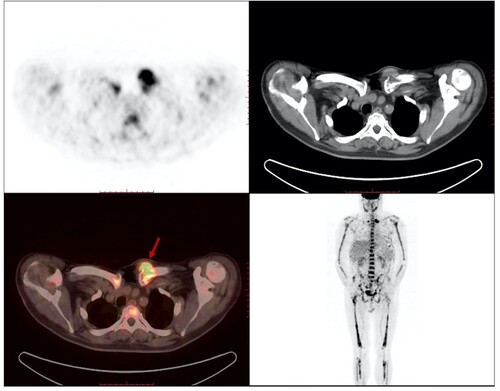
A total of six patients (two females and four males) were treated with pomalidomide regimens. The median age at the time of diagnosis was 55 (range: 47–70) years. Two patients had IgA and four had IgG. There are two patients in Durie–Salmon (D-S) stage ⅢB, two in stage ⅡA, and two in stage ⅢA. For the International Staging System (ISS), one was in stage Ⅰ, one in stage Ⅱ, and four in stage Ⅲ. Four patients had EM-B, one had malignant pleural effusion and one had parenchymal organ involvement (liver and spleen). Four patients had needle biopsy: ascites flow cytometry in patient 1 analyzed 182696 cells; of these, 18.6% were abnormal plasma cells with CD38, CD56, cKAPPA expression and CD45, CD19, CD27, LAMBDA non-expression. Pleural effusion flow cytometry in patient 4 showed a marked number of plasmablasts, indicating EMD. Ultrasound-guided needle biopsy of soft tissue in patients 3 and 5 also revealed EMD because of the presence of monoclonal plasma cells. PET/CT of patient 6 showed a soft tissue mass in the left anterior chest wall with increased FDG metabolism and extramedullary infiltration was considered (). Patients’ characteristics are summarized in .
Table 1. Patients’ characteristics.
3.2. Treatment, response and survival analysis
Median prior lines of therapy were four (range 2–5). Before extramedullary relapse, all the patients had received first-line regimens with a three-drug combination: three of them had received lenalidomide/bortezomib/dexamethasone (RVD) and bortezomib/cyclophosphamide/dexamethasone (VCD) had been administered to the remaining three. Four patients had been treated with autologous hematopoietic stem cell transplantation (auto-HSCT) and two patients had received CAR-T cells infusion at doses of 6.0*106/kg. It is worth noting that patient 2 appeared four para-osseous extramedullary soft tissues half month after BCMA CAR-T cells infusion and CAR-T cells could be detected for at least 60 days in peripheral blood. Patient 5 developed EMD nine months after BCMA CAR-T cells infusion and CAR copy number was below the lower limit of detection in both peripheral blood and bone marrow at the time of extramedullary relapse.
After EMD relapse, pomalidomide was administered at a dose of 4 mg from days 1–21 in a 28-day cycle, and patients received a median of two (range 1–3) cycles. Six patients were treated with different pomalidomide-based regimens according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology version 3.2021 [Citation17]. Pomalidomide/ixazomib/dexamethasone [Citation18,Citation19] and bortezomib/dexamethasone/pomalidomide [Citation20] are preferred regimens, and daratumumab/pomalidomide/dexamethasone [Citation21] is another recommended regimen for previously treated MM patients. Dexamethasone/pomalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide is not widely used in clinical practice but is one of the options for patients with an aggressive relapse requiring multi-drug combinations after previous heavily treatments, including bortezomib, lenalidomide, and ixazomib.
All patients responded to the treatment after one cycle. Patients 1’s ascites disappeared and hepatosplenomegaly reduced from previous size (). Patient 2 was a 47-year-old male with a significant reduction in the size of extramedullary soft tissues. Four soft tissue swellings were noted in the right mandible (6 cm× 5 cm), right neck (6 cm×6 cm), left precordial area (5 cm×4 cm), and right inferior rib (5 cm×4 cm) disappeared after dexamethasone/pomalidomide/cisplatin/doxorubicin/cyclopho-sphamide/etoposide therapy (). Patient 3 was a 70-year-old woman with reduced swelling in the soft tissues in the right ilium (14 cm×10 cm×15 cm) and right knee (12.8 cm×7.3 cm) after using pomalidomide but had two new swellings. Malignant pleural effusion with a large number of plasmablast in patient 4 reduced after bortezomib/pomalidomide/dexamethasone(VPD) treatment and assessed as PR in both serology and EMD. But four months after EMD relapse, the peripheral blood showed plasma cell leukemia and he died one month later due to lung infection and heart failure. Patient 5 and patient 6 were both EM-B relapse and got PR and CR after dexamethasone/pomalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide therapy, respectively.
Figure 2. Abdominal ultrasound showed reduction in hepatosplenomegaly and ascites after receiving pomalidomide based therapy. (A: At diagonisis of EMD; a: Several hypoechoic areas, largest 26*22 mm in size, were seen in liver; b: Splenomegaly, 125*51 mm; c: a dark area of fluid was seen in the abdomen, approximately 87 mm deep. B: After receiving pomalidomide based therapy for three months; d: Several hypoechoic intraheptic areas were smaller than before, the largest one was 18*16 mm; e: Splenomegaly was smaller than before, 113*48 mm; f: inferior abdominal fluid dark area was 65 mm in depth).
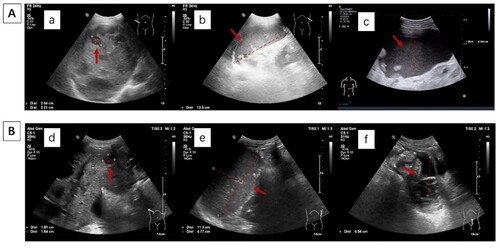
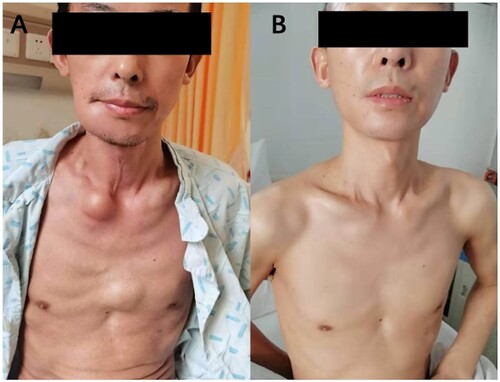
Figure 3. A: Patient 2 had para-osseous extramedullary soft tissues in the right mandible (6 cm ×5 cm), right neck (6 cm × 6 cm), left precordial area (5cm×4 cm), and right inferior rib (5 cm × 4 cm); B: His four soft tissue swellings had a significant reduction after pomalidomide based therapy.
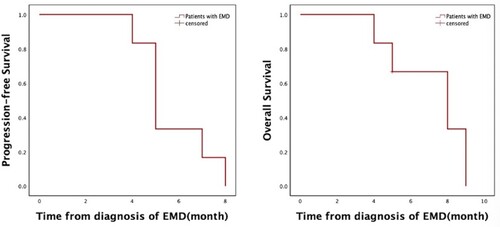
The best medullary response in the six patients was analyzed. Four patients got PR, one got CR, and one experienced medullary progression during treatment with pomalidomide. Next, we detected that extramedullary overall response rate (ORR) was 83% (n = 5) with 50% (n = 3) CR, 33% (n = 2) PR, and extramedullary progression in one patient. The median PFS was 5 months and median OS was 8 months from diagnosis of EMD (). Patients’ responses to treatment are compiled in . Overall, our data manifested an adequate ORR in these heavily pre-treated EMD patients. Treatment regimens and response to treatment are summarized in .
Table 2. Treatment regimens and response to treatment.
4. Discussion
The imaging techniques, such as X-ray, MRI, and PET/CT, gradually increased the detection rate of EMD. An analysis showed that PET/CT had a 96% sensitivity and 77.8% specificity in identifying EMD [Citation22]. The presence of EMD negatively affects survival [Citation3,Citation13]. Varettoni et al. [Citation23] showed that the appearance of EMD at any time of the disease was significantly related to the shortening of OS, and the survival time of patients with EMD was <6 months. Gaelmann et al. [Citation24] reported that in 3744 cases of ASCT patients, 3-year PFS (39.9%, P = 0.001) and OS (58%, P < 0.001) of EMD patients was worse than that of non-EMD patients. Usmani et al. [Citation4] reported that the 5-year OS rate was shorter in EMD (31%) than in non-EMD (59%) patients. The clinical situations of EMD are heterogeneous and the optimal administration of EMD is yet unclear.
In recent years, the treatment methods of EMD mainly include new chemotherapeutics, HSCT, cellular immunotherapy, and targeted molecular therapy. New chemotherapeutics include proteasome inhibitor (PI) with bortezomib as the representative drug and IMiDs with lenalidomide as the representative drug.
Bortezomib is currently the commonly used treatment for EMD patients, which has been recommended by Mayo Clinic in 2017 for EMD patients [Citation25]. Lakshman et al. [Citation26] studied 141 patients with relapsed or refractory multiple myeloma (RRMM) who had been treated with VDT-PACE. The study found that the ORR of EMD and non-EMD patients was 57.1% (29/51) and 52.9% (48/90), respectively, and the difference was not statistically significant between the two groups (P = 0.631), indicating that VDT-PACE regimen might reduce the effect of EMD on the adverse prognosis of RRMM patients. However, VDT-PACE regimen has many adverse reactions, such as hemocytopenia, thrombus, and neurologic disorder [Citation27,Citation28].
Carfilzomib has good efficacy, safety, and tolerability in RRMM patients. Muchtar et al. [Citation29] conducted a retrospective clinical study on 135 RRMM patients who had received carfilzomib-based treatment. The outcomes showed that the presence of EMD was associated with lower ORR (40% vs. 49%, P = 0.39) and significant decrease in clinical benefit rates (CBR) (43.3% vs. 63.5%, P = 0.06), indicating a higher rate of non-response in EMD patients.
The first generation of IMiD thalidomide is commonly used in the treatment of MM, but its effect on EMD is unsatisfactory [Citation30]. In the study by Short et al. [Citation31], 13 patients with EMD received pomalidomide plus low-dose dexamethasone treatment, and the response rate was 31% (4/13), of which two patients obtained CR and two patients obtained PR.
There is no unified conclusion on whether ASCT can overcome the adverse prognosis. Li et al. [Citation32] demonstrated that bortezomib induction therapy combined with ASCT is a better choice for EMD patients, and the regimen does not increase the extramedullary relapse rate of patients. In Lee et al.’s study of 275 MM patients, 27 of 54 patients with EMD were transplant-eligible. No significant difference was noted in OS and PFS between patients with and without EMD in the transplantation groups (P > 0.05), which suggested that auto-HSCT can overcome the adverse prognostic effect of EMD [Citation33]. However, Kumar et al. [Citation34] analyzed 271 MM patients who had received ASCT; the CR rate, median PFS, and median OS of EMD patients were significantly worse than those of non-EMD patients (CR rate was 36.4% and 89.9%, P < 0.002; median PFS period was 18 months and 44 months, P < 0.001; median OS period was 32 months and 100 months, P < 0.001). The results showed that ASCT could not overcome the poor prognosis of EMD.
Jennifer et al. [Citation35] showed one EMD patient whose soft tissue swelling was not appreciable on CT 55 weeks after anti-BCMA CAR-T cell infusion. Xu et al. [Citation36] reported that five patients with EMD who received anti-BCMA CAR-T cell therapy relapsed within one year, and all of the relapsed extramedullary lesion appeared in a new location with medullary progression.
In the current study, we attempted to find effective treatment strategies for MM patients with EMD. To the best of our knowledge, the experience in pomalidomide-based therapy is still limited; hence, we performed a retrospective analysis regarding the effect of pomalidomide in the treatment of EMD. Notably, pomalidomide played an essential role in reducing the EMD in the previously treated patients with malignant pleural effusion in our study. Previously, 14 EMD patients had received treatment with novel agents from December 2007 to May 2014 without pomalidomide. Among them, seven patients received bortezomib-based regimens, two patients received thalidomide-based therapy, three patients received lenalidomide-based regimens, one patient received radiotherapy only, and one patient refused further therapy. The median OS for EMD was only 5 months [Citation37]. The current data demonstrated that EMD patients who received pomalidomide therapy had longer survival time; nonetheless, additional cases need to be explored.
In conclusion, pomalidomide-based treatment strategies have an evident effect on the treatment of EMD but are limited due to a small number of cases.
EMD suggests poor prognosis and should be considered as high-risk MM. Notably, identifying novel treatment strategies and better supportive care for patients suffering from EMD and prolonging their survival time still needs an in-depth investigation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi:10.1038/nrdp.2017.46
- Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127(8):971–976. doi:10.1182/blood-2015-07-635383
- Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761–1767. doi:10.3324/haematol.2012.065698
- Mangiacavalli S, Pompa A, Ferretti V, et al. The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann Hematol. 2017;96(1):73–80. doi:10.1007/s00277-016-2847-z
- Bladé J, Fernández de Larrea C, Rosiñol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805–3812. doi:10.1200/jco.2011.34.9290
- Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99(2):360–364. doi:10.3324/haematol.2013.094409
- Rosinol L, Beksac M, Zamagni E, et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br J Haematol. 2021;194(3):496–507. doi:10.1111/bjh.17338
- Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54(6):1135–1141. doi:10.3109/10428194.2012.740562
- Pasmantier MW, Azar HA. Extraskeletal spread in multiple plasma cell myeloma. A review of 57 autopsied cases. Cancer. 1969;23(1):167–174. doi:10.1002/1097-0142(196901)23:1<167::aid-cncr2820230122>3.0.co;2-0
- Dawson MA, Patil S, Spencer A. Extramedullary relapse of multiple myeloma associated with a shift in secretion from intact immunoglobulin to light chains. Haematologica. 2007;92(1):143–144. doi:10.3324/haematol.10297
- Cerny J, Fadare O, Hutchinson L, et al. Clinicopathological features of extramedullary recurrence/relapse of multiple myeloma. Eur J Haematol. 2008;81(1):65–69. doi:10.1111/j.1600-0609.2008.01087.x
- Sevcikova S, Minarik J, Stork M, et al. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. 2019;36:32–39. doi:10.1016/j.blre.2019.04.002
- Perez-Simon JA, Sureda A, Fernandez-Aviles F, et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20(3):542–545. doi:10.1038/sj.leu.2404085
- Bhutani M, Foureau DM, Atrash S, et al. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1–20. doi:10.1038/s41375-019-0660-0
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi:10.1038/sj.leu.2404284
- Kumar SK, Callander NS, Adekola K, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network. 2020;18(12):1685–1717. doi:10.6004/jnccn.2020.0057
- Voorhees PM, Mulkey F, Hassoun H, et al. Alliance A061202. a phase I/II study of pomalidomide, dexamethasone and ixazomib versus pomalidomide and dexamethasone for patients with multiple myeloma refractory to lenalidomide and proteasome inhibitor based therapy: phase I results. Blood. 2015;126(23):375. doi:10.1182/blood.V126.23.375.375
- Krishnan AY, Kapoor P, Palmer J, et al. A phase I/II study of ixazomib (Ix) pomalidomide (POM) dexamethasone (DEX) in relapsed refractory (R/R) multiple myeloma: initial results. J Clin Oncol. 2016;34(15_suppl.):8008. doi:10.1200/JCO.2016.34.15_suppl.8008
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781–794. doi:10.1016/s1470-2045(19)30152-4
- Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. doi:10.1182/blood-2017-05-785246
- Lu YY, Chen JH, Liang JA, et al. 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: a systematic review and meta-analysis. Nucl Med Commun. 2014;35(7):697–703. doi:10.1097/MNM.0000000000000122
- Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21(2):325–330. doi:10.1093/annonc/mdp329
- Gagelmann N, Eikema DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the chronic malignancies working party of the EBMT. Haematologica. 2018;103(5):890–897. doi:10.3324/haematol.2017.178434
- Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for relapsed multiple myeloma: guidelines from the mayo stratification for myeloma and risk-adapted therapy. Mayo Clin Proc. 2017;92(4):578–598. doi:10.1016/j.mayocp.2017.01.003
- Lakshman A, Singh PP, Rajkumar SV, et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am J Hematol. 2018;93(2):179–186. doi:10.1002/ajh.24954
- Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138(2):176–185. doi:10.1111/j.1365-2141.2007.06639.x
- Muchtar E, Ram R, Raanani P, et al. First line and salvage therapy with total therapy 3-based treatment for multiple myeloma––an extended single center experience. Leuk Res. 2014;38(12):1401–1406. doi:10.1016/j.leukres.2014.06.024
- Muchtar E, Gatt ME, Rouvio O, et al. Efficacy and safety of salvage therapy using Carfilzomib for relapsed or refractory multiple myeloma patients: a multicentre retrospective observational study. Br J Haematol. 2016;172(1):89–96. doi:10.1111/bjh.13799
- Laura Rosiñol MTC, Bladé J, Esteve J, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89(7):832–836.
- Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25(6):906–908. doi:10.1038/leu.2011.29
- Li J, Shen KN, Huang WR, et al. Autologous stem cell transplant can overcome poor prognosis in patients with multiple myeloma with extramedullary plasmacytoma. Leuk Lymphoma. 2014;55(7):1687–1690. doi:10.3109/10428194.2013.853296
- Lee SE, Kim JH, Jeon YW, et al. Impact of extramedullary plasmacytomas on outcomes according to treatment approach in newly diagnosed symptomatic multiple myeloma. Ann Hematol. 2015;94(3):445–452. doi:10.1007/s00277-014-2216-8
- Kumar L, Gogi R, Patel AK, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transplant. 2017;52(10):1473–1475. doi:10.1038/bmt.2017.165
- Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis Relapsed Multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. doi:10.1200/JCO.2018.77.8084
- Xu J, Chen LJ, Yang SS, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116(19):9543–9551. doi:10.1073/pnas.1819745116
- Qu X, Chen L, Qiu H, et al. Extramedullary manifestation in multiple myeloma bears high incidence of poor cytogenetic aberration and novel agents resistance. Biomed Res Int. 2015;2015:787809. doi:10.1155/2015/787809