ABSTRACT
Objectives
Several studies have demonstrated the expression status of CD56 was associated with prognosis in multiple myeloma (MM) patients, the prognostic significance remains controversial. Here, the prognostic value of CD56 is further investigated in MM patients.
Methods
We systematically searched the databases including PubMed, EMBASE, Web of Science and the Cochrane Library. The hazard ratios (HRs) with their 95% confidence intervals (CIs) were pooled to evaluate the relationship between CD56 and overall survival (OS) and progress-free survival (PFS).
Results
A total of 14 studies covering 1365 patients were included in this meta-analysis. The results revealed that CD56 negativity in MM was associated with poorer OS (HR = 1.83, 95% CI = 1.29–2.60, P = 0.001) and PFS (HR = 1.57, 95% CI = 1.27–1.95, P = 0.000). Subgroup analysis further demonstrated that there was an effect of treatment regimen, detection method, survival analysis, study region and the cut-off value of CD56 on CD56 expression.
Discussion and conclusion
The meta-analysis suggested that CD56 negative patients had a poor prognosis for OS in Asian patients and for PFS in non-Asian patients. Novel drugs didn’t show a significant improvement or overcome on the adverse prognosis brought by CD56 negativity. For patients undergone autologous stem-cell transplantation (ASCT), the poor prognosis was overcome by the treatment, which shed a light on the prognostic judgment and individualized treatment.
Introduction
Multiple myeloma (MM) is the second most common hematological malignancy next only to lymphoma, accounting for 13% of all hematological malignancies [Citation1]. At present, it still remains an incurable disease. The development of novel therapeutic drugs including immunomodulatory drugs (e.g., lenalidomide) and proteasome inhibitors (e.g., bortezomib) and the wide application of autologous stem-cell transplantation (ASCT) improve the prognosis of patients with MM considerably in recent years. While not all patients can benefit from the therapy and the response rates differ significantly. The prognosis differs a lot with patients, for some patients, the prognosis is only 1–2 years, while some other patients with a disease duration for over 10 years without progression [Citation2]. Therefore, MM is a plasma cell disease with great heterogeneity in both disease progression and prognosis, it is particularly important to explore the prognostic factors and carry out individualized therapy. Currently, the distinct immune phenotypes of myeloma cells have been gradually recognized with the application of multiparameter flow cytometry. Meanwhile, some studies have demonstrated that immune phenotypes have a close association with the prognosis of MM, which is of great clinical value in the diagnosis and prognostic assessment of diseases [Citation3–5]. But the function of some immune phenotypes, such as CD56, is mainly unknown to date, although it is frequently expressed in myeloma plasma cells, the association between CD56 and prognosis is still disputable.
CD56, a membrane glycoprotein belonging to the immunoglobulin superfamily, is also referred to as neural cell adhesion molecule (NCAM) [Citation6,Citation7]. It is first reported that the expression of CD56 was strongly positive in myeloma plasma cells in 1990 by Van Camp et al [Citation8]. Subsequently, studies have reported that CD56 is expressed in 70–80% of MM patients [Citation9]. It is widely accepted that CD56 is one of the important hallmarks of distinguishing benign and malignant diseases at present because it is hardly expressed on normal cells [Citation10–12]. However, the expression of CD56 did not occur on all myeloma plasma cells, CD56 negative MM patients are also not uncommon. A large number of researches have focused on the clinical characteristics and prognostic factors of CD56 negative MM patients. Nevertheless, the results have not reached a consensus. In this study, a meta-analysis was performed for further comprehensive understanding of the prognostic significance of CD56 in MM.
Materials and methods
Search strategy
The electronic databases including PubMed, Embase, Web of Science and Cochrane Library were systematically searched and the search time limit was from the establishment of each database to March 2021. The search terms were: ‘Multiple Myeloma’ or ‘Myeloma, Multiple’ or ‘Myeloma, Plasma Cell’ or ‘Cell Myeloma, Plasma’ or ‘Myeloma Multiple’ and ‘CD56 Antigen’ or ‘Antigen, CD56’ or ‘CD56’. Our search was restricted to studies of human beings without language restrictions, reference lists of literature were also manually examined for eligible studies to ensure the recall.
Selection criteria
The following inclusion criteria were applied: (1) Study objects were patients diagnosed of multiple myeloma. (2) Evaluation of the relationship between CD56 expression status and prognosis of patients with MM. (3) The studies provided sufficient data to extract or calculate the Hazard ratio (HR) and 95% confidence interval (CI) for Overall survival (OS) and Progression-free survival (PFS). Articles were excluded if they belonged to any of the following circumstances: (1) Case report, review, meta-analysis, letters and conference abstracts. (2) Incomplete or unavailable data. (3) Repeatedly published studies. (4) Studies on non-humans.
Data extraction and quality assessment
The literature screening, data extraction and quality assessment were performed independently by two authors according to the literature inclusion and exclusion criteria. The data extraction content mainly includes: first author, year of publication, country, age of patients, sample size, number of patients with CD56 positivity, treatment regimen, detection method, survival analysis and the cut-off value of CD56 positivity. We also extracted HRs and 95% CIs to evaluate the efficacy of OS and PFS. If HR and 95% CI were not directly presented in the original article, the software Engauge Digitizer 4.1 was used to read the Kaplan-Meier curve and extract cumulative survival rates at each time point, then the resulting data was incorporated into the 1745-6215-816-S1 worksheet provided by Tierney et al to estimate the HR and 95% CI [Citation13]. If univariate and multivariate HRs and 95% CIs were both reported, multivariate results were preferentially considered. The Newcastle-Ottawa Scale (NOS) [Citation14] was used to evaluate the study quality and studies scoring 6–9 are regarded as high quality.
Statistical analysis
All statistical analyses were carried out using Stata ver.16 software (College Station, TX, U.S.A.). HR and odds ratio (OR) with their 95% CI were pooled to assess the associations between CD56 and OS, PFS and clinicopathological characteristics. The χ2-based Q test and the I2 statistics were used to evaluate the heterogeneity among the studies. Heterogeneity was considered significant when P < 0.10 or I2 ≥ 50% and a random effects model was performed, otherwise a fixed effects model was employed. Egger’s test and Begg’s test were applied to assess publication bias. The asymmetric funnel plot or P < 0.05 of Begg’s test indicated the potential of publication bias [Citation15, Citation16]. It was considered to be statistically significant when Bilateral P < 0.05.
Results
Literature selection
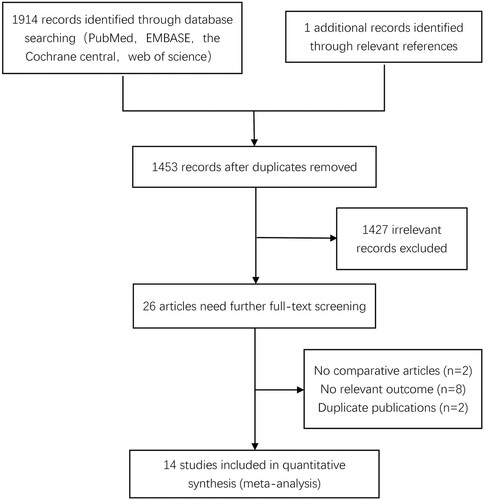
As shown in , 1914 documents were retrieved in databases and one article was identified through the related references. After excluding duplications (n = 462) 1453 articles remained for further screening. A total of 26 literature records were selected by browsing the title and abstract and required further screening through the full text. Twelve studies were excluded for the following reasons according to reading the full text carefully: no relevant outcome, no comparison and duplicated publication. Fourteen articles fulfilling the criteria were included finally [Citation5, Citation17–29].
Study characteristics
The basic characteristics of the 14 eligible studies including 1365 patients were summarized in . All the included studies were published between 2002 and 2020. The sample size in the individual studies ranged from 46 to 215. There were 959 CD56-positive and 406 CD56-negative patients. The positive expression rate of CD56 was within the range of 57.14%–78.57%, which was consistent with previously reports (70%–80%). Nine of the studies were from Asia [Citation17–20, Citation22, Citation26–29], four from Europe [Citation5, Citation21, Citation24, Citation25], and one from Canada [Citation23]. Among these studies, seven studies included patients treated with novel drugs (bortezomib-based or lenalidomide-based therapy) [Citation19–22, Citation27–29], two studies included patients undergone ASCT [Citation5, Citation23], two other studies received traditional therapies [Citation17, Citation25], two further studies received mixed treatment regimen [Citation18, Citation24] and the treatment description was not available in one study [Citation26]. All the studies achieved a NOS score ≥ 6.
Table 1. Characteristics of the 14 included studies.
Analysis of CD56 and OS
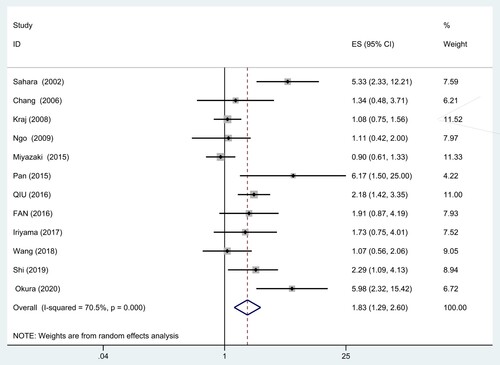
Twelve studies comprising 1211 patients provided the data regarding CD56 expression and OS in MM (). Owing to the significant heterogeneity (I2 = 70.5%, P = 0.000), a random-effect model was applied. The result showed that CD56 negative patients had a significantly shorter OS (HR = 1.83, 95% CI = 1.29–2.60, P = 0.001). Subgroup analysis based on treatment regimen, survival analysis, detection method, study region and the cut-off value of CD56 positivity was carried out to find out the sources of heterogeneity ().
Table 2. Results of subgroup analysis for OS and PFS.
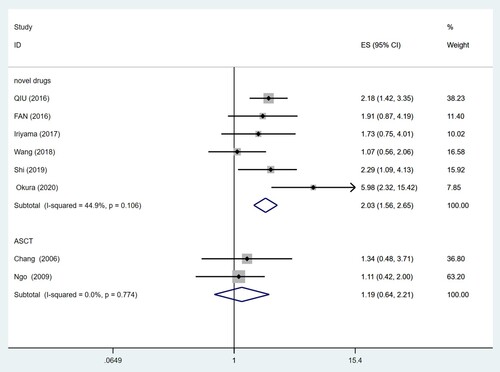
Stratified analysis by treatment regimen suggested that CD56 negativity was significantly associated with poorer OS (HR = 2.03, 95% CI = 1.56–2.65, P = 0.000) even in the case of novel therapeutic drugs. However, similar results were not noticed in the treatment of ASCT (HR = 1.19, 95% CI = 0.64–2.21, P = 0.583). There was no significant heterogeneity among the two subgroups (I2 = 44.9%, P = 0.106; I2 = 0.0%, P = 0.774) ().
The pooled HR for univariate analysis and multivariable analysis were 1.77 (95% CI = 1.18–2.65, I2 = 74.4%) and 2.14 (95% CI = 0.96–4.76, I2 = 58.4%) respectively.
A variety of detection methods were used in the studies, flow cytometry (FCM) was applied in most of these studies. Immunohistochemistry (IHC), an additional detection method, was applied in only a few studies. In subgroup analysis of FCM, CD56 negativity was significantly associated with poorer OS (HR = 1.99, 95% CI = 1.33–2.96, I2 = 75.4%). No such correlation was found in the subgroup of IHC (HR = 1.19, 95% CI = 0.64–2.21, I2 = 0.0%).
The correlation of CD56 negativity with worse OS was maintained only in patients of Asian (HR = 2.20, 95% CI = 1.40–3.45, I2 = 74.6%) but not non-Asian patients (HR = 1.11, 95% CI = 0.81–1.52, I2 = 0.0%).
The cut-off value of CD56 positivity most commonly used in studies was 20%. There were also other cut-offs (50%, 10%) that were used only in a few studies, so the meta-analysis was not performed in the latter. The significant effect and heterogeneity of CD56 negativity on OS were observed in group of 20% (HR = 1.83, 95% CI = 1.18–2.85, I2 = 69.6%).
Analysis of CD56 and PFS
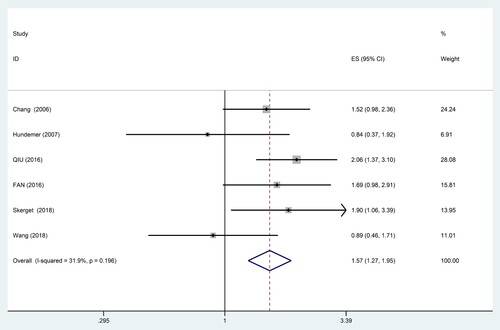
Effect of CD56 on PFS could be evaluated in 6 studies including 530 patients. The result indicated that lack of CD56 expression was significantly associated with poorer PFS in MM (HR = 1.57, 95% CI = 1.27–1.95, P = 0.000), with moderate heterogeneity (I2 = 31.9%, P = 0.196) (). The subgroup analysis was also performed based on treatment regimen, survival analysis, detection method, study region and the cut-off value of CD56 positivity ().
Stratified analysis by treatment regimen suggested that CD56 negativity was significantly associated with poorer PFS (HR = 1.91, 95% CI = 1.44–2.54, P = 0.000) even in the case of novel therapeutic drugs. There was no significant heterogeneity (I2 = 0.0%, P = 0.849).
The pooled HR for univariate analysis was 1.65 (95% CI = 1.32–2.06, I2 = 19.3%).
In subgroup analysis by detection method, significant effect of CD56 negativity on PFS was observed in FCM subgroup (HR = 1.59, 95% CI = 1.24–2.04, I2 = 45.3%).
The correlation of CD56 negativity with worse PFS was maintained only in patients of non-Asian (HR = 1.49, 95% CI = 1.08–2.05, I2 = 21.2%) but not Asian patients (HR = 1.54, 95% CI = 0.97–2.44, I2 = 56.5%).
The significant effect of CD56 negativity on PFS was not observed in group of 20% (HR = 1.41, 95% CI = 0.62–3.20, I2 = 78.2%).
The treatment of ASCT, multivariable analysis, IHC and 50% cut-off were used only in one study respectively and, therefore, meta-analysis were not performed.
Association of CD56 and clinicopathological features
In addition, we evaluated the possible correlation between CD56 and different clinicopathological features of MM patients (). We found CD56 negative patients were less likely to have bone destruction (OR = 0.50, 95% CI = 0.34–0.74, I2 = 15.4%, P = 0.001), but there was no significant correlation between CD56 and age, sex, β2-microglobulin, serum creatinine, serum calcium levels, clinical stage, bone marrow plasma cell percentage and extramedullary disease.
Table 3. The association between CD56 and clinicopathological characteristics of MM.
Sensitivity analysis and publication bias
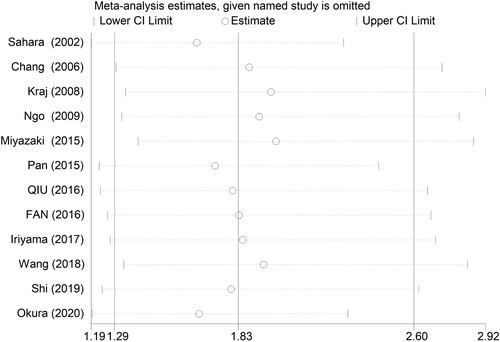
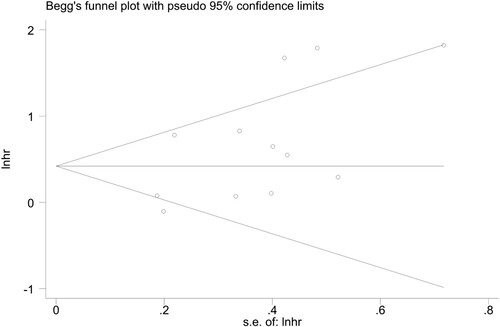
Sensitivity analysis was performed by omitting one individual study each time to explore whether the results were robust. We found no individual study significantly influenced the overall HR for OS (). As shown in , the funnel plot of Begg’s seemed symmetrical and there was no significant evidence of publication bias in the current meta-analysis (Begg’s P = 0.086).
Discussion
It is very important to predict the clinical outcome and identify at-risk patients in clinical practice. R-ISS stratification system is recommended to evaluate the prognosis by the international myeloma working group (IMWG) in 2013 [Citation30]. Several previous studies have demonstrated that the treatment based on bortezomib can overcome the effect of cytogenetic abnormalities on the poor prognosis of MM [Citation31, Citation32]. Consequently, R-ISS is not highly predictive of prognosis in patients with MM in the novel agents era. In recent years, immunophenotyping by flow cytometry is playing a more and more crucial role in the diagnosis and prognosis of MM. It is a simpler method to use immunophenotype as a prognostic marker than R-ISS.
In this meta-analysis, 14 eligible studies covering 1365 patients were included. Results showed CD56 negativity was a poor prognostic factor for OS (HR = 1.83, 95% CI = 1.29–2.60, P = 0.001) and PFS (HR = 1.57, 95% CI = 1.27–1.95, P = 0.000) in MM patients. Besides, we found that CD56 negative patients were less likely to have bone destruction. The lack of CD56 expression may lead to a higher trend circulating in peripheral blood for myeloma cells and a lower occurrence probability of osteolytic complications [Citation33], which also explains that CD56 negative patients had a poorer prognosis, to a certain extent. Heterogeneity was found in analysis of the correlation between CD56 and OS (I2 = 70.5%) and PFS (I2 = 31.9%), which seemed to reflect the association between treatment regimen, survival analysis, detection method, study region and the cut-off value of CD56 positivity and OS and PFS.
Stratified analysis by treatment regimen showed that novel drugs failed to overcome or improve the adverse prognosis brought by CD56 negativity. CD56 expression status had no significant correlation with the prognosis of MM in the case of ASCT, which indicated that ASCT may have overcome the adverse prognostic effect. The present result also supports that ASCT is considered as an essential component of induction treatment for eligible patients. However, CD56 negativity was not independently associated with poor prognosis in MM in the studies that carried out multivariate analysis. This is in accordance with the most recent study by Koumpis et al showing that absence of CD56 expression was not an independent prognostic factor [Citation34]. For CD56 to be included in the prognostic score for high risk it needs to be proved that it is an independent prognostic marker in prospective studies. We also found CD56 was a prognostic factor for OS only in Asian patients and the prognostic value was not reflected in non-Asian patients provisionally, but this was reversed in PFS. The reasons for this inconsistency might be due to the limited sample sizes or the small number of the included studies. With reference to technical factors, the 20% cut-off had significant prognostic value for OS but not PFS. Only FCM was significantly associated with poor OS and PFS. These results revealed that technical factors and study region can affect the relationship between CD56 and prognosis in MM patients, such differences should be fully considered in future studies of CD56 and the technical method should be unified as much as possible to make the results convincible.
CD56 plays a role in holding myeloma cells together and tethered to the marrow stroma, and limiting the extramedullary spreading of cells, while a lack of CD56 expression is prone to extramedullary infiltration [Citation19, Citation35, Citation36]. In addition, CD56 absence may weaken the interactions between cells and enhance the secretion of MMP-9 at the same time, which is involved in basal membrane degradation that furthers myeloma cells dissemination [Citation10, Citation33]. Thus, the lack of CD56 expression suggests a worse prognosis in theory, which is in line with the results of our study.
However, some limitations exist in the meta-analysis. First, the relatively small sample sizes (n < 200) were included in most of the studies. Second, there are no standard schemes and technical provisions of MM by flow cytometry at home and abroad and the gate scheme was not uniform among the healthcare structures, which may have some influence on the results. Third, stratified analysis by survival analysis suggested that CD56 negativity was not an independent prognostic factor for MM patients. But only 3 studies evaluated CD56 using multivariate analysis, and the remaining studies performed univariate analysis. The result should be further validated by incorporating more studies that carried out multivariate analysis. Fourth, part of the survival data was extracted from the survival curves due to the lack of original survival data. It was potentially prone to bias during extraction although it had been recognized by many scholars. Finally, the reliability of the results still needs to be further validated because fewer data were available for clinicopathological features.
Conclusions
In conclusion, our meta-analysis suggested that CD56 negative patients had a poor prognosis for OS in Asian patients and for PFS in non-Asian patients. Novel drugs even failed to overcome or improve the adverse prognosis brought by CD56 negativity. For patients undergone ASCT, the poor prognosis was overcome by the treatment. Therefore, in the novel agents era, it still needs ASCT to be used as part of induction therapy in CD56 negative patients and more positive and effective treatment measures for individualized treatment should be taken to improve the prognosis. However, CD56 lost independent prognostic value when a multivariate analysis was performed, so prospective studies are needed in order to evaluate the role of expression of CD56 as a predictive biomarker in the era of novel regimens.
Acknowledgements
We would like to thank all of the study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Palumbo A, Anderson K. Multiple myeloma. New Engl J Med. 2011;364(11):1046–1060.
- Smith D, Yong K. Advances in understanding prognosis in myeloma. Brit J Haematol. 2016;175(3):367–380.
- Ayed A, Chang L, Moreb J. Immunotherapy for multiple myeloma: Current status and future directions. Critl Rev Oncol Hematol. 2015;96(3):399–412.
- Robillard N, Wuillème S, Lodé L, et al. CD33 is expressed on plasma cells of a significant number of myeloma patients, and may represent a therapeutic target. Leukemia. 2005;19(11):2021–2022.
- Ngo NT, Brodie C, Giles C, et al. The significance of tumour cell immunophenotype in myeloma and its impact on clinical outcome. J Clin Pathol. 2009;62(11):1009–1015.
- Cunningham B, Hemperly J, Murray B, et al. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236(4803):799–806.
- Lanier L, Testi R, Bindl J, et al. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169(6):2233–2238.
- Van Camp B, Durie B, Spier C, et al. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood. 1990;76(2):377–382.
- Harrington A, Hari P, Kroft S. Utility of CD56 immunohistochemical studies in follow-up of plasma cell myeloma. Am J Clin Pathol. 2009;132(1):60–66.
- Bataille R, Jégo G, Robillard N, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006;91(9):1234–1240.
- Damgaard T, Knudsen LM, Dahl IM, et al. Regulation of the CD56 promoter and its association with proliferation, anti-apoptosis and clinical factors in multiple myeloma. Leuk Lymph. 2009;50(2):236–246.
- Rawstron AC, Orfao A, Beksac M, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–438.
- Tierney J, Stewart L, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Sahara N, Takeshita A, Shigeno K, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br J Haematol. 2002;117(4):882–885.
- Pan Y, Wang H, Tao Q, et al. Absence of both CD56 and CD117 expression on malignant plasma cells is related with a poor prognosis in patients with newly diagnosed multiple myeloma. Leuk Res. 2016;40:77–82.
- Qiu Q, Zhu P, Wang MJ, et al. Expression of CD56 and CD19 in patients with newly diagnosed multiple myeloma and their relationship with karyotypes and prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(4):1071–1078.
- Shi J, Sun K, Zhu ZM, et al. Prognostic significance of CD56 and CD117 expression in patients with newly diagnosed multiple myeloma treated with bortezomib-based first-line therapy. Zhonghua Xue Ye Xue Za Zhi. 2019;40(8):693–696.
- Skerget M, Skopec B, Zadnik V, et al. CD56 expression is an important prognostic factor in multiple myeloma even with Bortezomib induction. Acta Haematol. 2018;139(4):228–234.
- Okura M, Ida N, Yamauchi T. The clinical significance of CD49e and CD56 for multiple myeloma in the novel agents era. Med Oncol. 2020;37(11):103.
- Chang H, Samiee S, Yi QL. Prognostic relevance of CD56 expression in multiple myeloma: a study including 107 cases treated with high-dose melphalan-based chemotherapy and autologous stem cell transplant. Leuk Lymphoma. 2006;47(1):43–47.
- Kraj M, Sokołowska U, Kopeć-Szlezak J, et al. Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leuk Lymphoma. 2008;49(2):298–305.
- Hundemer M, Klein U, Hose D, et al. Lack of CD56 expression on myeloma cells is not a marker for poor prognosis in patients treated by high-dose chemotherapy and is associated with translocation t(11;14). Bone Marrow Transpl. 2007;40(11):1033–1037.
- Miyazaki K, Suzuki K. Clinical and laboratory significance of CD56 (a neural cell adhesion molecule) positivity in multiple myeloma and AL amyloidosis. Int J Myelom. 2015;50(3):30–35.
- Fan QY, Wang Y, Mi JQ. Study on the efficacy of bortezomib on CD56 negative multiple myeloma patients. J Shanghai Jiaotong Univ. 2016;36(10):1445–1450.
- Iriyama N, Miura K, Hatta Y, et al. Clinical effect of immunophenotyping on the prognosis of multiple myeloma patients treated with bortezomib. Oncol Lett. 2017;13(5):3803–3808.
- Wang H, Zhou X, Zhu JW, et al. Association of CD117 and HLA-DR expression with shorter overall survival and/or progression-free survival in patients with multiple myeloma treated with bortezomib and thalidomide combination treatment without transplantation. Oncol Lett. 2018;16(5):5655–5666.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869.
- Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634.
- Jagannath S, Richardson P, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21(1):151–157.
- Pellat-Deceunynck C, Barillé S, Jego G, et al. The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia. 1998;12(12):1977–1982.
- Koumpis E, Tassi I, Malea T, et al. CD56 expression in multiple myeloma: correlation with poor prognostic markers but no effect on outcome. Pathol Res Pract. 2021;225:1–5.
- Blaheta R, Beecken W, Engl T, et al. Human cytomegalovirus infection of tumor cells downregulates NCAM (CD56): a novel mechanism for virus-induced tumor invasiveness. Neoplasia. 2004;6(4):323–331.
- Ceran F, Falay M, Dağdaş S, et al. The assessment of CD56 and CD117 expressions at the time of the diagnosis in multiple myeloma patients. Turk J Haematol. 2017;34(3):226–232.