ABSTRACT
Fertility is a concern in young female survivors of hematological malignancies. We evaluated post-treatment ovarian function in patients by measuring anti-Müllerian hormone (AMH) and conventional hormone levels to correlate with menstruation and fertility.
The prospective cohort study included 29 reproductive-aged women diagnosed with Hodgkin lymphoma (n = 11), non-Hodgkin lymphoma (n = 9) or acute myeloid leukemia (n = 9). Hormone assays were measured after treatment was completed and compared to age-matched healthy controls. Menstrual changes and postmenopausal symptoms were assessed annually.
Serum AMH levels were significantly lower compared to controls at 12 months after treatment [1.0 (0.18–1.8) vs. 2.2 (1.8–4.8) ng/mL; P < .001). At 12 months, FSH and LH levels were significantly higher compared to controls. The interruption of menstrual cycles was observed in 80% (22/27) of patients. Normal menstruation returned at a median of 1.5 months after cessation of treatment in 71% of patients, while 29% of patients had persistent amenorrhea. Low AMH levels at 12 months after therapy (<1 ng/mL) correlated more strongly with abnormal menstrual cycles than normal AMH levels (46% vs. 0%, P = .04). Four patients with low AMH consulted an infertility clinic.
In summary, low serum AMH at 12 months after chemotherapy was associated with persistent menstrual abnormalities.
Introduction
Recent advances in cancer therapy have improved long-term survival of patients with hematological malignancies [Citation1,Citation2]. Although the majority of these patients are post-menopausal, a significant number are adolescents and young adults. The most common childhood cancer is leukemia constituting 28% of cases. More than 90% of adolescent Hodgkin lymphoma patients, 60% of young acute myeloid leukemia patients and nearly 100% of young acute lymphoblastic leukemia patients are expected to be in remission after treatment [Citation3]. As the number of young survivors grows, the long-term complications from cancer therapies need to be addressed [Citation4].
Reproductive-aged women who survive cancer therapy are at high risk for premature ovarian failure and infertility depending on treatment modality [Citation5]. Alkylating agents and radiation therapy are known to jeopardize ovarian function in a dose-dependent manner [Citation6,Citation7]. The effects of chemotherapy that lead to ovarian suppression may lead to infertility, premature menopause, and a considerable risk of osteoporosis [Citation8,Citation9]. Concerns of various conditions associated with chemotherapy treatment in reproductive-aged women significantly contribute to mental distress and impact quality of life [Citation10].
According to a previous study, it was showed that serum anti-Müllerian hormone (AMH) levels are a sensitive marker of ovarian reserve and fertility in reproductive-aged women receiving chemotherapy [Citation11–13]. AMH is a dimeric glycoprotein in the TGF-β family and is produced by the granulosa cells of ovarian follicles [Citation14,Citation15]. Furthermore, studies in mice demonstrated that AMH has an inhibitory effect on follicular growth and prevents premature follicular exhaustion [Citation16,Citation17]. Previous data showed that serum AMH levels are more strongly correlated with early antral follicles than conventional hormone measurements [Citation18,Citation19]. Among hematological malignancy patients, serum AMH is a reliable marker indicating an ovarian reserve in young women treated by chemotherapy or stem cell transplantation [Citation20–24]. Indeed, before receiving chemotherapy, female lymphoma patients have significantly lower serum AMH levels than breast cancer patients and healthy age-matched controls [Citation20]. The low AMH levels are associated with fewer oocytes retrieving from lymphoma patients compared to those with breast cancer and healthy controls. This is suggestive of lymphoma posing a significant risk for compromised ovarian functions [Citation20,Citation25].
Hematological malignancies usually require urgent treatment. Patients frequently present with cytopenia, rending invasive procedures infeasible. Therefore, preserving fertility is challenging during this period. Before chemotherapy initiation, patients should be aware of the reproductive risk posed. Informing patients about this risk and potential solutions may reduce stress and improve quality of life.
In this study, we aimed to assess longitudinal ovarian reserve using post-treatment serum AMH levels and conventional hormone assays correlating with the long-term menstruation and fertility.
Materials and methods
Patients
Reproductive-aged women diagnosed with Hodgkin lymphoma, non-Hodgkin lymphoma and acute myeloid leukemia were prospectively recruited at King Chulalongkorn Memorial hospital between January 2018-January 2019. Female acute lymphoblastic leukemia patients were not included in the study because the extended period of the treatment protocol. All eligible patients received either chemotherapy, or chemotherapy followed by hematopoietic stem cell transplantation (HSCT).and were in remission at the time of the study. Chemotherapy regimens are provided in the Supplementary Table 1. Age-matched healthy female subjects who were not currently using any hormonal treatment and had regular menstrual cycles served as controls. Patients who were diagnosed with acute promyelocytic leukemia, ovarian lymphoma, those with a history of unilateral or bilateral oophorectomy, previous malignancies, pregnancies after treatments, relapsed diseases, or patients with a history of preexisting abnormal menstrual cycles were excluded.
Informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (No. IRB 004/61) and followed the Declaration of Helsinki.
Hormone level measurements
Blood samples from patients for AMH, estradiol, FSH, and LH levels were collected at 3, 6 and 12 months after the end of treatments and compared to age-matched healthy controls. Blood for hormonal analysis was drawn from the antecubital vein. Serum concentrations of AMH, estradiol, FSH and LH were measured in the same laboratory using immunoassay for in vitro quantitative determination and analyzed with specific ELISA kits (Elecsys, Roche, Mannheim, Germany) and a Cobas e411 analyzer at the central laboratory of King Chulalongkorn Memorial Hospital. The detection limits of AMH, estradiol, FSH and LH ranged from 0.01–23 ng/mL, 5–3000 pg/mL, 0.1–200 IU/mL, and 0.1–200 IU/mL, respectively.
Menstruation and postmenopausal symptoms assessment
Questionnaires regarding longitudinal changes in menstruation and postmenopausal symptoms (provided in supplementary material) at pre-treatment, during treatment, and post-treatment were completed by patients upon completion of chemotherapy and then annually until the study was completed.
Statistical analysis
Normality of the data was tested using the Shapiro–Wilk test. Continuous data are presented as means (± standard deviations, SD) or median (± interquartile range, IQR) as appropriate. Comparisons of continuous data and categorical data were analyzed by the unpaired Student t-test or Mann–Whitney U test and Chi-square method, respectively. Statistically significant differences between two or more groups were tested using the Kruskal–Wallis test. A p-value of < .05 constituted statistical significance. All statistical parameters were analyzed using GraphPad Prism version 9.1.2 for Windows, GraphPad Software, San Diego, California USA.
Results
Patients
Twenty-nine reproductive-aged women diagnosed with Hodgkin lymphoma (n = 11), non-Hodgkin lymphoma (n = 9) and acute myeloid leukemia (n = 9) were enrolled in the study. Patient ages ranged from 18–40 years old with a median of 32 years. Patients either received chemotherapy (n = 24; 83%) or underwent hematopoietic stem cell transplantation (HSCT) (n = 5; 17%). Eight patients received a non-alkylating agent containing regimen (ABVD), sixteen patients received a cyclophosphamide/ifosfamide containing regimen and five patients underwent HSCT. Baseline clinical characteristics of patients were summarized in .
Table 1. Clinical characteristics and treatment data of the patients.
Hormone levels
Serum AMH levels of patients were slightly lower compared to controls at 3 months [1.6 (0.0–4.2) vs. 2.2 (1.8–4.8); P = .06], but statistical significance was not reached until 6 months [0.3 (0–1.8) vs. 2.2 (1.8–4.8); P = .004]. For conventional hormone assays, FSH levels were statistically higher than controls at each time point [3 months: 8.2 (4.2–91.0), 6 months: 8.4 (5.0–90.0) and 12 months: 7.1 (5.2–9.8) vs. 4.3 (2.2–5.0); P = .01, .02 and <.001, respectively], but LH levels were only statistically higher at 12 months [8.4 (4.1–15.0) vs. 4.7(3.6–7.6); P = .02. No differences were found between patient estradiol levels and those of healthy subjects as shown in . AMH, estradiol, FSH, and LH levels of patients were compared to age-matched healthy controls in . Patients undergoing HSCT had markedly low AMH followed by alkylating base chemotherapy, while AMH levels of patients who received non alkylating agents were within normal limits (<0.01 ng/mL vs. 0.48 (0.02–0.82) vs. 1.6 (0.5–2.5) ng/mL; P = .001 and P = .005, respectively) ().
Figure 1. Longitudinal changes in hormone levels among patients with hematologic malignancies compared to those of age-matched healthy controls. The dot lines indicated median ± interquartile range. AMH, Anti-Mullerian hormone; FSH, Follicle stimulating hormone; LH, Luteinizing hormone.
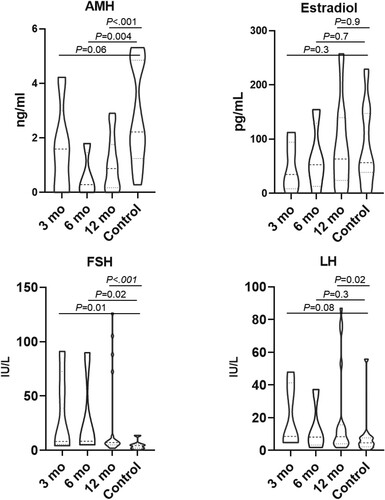
Figure 2. Hormone levels of patients classified by treatment modalities. The lines in the indicated median and the boxes indicated interquartile ranges. AMH, Anti-Mullerian hormone; FSH, Follicle stimulating hormone; LH, Luteinizing hormone; CMT, Chemotherapy; SCT, Stem cell transplantation.
Table 2. Hormone levels of patients compared with age-matched healthy controls.
Conventional hormone levels including estradiol, FSH and LH levels significantly differed between patients who underwent HSCT vs. chemotherapy (Estradiol: 0 (0–5.2) vs. 75.6 (47.0–141.5), P < .001; FSH: 91.0 (80.2–115.7) vs. 4.6 (2.8–7.0) P < .001: LH: 73.8(45.1–86.9) vs. 6.9(4.0–11.4) P < .001). (Supplementary Figure 1) Levels were found to be similar between patients who received non-alkylating agents compared to the alkylating agent group [Estradiol: 69.1(39.1–137.6) vs. 86.12 (53.6–167.5), P = .5: FSH: 4.4 (3.0–7.1) vs. 4.8 (2.1–7.6), P = .8: LH: 7.15 (4.7–10.7) vs. 5.8(3.4–13.7) P = .8, .]
Long-term menstruation changes and postmenopausal symptoms
Interruption of menstrual cycles during treatment was observed in 80% of patients (22/27). Follow-up data were available for 21 patients with a median follow-up time of 51 months (range 32–99 months). Normal menstruation returned shortly after cessation of treatment with a median time of 1.5 months in 71% (15/21) of patients. Six patients (28.5%) had persistent amenorrhea, four of whom were HSCT patients. The other two NHL patients had received the alkylating agent containing regimen. Five patients (18%) reported post-menopausal symptoms during the 12-month period following chemotherapy. All symptoms were resolved during follow-up.
We classified patients into a low AMH group (serum AMH <1 ng/mL) and normal AMH group (AMH ≥1 ng/mL) based on the 12-month AMH levels. The low AMH group (N = 17) comprised eight patients receiving an alkylating agent containing regimen, four cases of a non-alkylating agent containing regimen, and five patients undergoing HSCT ((a)). In the normal AMH group, ten patients received a non-alkylating agent regimen, and two patients received an alkylating agent containing regimen ((b)).
Figure 3. Serum Anti-Mullerian hormone (AMH) levels and its clinical relevance A. Pie charts showing received treatments. The patient groups were classified according to their 12-month AMH levels B. Association of hormone levels and patient long-term outcomes.
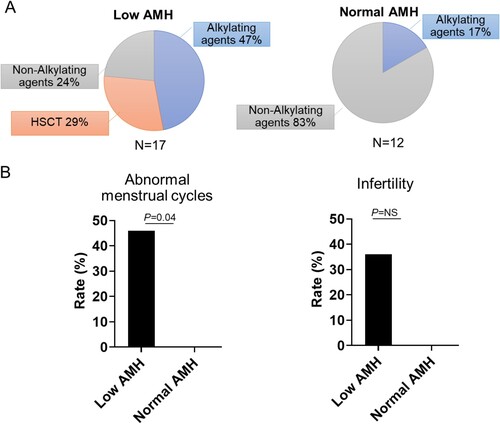
At 12 months after therapy, low AMH patients showed a higher rate of abnormal menstrual cycles compared to patients with normal AMH levels [ 46% (6/13) vs. 0% (0/8), P = .04] as shown in (c). The low AMH group experienced a higher rate of infertility [31% (4/13) vs. 0% (0/8) P = .2], although statistical significance was not reached due to the small sample size ((d)). FSH and LH levels were not significantly associated with long-term menstrual abnormalities.
Two patients from the cohort were able to conceive a child. One was an NHL patient with low AMH levels who received dose-adjusted EPOCH for six cycles. She conceived at 36 months after therapy. The other was a Hodgkin lymphoma patient who received ABVD and reported normal AMH levels.
Discussion
In this study, we performed assessments of AMH, estradiol, FSH, and LH levels to predict clinically relevant ovarian dysfunction after hematologic malignancy treatment using chemotherapy or HSCT. Our study showed that post-treatment AMH levels in the patients with hematologic malignancies were lower than those of age-matched controls, especially at 12 months. FSH and LH levels were also different at 12 months but not for estradiol.
Lie Fong et al. investigated a cohort of 25 patients with hematologic malignancies treated with multi-drug chemotherapy or high dose therapy followed by HSCT showing similar results. Patients who received chemotherapy had significantly lower level of serum AMH and high FSH than controls, while estradiol levels were maintained. In those who underwent HSCT, serum AMH concentrations were undetectable, as were estradiol levels, and FSH serum levels were significantly increased compared to controls [Citation26]. This suggests that the ovarian damage caused by gonadotoxic chemotherapy was due to a diminished primordial follicle rather than a total follicle loss [Citation27].
Chemotherapy is a well-known cause of ovarian damage, especially to primordial follicles [Citation28]. AMH is secreted from antral follicles reflecting ovarian function. The AMH levels of patients receiving non-alkylating agent containing regimens were normal after 12 months of cancer treatment, while patients receiving alkylating agent containing regimens or undergoing HSCT had persistently low AMH throughout the follow-up period consistent with previous reports [Citation21,Citation29]. On contrary, most conventional hormone assays were unable to detect differences between treatment regimens.
We demonstrated that normal AMH levels at 12 months after cancer treatments were associated with menstruation recovery, while AMH levels taken early after therapy was not reflect this outcome. Previous reports evaluated fertility of female patients who received high dose cyclophosphamide and total body irradiation with ovarian shielding. Serum AMH levels were extremely low immediately after HSCT. However, the serum AMH level gradually increased, starting after 1 year after transplants [Citation30,Citation31]. Menstrual recovery was 42% and 78% at 6 months and 1 year after HSCT, respectively [Citation30]. This indicated that early AMH levels did not reflect ovarian reserves after high dose therapy.
In this study, patients with low post-treatment AMH levels an increased risk of abnormal menstrual cycles. All patients with persistent amenorrhea had extremely low or undetectable post-treatment AMH levels. Our data reinforced that blood testing at one year post-treatment is an appropriate time point for long-term clinically relevant ovarian dysfunction. Data from patient questionnaires revealed that several patients sought infertility counseling and treatments.
Young female cancer survivors are at risk for long-term effects on ovarian functioning from cancer therapy. Previous studies have has shown that female cancer survivors typically receive incomplete fertility-related information from healthcare professionals during illness and treatment [Citation32]. In Southeast Asia, the awareness of reproductive health was still low [Citation33]. Woman reproductive health in these countries indeed deserves a special attention. In addition to medical concerns, reproductive problems among female cancer survivors may also lead to moderate to severe depression [Citation34]. In Thailand, most patients with hematologic malignancies are referred from community hospitals to tertiary care centers for treatment. When patients reach tertiary centers, evaluation and treatment need to be performed expeditiously. Fertility issues are often inadequately addressed at the time of diagnosis. After treatment, there is only a short fertility window for patients to receive counseling regarding family planning before proceeding to the subsequent therapy [Citation5].
The greatest strength of this study is the long-term follow-up of the patients with the median of 51 months. A major limitation is the small sample size. Future validation in a larger population is warranted.
Supplemental Material
Download MS Word (57.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ehrhardt MJ, Hochberg J, Bjornard KL, et al. Long-term survivors of childhood, adolescent and young adult non-Hodgkin lymphoma. Br J Haematol. 2019;185(6):1099–1110.
- Bartram J, Veys P, Vora A. Improvements in outcome of childhood acute lymphoblastic leukaemia (ALL) in the UK - a success story of modern medicine through successive UKALL trials and international collaboration. Br J Haematol. 2020;191(4):562–567.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Loren AW. Fertility issues in patients with hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2015;2015:138–145.
- Rossi BV, Missmer S, Correia KF, et al. Ovarian reserve in women treated for acute lymphocytic leukemia or acute myeloid leukemia with chemotherapy, but not stem cell transplantation. ISRN Oncol. 2012;2012:956190.
- Green DM, Sklar CA, Boice, Jr. JD, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2374–2381.
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–1312.
- Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf. 2005;28(5):401–416.
- Lower EE, Blau R, Gazder P, et al. The risk of premature menopause induced by chemotherapy for early breast cancer. J Womens Health Gend Based Med. 1999;8(7):949–954.
- Gargus E, Deans R, Anazodo A, et al. Management of primary ovarian insufficiency symptoms in survivors of childhood and adolescent cancer. J Natl Compr Canc Netw. 2018;16(9):1137–1149.
- Peigné M, Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reprod Biol Endocrinol. 2014;12:26.
- Li X, Liu S, Ma L, et al. Can anti-Müllerian hormone Be a reliable biomarker for assessing ovarian function in women postchemotherapy? Cancer Manag Res. 2020;12:8171–8181.
- Miyoshi Y, Ohta H, Namba N, et al. Low serum concentrations of anti-Müllerian hormone are common in 53 female childhood cancer survivors. Horm Res Paediatr. 2013;79(1):17–21.
- Josso N, Clemente N. Transduction pathway of anti-Müllerian hormone, a sex-specific member of the TGF-beta family. Trends Endocrinol Metab. 2003;14(2):91–97.
- Moolhuijsen LME, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–3373.
- Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796.
- Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142(11):4891–4899.
- Fanchin R, Schonäuer LM, Righini C, et al. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–327.
- Bhide P, Pundir J, Homburg R, et al. Biomarkers of ovarian reserve in childhood and adolescence: A systematic review. Acta Obstet Gynecol Scand. 2019;98(5):563–572.
- Lawrenz B, Fehm T, von Wolff M, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma–evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98(1):141–144.
- Decanter C, Morschhauser F, Pigny P, et al. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20(2):280–285.
- Martin HL, Ullah S, Abbas N, et al. Predicting chemotherapy-induced menopause using baseline and post-chemotherapy anti-Müllerian hormone levels: results of a pilot study. Cancer Reports (Hoboken, N.J.). 2021;4(3):e1342–e1342.
- Krawczuk-Rybak M, Leszczynska E, Poznanska M, et al. Anti-müllerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol. 2013;2013:125080–125080.
- van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92(10):3869–3874.
- Lekovich J, Lobel ALS, Stewart JD, et al. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet. 2016;33(5):657–662.
- Lie Fong S, Lugtenburg PJ, Schipper I, et al. Anti-müllerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23(3):674–678.
- Kimler BF, Briley SM, Johnson BW, et al. Radiation-induced ovarian follicle loss occurs without overt stromal changes. Reproduction (Cambridge, England). 2018;155(6):553–562.
- Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol. 2016;12(20):2333–2344.
- Nakano H, Ashizawa M, Akahoshi Y, et al. Assessment of the ovarian reserve with anti-Müllerian hormone in women who underwent allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning regimens or myeloablative regimens with ovarian shielding. Int J Hematol. 2016;104(1):110–116.
- Ashizawa M, Akahoshi Y, Nakano H, et al. Updated clinical outcomes of hematopoietic stem cell transplantation using myeloablative total body irradiation with ovarian shielding to preserve fertility. Biol Blood Marrow Transplant. 2019;25(12):2461–2467.
- Nakano H, Ashizawa M, Akahoshi Y, et al. Assessment of the ovarian reserve with anti-Müllerian hormone in women who underwent allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning regimens or myeloablative regimens with ovarian shielding. Int J Hematol. 2016;104(1):110–116.
- Armuand GM, Rodriguez-Wallberg KA, Wettergren L, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30(17):2147–2153.
- Feng C, Lai Y, Li R, et al. Reproductive health in Southeast Asian women: current situation and the influence factors. Global Health Journal. 2018;2(1):32–41.
- Gorman JR, Su HI, Roberts SC, et al. Experiencing reproductive concerns as a female cancer survivor is associated with depression. Cancer. 2015;121(6):935–942.
