 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Transfusion of blood from glucose-6-phosphate dehydrogenase (G6PD) enzyme deficient donors could cause a potentially unfavorable outcome, especially in newborns and those with hemoglobinopathies.
Aims
To determine the prevalence of G6PD deficiency in Thai blood donors, the characteristics of G6PD deficient blood, and the efficacy of fluorescent spot test (FST) to screen for G6PD deficiency in a hospital blood bank setting.
Methods
Blood samples were obtained from 514 Thai blood donors who donated blood at Siriraj Hospital (Bangkok, Thailand) during December 2020-February 2021. G6PD deficiency status was screened using FST, and in vitro hemolysis of red blood cell parameters of G6PD deficient blood units was compared with those of normal control units at different time points during 35 days of refrigerated storage.
Results
The prevalence of G6PD deficiency was 7.59% (35 [8.73%] males, 4 [3.54%] females). The sensitivity of FST was 100% (95% confidence interval [CI]: 90.97-100%), and the specificity was 99.58% (95%CI: 98.49-99.95%). In vitro hemolysis was not significantly different between G6PD deficiency and normal controls.
Conclusion
The prevalence of G6PD deficiency in this study was 7.59%. FST was demonstrated to be an effective and reliable method for G6PD deficiency screening among Thai blood donors in a hospital blood bank setting.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an X-linked inherited disorder that is characterized by the insufficiency of an enzyme that is used in the pentose phosphate pathway to generate nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is a crucial oxidation reduction molecule that protects red blood cells (RBC) from reactive oxygen species (ROS). Patients with G6PD deficiency manifest varying degrees of acute hemolysis in response to oxidative stress precipitated by certain medications and foods. Transfusion of red cell products from G6PD enzyme deficient donors could cause a potentially unfavorable outcome, especially in newborns and those with hemoglobinopathies [Citation1–3]. Current screening criteria of blood donors relative to red cell disorders in Thailand relies mostly on history taking and point-of-care hemoglobin (Hb) testing. The screening of G6PD deficiency is not performed in the donors at the moment. According to the World Health Organization (WHO) Blood Donor Selection guidelines, only donors with a previous history of hemolysis are to be permanently deferred [Citation4]. However, countries with a high prevalence of G6PD deficiency should establish their own criteria for screening at-risk donors, and they should establish their own transfusion guidelines [Citation5].
The distribution of this enzyme deficiency closely correlates with malaria endemic areas, including Thailand [Citation6]. We previously reported a rate of G6PD deficiency in Thai blood donors of 7.7% (9.2% in males, 6.3% in females) [Citation7]. Although G6PD deficiency is prevalent in Thai population, there is no report of hemolysis in RBC products from a Thai G6PD deficient blood donor. The incidence of this condition could be either underreported due to the unavailability of an established guideline to screen for G6PD deficiency in blood donors, or because the majority of Thai people with G6PD deficiency have a severity level of Class III (moderate to mild enzyme deficiency) [Citation8].
Accordingly, the aim of this study was to determine the prevalence of G6PD deficiency in Thai blood donors, the characteristics of G6PD deficient blood from Thai donors, and the efficacy of fluorescent spot test (FST) for screening of G6PD deficiency in a hospital blood bank setting.
Materials and methods
Blood donor recruitment and blood sample collection
Blood samples were obtained from 514 blood donors (401 males, 113 females) who attended the Department of Transfusion Medicine of the Faculty of Medicine Siriraj Hospital, Mahidol University, which is a national tertiary referral hospital that is located in Thailand, during December 2020 to February 2021. All included donors were eligible to donate according to the standard donor screening procedure used at our center. The mean age of donors was 33.9 ± 10.3 years (range: 18-58). After receiving approval from the Siriraj Institutional Review Board (SIRB) (COA no. R016435002), study participants were recruited and written informed consent was obtained. A whole blood donation of 450 ml was collected in either a triple blood bag system (JMS Triple Blood Bag, CPD-SAGM solution; JMS Pte Ltd., Singapore) or a quadruple blood bag system (Terumo BCT Europe, N.V., Belgium).
Red blood cell (RBC) products in Thailand
There are three types of red cells products available in Thailand. The whole blood units were centrifuged and separated into packed red cells (PRC) and platelet-rich plasma. After 100 ml of SAGM was added into PRC, the products were labeled as red cells in additive solution (AS-RBC). The second type of RBC was prepared by leukofiltration of the AS-RBC and labeled as pre-storage filtered blood (PFB). Lastly, the leukocyte poor blood (LPB) was leukoreduction by the method of centrifugation to separate RBC from the buffy coat and the platelet-poor plasma.
Fluorescent spot test (FST)
A commercial test kit (R&D Diagnostics Ltd, Holargos, Greece) was used to screen for G6PD enzyme deficiency in the enrolled Thai blood donors. Briefly, the test was performed by mixing 10 ul of acid citrate dextrose (ACD) blood sample with 100 ul G6PD Reagent Solution. The mixture was then transferred to a filter paper and incubated for 10 minutes. After the filter paper became dry, the blood spots were read under ultraviolet (UV) light at a wavelength of 340 nm in comparison to both positive and negative controls. The fluorescence intensity indicated the enzyme activity, with bright fluorescence indicating normal activity and no fluorescence indicating G6PD enzyme deficiency.
Quantitative methemoglobin (MetHb) reduction (MR) assay
Five ml of citrate phosphate dextrose adenine (CPDA) blood was tested via oxidization with sodium nitrite, which changes oxyhemoglobin to methemoglobin (MetHb). Nile blue and glucose were then added to the reaction. In a normal individual, NADPH in the blood transforms MetHb to oxyhemoglobin. The proportion of MetHb to total Hb was measured using a spectrophotometer at optical densities (OD) of 540 and 630 nm, respectively. The difference in the OD reflects the severity of G6PD enzyme deficiency, as follows: 0-5% normal, 10-25% heterozygote, and >25% homozygous deficiency.
Red blood cell indices and hemolysis
Red blood cell indices were obtained from complete blood count (CBC) using an automated hematology analyzer (Sysmex XS-800i; Sysmex Corporation, Kobe, Japan). Hemolysis of blood products was monitored on days 1, 7, 14, 21, and 35 from free plasma Hb using a Plasma/Low Hb Photometer system (Hemocue AB, Angelholm, Sweden) and the following formula:
Where the standard reference of hemolysis in RBC products must not exceed 0.8% at the end of the RBC storage period [Citation9].
Statistical analysis
All statistical analyses in this study were calculated using SPSS Statistics version 18 (SPSS Inc, Chicago, IL, USA). We used descriptive statistics to describe demographic and clinical data, including number, percentage, mean and range, mean plus/minus standard deviation, and mean plus/minus standard deviation and range. Sensitivity and specificity were calculated using the standard formula [Citation10]. All the data were tested for their normal distribution using Shapiro–Wilk test. Differences in the means of hemolysis between the number of days of storage were calculated using Friedman test. Mann–Whitney U test was used to compare RBC parameters and hemolysis between G6PD deficient and normal samples. Correlations were calculated using Spearman’s test due to the skewness of the data. A p-value less than 0.05 was considered statistically significant for all tests.
Results
Prevalence of G6PD deficiency and FST performance
A total of 514 Thai blood donors (78% male, 12% female) that presented at the Department of Transfusion Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University were screened for G6PD enzyme status using the FST. Of those, 473 donors (92.02%) had bright fluorescence, 34 had no fluorescence (6.61%), and 7 (1.36%) had indeterminate results. The 41 samples with dim or no fluorescence were then confirmed for their enzyme activity by quantitative MR assay. Of those, 2 samples with indeterminate results had normal enzyme activity (0, 0.7%), and the other 39 samples were found to be G6PD deficient. Among those 39 samples, four male donors had an MR test result of 100%, and all samples had an MR test result >25%. We used the MR assay to test 8 randomly selected donors with bright FST, and all of those donors had normal MR results (0-5%). The prevalence of G6PD deficiency was 7.59% (35 [8.73%] males, 4 [3.54%] females). The sensitivity of the FST was 100% (95% confidence interval [CI]: 90.97-100%), and the specificity was 99.58% (95%CI: 98.49-99.95%). The positive predictive value and negative predictive value of the FST were 95.12% (95%CI: 83.03-98.73%) and 100% (95%CI: 98.60-99.95%), respectively.
Red blood cell parameters
We then compared CBC results, obtained from the EDTA blood sample of the donors on the day of donation, between groups. The following RBC parameters were not significantly different between groups: Hb level: (p = 0.369), RBC count (p = 0.132), mean corpuscular volume (MCV) (p = 0.099), mean corpuscular hemoglobin (MCH) (p = 0.682). the The mean corpuscular hemoglobin concentration (MCHC) was significantly lower in the deficient group than in the normal control group (p = 0.009); and, red cell distribution width (RDW) was significantly higher in the normal control group than in the G6PD deficient group (p = 0.006). The values of these RBC parameters were demonstrated in . Blood products prepared from these 39 confirmed G6PD deficient units and from 10 normal control samples were tested for plasma Hb and hemolysis. Among the 39 G6PD deficient units, there were 3 units of AS-RBC, 29 units of LPB and 7 units of PFB ().
Table 1. Demographic data, types of blood products, and red blood cell (RBC) parameters compared between the normal control group and the glucose-6-phosphate dehydrogenase (G6PD) enzyme deficiency group.
Hemolysis in red cell products
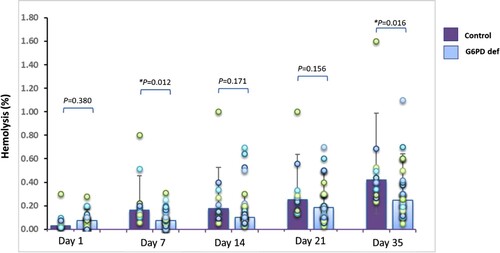
Hemolysis was evaluated on days 1, 7, 14, 21, and 35 of refrigerated storage. In the G6PD deficient samples, the percentage of hemolysis was 0.07 on day 1, 0.09 on day 7, 0.16 on day 14, 0.21 on day 21, and 0.30 on day 35. We assessed hemolysis in 10 normal control samples, and the percentage of hemolysis was 0.06 on day 1, 0.25 on day 7, 0.28 on day 14, 0.33 on day 21, and 0.53 on day 35 (). The spectrum of hemolysis was not found to be correlated with Hb level (G6PD deficiency p = 0.624, normal controls p = 0.864) or any other RBC parameters in both groups. However, the percentage of hemolysis in both groups increased over the storage time (p < 0.001) (). The rate of hemolysis in the majority of products did not exceed 0.8%. On day 35, the hemolysis rate in the control group was significantly higher than in the G6PD deficiency group (p = 0.016) as shown in . Of those two outlier samples, an AS-RBC unit from the normal control group and an LPB from the G6PD deficiency groups had a hemolysis percentage over 0.8% at day 7 and day 35 of storage, respectively. Samples of these two units were then sent for bacterial culture and both had a negative result. In addition, the severity of enzyme deficiency was not correlated with the degree of hemolysis. No significant correlations were found between the MR test results (%) and hemolysis (%) (data not shown).
Figure 1. Rate of hemolysis in all types of red blood cell (RBC) products compared between the normal control group and the glucose-6-phosphate dehydrogenase (G6PD) enzyme deficiency group at day 1, day 7, day 14, day 21, and day 35 of storage. Hemolysis were significantly higher (*p <0.05) in the normal control group on day 7 and day 35. Mann-Whitney U test was applied for this comparison. The bars within the chart reflect the median, and the error bars represent the interquartile range.
Discussion
The prevalence of G6PD deficiency in Thai blood donors was found to be 7.59%, and the prevalence is higher in males than in females (8.73% vs. 3.54%, respectively). Our finding is consistent with the previously reported rate of 7.7% based on enzyme activity testing [Citation7] and a 6.1% prevalence by FST [Citation11]. However, the frequency increased to 14.2% if based on molecular analysis [Citation11]. The most common genetic variant among Thai G6PD deficient individuals is G6PD Viangchan (871G > A), followed by G6PD Canton (1376G > T) and Mahidol (487G > A) [Citation7, Citation8, Citation11]. All 3 of these variants are in Class III of the WHO severity classification, which is defined as intermittent hemolysis when exposed to oxidative stress [Citation12]. However, there is a growing number of studies that are reporting G6PD deficient blood transfused to neonates or patients with hemoglobinopathies to be associated with more unfavorable outcomes when compared to other types of blood product recipients [Citation2, Citation5, Citation13]. We, therefore, set forth to determine and implement a screening method to lower the risk for these at-risk patients. Our results showed FST to be a feasible screening method for detecting G6PD deficient blood products in routine clinical practice in a hospital blood bank setting. This qualitative test gives binary results, and samples with intermediate fluorescence are further tested by MR assay. The sensitivity and specificity of the FST, when compared to the MR assay, are very high at 100% (95%CI: 90.97-100) and 99.58% (95%CI: 98.49-99.95), respectively. These rates are comparable to those previously reported in the Southeast Asian population [Citation14, Citation15].
We also explored whether G6PD deficient blood products are appropriate for transfusion. According to European Directorate for the Quality of Medicines & Healthcare (EDQM) standard, RBC products that have hemolysis less than 0.8% can be transfused [Citation9]. The hemolysis rate in our study peaked at day 14 of storage and increased over the storage time (p < 0.001). This finding corresponds with previous literature regarding storage lesions in refrigerated RBC products [Citation16, Citation17]. However and interestingly, the hemolysis rate in the normal control products was significantly higher than in the G6PD deficient products (). One of the unit (AS-RBC) in the normal control group had a hemolysis rate of 1.6%, and one of the unit (LPB) in the G6PD deficiency group had a hemolysis rate of 1.1%. The increase in hemolysis in those 2 units was higher than 0.8% on day 7 and day 35 of storage, respectively. No significant correlation was found between the hemolytic spectrum and Hb concentration or any other RBC parameters of the blood products including the MR test results. No bacterial contamination was detected and the two products were prepared from different types of bags (AS-RBC and LPB). Thus and consistent with previous reports, infection and bag type should not be considered likely causes of unusual hemolysis [Citation18, Citation19]. In Thailand and other Southeast Asian countries, mild thalassemia disease or thalassemic trait among blood donors is not uncommon since we rely only on Hb concentration for donor screening [Citation7, Citation20, Citation21]. This could be one of the potential explanations for the strikingly high hemolysis identified in this study.
Also in agreement with our study, the in vitro hemolysis in bags did not differ between the G6PD deficiency and normal control groups [Citation22]. The reason is probably that under refrigerated storage, there is less oxidative stress and a higher level of glucose, which is a substrate for glycolysis, for the enzyme-deprived red blood cells when compared to the in vivo environment [Citation23].
Limitations
This study has some mentionable limitations. We investigated only the in vitro parameters that are routinely used in our laboratory to verify the quality of red cell products before transfusion. As such, our findings may not reflect in vivo post-transfusion recovery. Our finding of a higher hemolysis level in the normal control group has increased our awareness of thalassemia, which is another red cell disorder that is more prevalent in Thai population [Citation7]. Future study is needed to determine if thalassemia screening is needed among Thai blood donors, and to determine the effects of thalassemia on blood products.
Conclusion
The prevalence of G6PD deficiency in this study was 7.59%. FST was demonstrated to be an effective and reliable method for G6PD deficiency screening among Thai blood donors in a hospital blood bank setting. Changes to the blood management policies at our center based on the findings of this study include: 1) G6PD deficient products will not be stored for more than 14 days, and 2) G6PD deficiency blood products will not be transfused to neonates for exchange transfusion or chronic transfusion dependent patients. To our knowledge, this is the first study to assess the in vitro quality of G6PD deficient RBC products in Thailand, which is a country with a high prevalence of moderate to mild G6PD enzyme deficiency.
Acknowledgments
The authors gratefully acknowledge the volunteers who generously agreed to participate in this study; Dr. Saowalak Hunnangkul for assistance with statistical analysis; and, The Division of Hematology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University for assistance with methemoglobin reduction testing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shalev O, Manny N, Sharon R. Posttransfusional hemolysis in recipients of glucose-6-phosphate dehydrogenase-deficient erythrocytes. Vox Sang. 1993;64(2):94–98.
- Sagiv E, Fasano RM, Luban NLC, et al. Glucose-6-phosphate-dehydrogenase deficient red blood cell units are associated with decreased posttransfusion red blood cell survival in children with sickle cell disease. Am J Hematol. 2018;93(5):630–634.
- Raciti PM, Francis RO, Spitalnik PF, et al. Acquired hemoglobin variants and exposure to glucose-6-phosphate dehydrogenase deficient red blood cell units during exchange transfusion for sickle cell disease in a patient requiring antigen-matched blood. J Clin Apher. 2013;28(4):325–329.
- WHO Guidelines Approved by the Guidelines Review Committee. Blood Donor Selection: Guidelines on assessing donor suitability for blood donation. Geneva: World Health Organization Copyright © 2012, World Health Organization; 2012.
- Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang 2013;105(4):271–282.
- Nkhoma ET, Poole C, Vannappagari V, et al. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells, Mol Diseases. 2009;42(3):267–278.
- Kittisares K, Palasuwan D, Noulsri E, et al. Thalassemia trait and G6PD deficiency in Thai blood donors. Transfus Apher Sci. 2019;58(2):201–206.
- Nuchprayoon I, Sanpavat S, Nuchprayoon S. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Thailand: G6PD viangchan (871G > A) is the most common deficiency variant in the Thai population. Hum Mutat. 2002;19(2):185.
- EDQM. Blood component monographs. Guide to the preaparation, use and quality assurance of blood components. 20th ed. Strasbourg: Counsil of Europe; 2020. p. 195–269.
- Domingo GJ, Satyagraha AW, Anvikar A, et al. G6pd testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391.
- Nantakomol D, Paul R, Palasuwan A, et al. Evaluation of the phenotypic test and genetic analysis in the detection of glucose-6-phosphate dehydrogenase deficiency. Malar J. 2013;12:289.
- Updating The WHO G6PD classification of variants and the international classification of diseases, 11th revision). Malaria policy advisory Committee meeting; 2019; Geneva, Switzerland.
- Renzaho AM, Husser E, Polonsky M. Should blood donors be routinely screened for glucose-6-phosphate dehydrogenase deficiency? A systematic review of clinical studies focusing on patients transfused with glucose-6-phosphate dehydrogenase-deficient red cells. Transfus Med Rev. 2014;28(1):7–17.
- Espino FE, Bibit JA, Sornillo JB, et al. Comparison of three screening test kits for G6PD enzyme deficiency: implications for Its Use in the radical cure of vivax malaria in remote and resource-poor areas in the Philippines. PLoS One. 2016;11(2):e0148172.
- Henriques G, Phommasone K, Tripura R, et al. Comparison of glucose-6 phosphate dehydrogenase status by fluorescent spot test and rapid diagnostic test in Lao PDR and Cambodia. Malar J. 2018;17(1):243.
- Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127(1):375–382.
- Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51(4):844–851.
- Brecher ME, Foster M, Mair D. Glucose and haemolysis as a rapid screen for contamination of red blood cells with Yersinia and Serratia. Vox Sang 2001;81(2):136–138.
- Sawant RB, Jathar SK, Rajadhyaksha SB, et al. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1(2):47–51.
- Nuinoon M, Kruachan K, Sengking W, et al. Thalassemia and hemoglobin e in southern Thai blood donors. Adv Hematol. 2014;2014:932306.
- Rosline H, Ahmed SA, Al-Joudi FS, et al. Thalassemia among blood donors at the hospital universiti sains Malaysia. Southeast Asian J Trop Med Public Health. 2006;37(3):549–552.
- Francis RO, D'Alessandro A, Eisenberger A, et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J Clin Invest. 2020;130(5):2270–2285.
- Karafin MS, Francis RO. Impact of G6PD status on red cell storage and transfusion outcomes. Blood Transfus. 2019;17(4):289–295.
