?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
To establish a reliable XN-HPC cutoff, for an effective CD34 + cell count of ≥2 × 106cells/kg of the recipient’s body weight, in harvested bone marrow products in allogenic transplantation.
Methods
The study was carried out in two phases. In retrospective Phase 1, data from 47 donors were analyzed. Sysmex analyzer XN-20 and BD FACS Calibur were employed to process XN-HPC and CD34 + cell enumeration, respectively. To make the two variables comparable, both XN-HPC and CD34 + cell counts were reported as the number of cells/kg of the recipient’s body weight. Spearman’s rank correlation coefficient was calculated for CD34 + cells and XN-HPC, followed by the calculation of the receiver operating characteristic (ROC) curve to identify the XN-HPC value which could effectively predict the cutoff of ≥2 × 106 CD34 + cells/kg of the recipient’s body weight. In Phase 2, the computed XN-HPC cutoff was validated in a prospective set of 53 donors by obtaining the positive and negative predictive values.
Results
Statistically significant correlation was obtained between XN-HPC and CD34 + cell count with Spearman’s rho of 0.54 (p-value <0.001). The optimal XN-HPC cutoff, for the required CD34 + ve cell count of ≥2 × 106 cells/kg of the recipient’s body weight, was calculated to be ≥2.80×106 cells/kg of the recipient’s body weight with the specificity and sensitivity of 100% and 31%, respectively. The ROC curve demonstrated the area under the curve to be 0.74. Phase 2 validation revealed 100% PPV.
Conclusions
For harvested bone marrow products with XN-HPC of ≥2.80×106 cell/kg of the recipient’s body weight, CD34 + cell enumeration by flow cytometry can safely be disposed of.
Introduction
In recent years, several stem cell–based therapeutic avenues have been explored owing to the advent of stem cell research and extraordinary advances in cellular and molecular biology. Stem cells are defined by their unlimited capacity of self-renewal and ability to differentiate into various healthy specialized cells [Citation1–3]. The term ‘stem cell’ encompasses diverse cellular origins and biological characterization. Based on their origin, stem cells are designated either as embryonic stem cells or as adult/somatic stem cells. Attributable to their distinct biological properties, embryonic stem cells exhibit pleuripotency, i.e. the ability to differentiate into all cell types, whereas adult stem cells are multipotent and demonstrate more restricted plasticity and differentiation potential [Citation4].
One of the most established and well-studied therapeutic applications of adult stem cells is hematopoietic stem cell transplantation (HSCT) [Citation5]. It is considered to be the only curative option for various benign and malignant hematopoietic disorders. For allogenic HSCT, multipotent stem cells are obtained either through mobilized peripheral blood (PB) or through a direct bone marrow (BM) harvest. Harvested BM stem cells are considered to be the preferred source for the treatment of children suffering from inherited bone marrow failure syndromes due to the associated lower risk of chronic extensive graft versus host disease as compared with a PB stem cell source [Citation6–8].
The success of HSCT depends on the infusion of adequate doses of adult hematopoietic progenitor cells (HPCs), i.e. ≥ 2million CD34 + cells/kg of the recipient’s body weight, resulting in complete and sustained hematopoietic reconstitution [Citation9–14]. Various modalities like total mononuclear cell count, colony-forming unit granulocyte-monocyte, and CD34 + cell enumeration have been used to establish the adequacy of harvested stem cell products. Of these, CD34 + cell count by flow cytometry is universally accepted to be the most efficient and reliable indicator of HPCs [Citation15–23]. This modality, though effective, is highly expensive, operator-dependent, and labor intensive [Citation24–26].
Sysmex hematology analyzer XN-20 series separates a population of immature myeloid precursors (XN-HPC) through a combination of optical detection and fluorescent flow cytometry [Citation27–30]. Enumeration of XN-HPC by sysmex XN series is a cost-effective, operator-independent, non-labor-intensive process with a rapid turnaround time of 90 s. Comparable efficacy of preharvest XN-HPC to harvested product CD34 + cell count has been established in autologous stem cell transplantation. Similarly in our recent paper, the predictive potential and optimal cutoff of XN-HPC, to serve as a surrogate for CD34 + cell count in a postharvest PB stem cell product, has also been effectively demonstrated.
The aim of this paper is to establish reliable XN-HPC cutoff, for an optimal CD34 + cell count of ≥ 2 million CD34 + cells/kg of the recipient’s body weight, in harvested BM products in allogenic stem cell transplantation. To the best of our knowledge, no such analysis has been performed so far.
Methodology
Study design and participants
The study was carried out at the National Institute of Blood Diseases and Bone Marrow Stem Cell Transplantation in two phases. In Phase 1 of the study, retrospective data from 49 donors (from December 2013 to December 2015) were collected, sorted, and analyzed for the variables of interest i.e. XN-HPC and CD34 + cell count by flow cytometry. In Phase 2 of this study, data from a prospective set of 53 BM transplant donors were analyzed for XN-HPC and CD34 + cell count from August 2016 to September 2018. The study was IRB-approved, and informed consent/assent was taken by each participant.
For minor donors, all five recommended points of the American Academy of Pediatrics Committee on Bioethics were met as follows:
In Pakistan, volunteer donor bone marrow registries have not been established yet and only available potential donors are family members.
All minor donors were biological siblings to their respective recipients.
All patients were suffering from benign life-threatening disorders such as aplastic anemia, immunodeficiency disorders, and hemoglobinopathies. In developing countries like Pakistan, hemoglobinopathies eventually prove to be fatal owing to the nonavailability of adequate blood product support, iron chelators, and a very high incidence of transfusion-transmitted infections.
Optimal measures were taken to keep the donor’s physical and psychological stress to the minimum.
For all minor donors, both donors’ assent and parental consent were obtained.
Bone marrow stem cell harvesting
Bone marrow harvesting for all donors was performed, on the day of transplantation, under general anesthesia in the operating room. Donors were positioned prone and bilateral posterior superior iliac spine and iliac crests were punctured multiple times with bone marrow harvesting needles to collect the maximum aspirate of not more than 20 mL/kg of the donor’s body weight. All harvested products were collected in sterile blood bags and were analyzed for XN-HPC and CD34 + cell count before infusion. Separate aliquots in EDTA were sent for the enumeration of XN-HPC and CD34 + hematopoietic progenitor cells to the respective laboratories.
Enumeration of XN-HPC
Sysmex hematology analyzer XN-20 was employed to determine the harvested bone marrow products for hematopoietic progenitor cells. No sample preprocessing was performed. XN-HPC was reported as the number of HPCs × 103/μL by Sysmex XN-20. To make XN-HPC easily comparable to CD34 + cell count, it was subsequently converted to a number of XN-HPC cells/kg of the recipient’s body weight for each harvested product.
Enumeration of CD34 + cell count by flowcytometry
Harvested bone marrow stem cell products were analyzed by using FACSCalibur (Becton Dickinson) analytical flow cytometer employing the gold standard ISHAGE gating strategy. Each sample was adjusted for the leukocyte count of 10 × 109 leukocytes/L with phosphate buffer saline when necessary. Cell viability was examined by light microscopy using trypan blue staining (GIBCO BRL, Germany).
Each sample was run along with unstained control and isotype control. Unstained control determined the forward and side scatter characteristics of the population of interest, whereas isotype control ensured that the observed staining was due to specific binding rather than background /autofluoresence and/or artifact.
Hemodiluted samples and samples with inadequate lysis or washing of red blood cells were rejected. All such samples were restained and rerun on cytometer on the same day.
Staining
BD polystyrene tubes (12 × 75 mm) were used for each donor sample. With the reverse pipetting technique, 100 µL of the each sample was collected and incubated with 5 µL of CD38 (Fluorescein isothiocyanate) FITC, 5 µL of HLA-DR (Phycoerythrin) PE, 5 µL of CD45 (Peridinin-Chlorophyll-Protein) PerCP, and 2.5 µL of CD34 (Allophycocyanin) APC. After gentle vortex, the tubes were incubated for 20 min at room temperature in the dark. In the next step, 1 mL 1× FACS lysing solution was added to each tube and reincubated for another 20–25 min in the dark at room temperature. It was followed by centrifugation (1750 rpm for 5 mins) and washing with sheath fluid. Isotype control, to determine negative events, was run simultaneously with each sample with similar methodology as for the samples.
Acquisition
The photomultiplier tube (PMT) voltages and the fluorescence compensation were set according to the recommendations of the manufacturer with fluorescent reference beads (Calibrite; Becton Dickinson, Franklin lakes, New Jersey, USA). Using the CellQuest software, 60 000 events were acquired for each sample. After acquiring data, an analysis was done.
Analysis
Dot plots were created for analysis with CellQuest Pro software. In the first dot plot, FSC vs. SSC of all events was displayed. A second dot plot was set up with CD45 PerCP vs. SSC of all the events, and Region R1 was set around the lymphocyte population. A third dot plot was set up with CD34 APC vs. SSC of gated events from R1. Another region R2 was created with CD34 positive cell population in this dot plot. In the next dot plot, CD45 and CD34 double-positive events were taken as CD34 positive cells. ISHAGE gating protocol was followed.
Absolute CD34-count was calculated using the following equation:
Statistical analysis
Statistical analyses were performed using STATA version 11. Median and range were reported for continuous variables as the data were not normally distributed, whereas frequencies and percentages were reported for all categorical variables. Spearman’s rank correlation coefficient (ρ) (considering the smaller number and non-normal distribution of observations) was calculated for CD34 + cells and XN-HPC after removing two outliers, followed by the calculation of the receiver operating characteristic (ROC) curve to identify the HPC value, which could optimally distinguish the cutoff of ≥2 million or more for CD34 + cells/kg of the recipient’s body weight. CD34 + cell count obtained in the harvested bone marrow product was used as the predicted variable. P values less than or equal to .05 were considered statistically significant.
Results
Donor characteristics phase 1
All harvested bone marrow products were obtained from healthy full matched donors of patients suffering from hematological diseases. A total of 49 donors were enrolled, of which 23 were male with the median age of 6 years (IQR 6 years). The median weight of the recipients was 20 kg (IQR 12). The median product volume was 255 mL. The median XN-HPC and CD34 + cell counts were 1.7 × 106 and 4.4 × 106 cells per kg of the recipient’s body weight, respectively.
Correlation of XN-HPC and CD34 + cell count
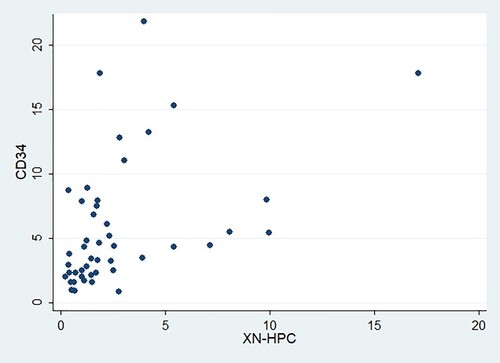
Spearman’s correlation was assessed in 47 phase 1 harvested bone marrow products. Spearman’s rho was found to be 0.54 (p-value <0.001), indicating a positive and statistically significant correlation between XN-HPC and CD34 + cell count. represents the distribution of XN-HPC and CD34 + cell counts.
XN-HPC cutoff for CD34 + cell count of ≥ 2million cells/kg of the recipient’s body weight
Keeping CD34 + cell count of ≥ 2million cells/kg of the recipient’s body weight as the target, the corresponding XN-HPC values were computed. The optimal cutoff of XN-HPC was calculated to be ≥2.80×106 cell/kg of the recipient’s body weight with a specificity and sensitivity of 100% and 31%, respectively (). shows that the ROC curve demonstrated the area under the curve to be 0.74.
Figure 2. Receiver operating characteristic curve for XN-HPC and CD34 + cell count of ≥ 2.0 x106 cells/kg of the recipient’s body weight.
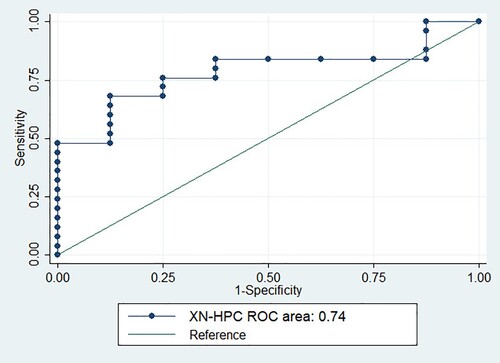
Table 1. Cutoffs for HPC count and the corresponding sensitivities and specificities.
Donor characteristics Phase 2
A separate set of 53 healthy donors was enrolled for the validation phase; 29 donors were male with a median age of 6 years (IQR 6). The median weight of the recipient was 16 kg (IQR 7.2). The median product volume was 270 ml. The median XN-HPC and CD34 + cell counts were 2.65 × 106 and 3.9 × 106 cells per kg of the recipient’s bodyweight, respectively.
Validation of XN-HPC cutoff value
Phase 2 of the study successfully validated the computed XN-HPC cutoff, demonstrating a positive predictive value (PPV) of 100% ().
Table 2. Validation of XN-HPC cutoff.
Discussion
Sysmex XN-20 hematology analyzers are unique in their ability to identify hematopoietic progenitor cells (XN-HPC) through an inbuilt white precursor cell (WPC)/stem cell channel. Hematopoietic progenitor cells have high membrane lipid content, which makes them inherently resistant to the distinct reagent system of the WPC channel. Subsequent analysis with an optical detection system and fluorescent flow cytometry segregates progenitor cells (XN-HPC) owing to their medium forward scatter (medium size), low side scatter (low granularity), and low fluorescence intensity. The process is cost-effective, requires no expertise, no sample pretreatment or manual gating, and has a turnaround time of only a few seconds.
Successful hematopoietic reconstitution is dependent on the infusion of an adequate dose of progenitor cells, which is universally defined as a CD34 + cell count of ≥ 2million cells/kg of the recipient’s body weight in the harvested product. Functional equivalence of peripheral blood XN-HPC, to peripheral blood and harvested product CD34 + cell count, has been evaluated by various studies to effectively determine the day of apheresis in autologous and allogenic stem cell transplantation. Mishra et al. concluded the comparable efficacy of XN-HPC and CD34 + cell count for both peripheral blood and harvested peripheral blood stem cell product with a statistically significant p value of <0.01 [Citation31].
Effective peripheral blood XN-HPC cutoff for optimal day of apheresis in autologous stem cell transplant setting has been proposed by Dami et al. For the targeted peripheral blood CD34 + cell count of ≥20 × 106/L, Dami et al. demonstrated that XN-HPC cutoff of ≥62 × 106/L for multiple myeloma and ≥30 × 106/L for other diseases had a sensitivity and specificity of 100% [Citation32]. Similarly, peripheral blood XN-HPC cutoff for an adequately harvested PBSC product has been reported by Moung et al. They successfully established that the peripheral blood XN-HPC cutoff of ≥ 20 × 106 cells/L had a NPV and PPV of 100% and 55%, respectively [Citation33].
Although the robust predictive potential of peripheral blood XN-HPC for harvested product CD34 + cell count has been verified by many studies, only a few have investigated the direct correlation of XN-HPC and CD34 + cell count in the harvested stem cell product. In our recently published study, correlation and prognostication potential of harvested PBSC XN-HPC count to harvested PBSC CD34 + cell count was investigated. The study revealed a fairly good correlation of the two entities and also reported and validated an XN-HPC cutoff of ≥1.84 million cells/kg of the recipient’s body weight as indicative of an adequately harvested PBSC product with a sensitivity and specificity of 78.2% and 100%, respectively [Citation34]. To the best of our knowledge, no such correlation study has been performed so far on a harvested bone marrow product.
The aim of this study was to establish an effective XN-HPC cutoff, for the optimum CD34 + cell count of ≥ 2 million/kg of the recipient’s body weight, in a harvested bone marrow product, so that this effective, time-efficient, affordable, and non-labor-intensive modality can replace the time-consuming and expensive option of CD34 + cell count enumeration by flow cytometry. It is not only the case that CD34 enumeration by flow cytometry requires significant expertise, it is also plagued by delayed reporting on account of a single analytical platform when multiple samples are required to be analyzed. This further reduces the feasibility of CD34 enumeration due to longer patient bed occupancy and disrupted staff and physician schedules. Similarly, CD34 enumeration cannot be utilized feasibly to evaluate intermediate products during apheresis/bone marrow harvesting to monitor stem cell yield and optimize collection timing.
The study has effectively demonstrated the statistically significant correlation between XN-HPC and CD34 + cell enumeration in a harvested bone marrow product. Also, the study has successfully computed the highly specific XN-HPC cutoff of ≥ 2.80 × 106 cell/kg of the recipient’s body weight for the adequately harvested bone marrow product, i.e. one with the CD34 + cell count of ≥ 2 million/kg of the recipient’s body weight. This XN-HPC cutoff was further validated in phase 2 of the study, which successfully verified the XN-HPC cutoff of ≥2.80 × 106 cell/kg of the recipient’s body weight and reported a positive predictive value of 100%.
The high specificity of this cutoff will allow to confidently establish the progenitor cell efficacy of any harvested bone marrow product with the XN-HPC value of ≥2.80 × 106 cell/kg of the recipient’s body weight. However, lower sensitivity is an important issue as it will mislabel a number of competent products as inadequate.
A limitation of this study is underrepresentation of adult bone marrow stem cells donors in both retrospective and prospective cohorts. Various non-HLA donor characteristics contribute to the overall outcome of HSCT. Of these, donor age has been postulated to be the most relevant factor influencing HSCT outcome. A survival advantage has been reported for recipients receiving a graft from younger donors in many unrelated donor (URD) studies [Citation35–37]. The phenomenon of stem cell ageing has been addressed in this regard, and a cumulative decline in quality and function has been reported due to increased DNA damage [Citation38]. Furthermore, clonal hematopoiesis also tends to increase with increasing age [Citation39]. Keeping all of this in consideration, the result of our study will not be directly transferable/ applicable to adult bone marrow stem cell transplant donors. Focused studies on adult bone marrow stem cell donors will shed light on the utility of XN-HPC as a surrogate for CD34 + cells in adult stem cell donors.
Conclusion
On the basis of these results, we conclude that for all harvested bone marrow products with the XN-HPC value of ≥2.80 × 106 cell/kg of the recipient’s body weight, CD34 + cell enumeration by flow cytometry can safely be disposed of. However, for harvested bone marrow products with XN-HPC values lower than the defined cutoff, CD34 + cell count enumeration by flow cytometry should be carried out to minimize the wastage of time and resources in subsequent unnecessary collections.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the NIBD Ethics Committee (NO. NIBD/RD-158/40-2013), and individual consent for this analysis was taken by each individual.
Supplemental Material
Download MS Excel (13.6 KB)Acknowledgements
(I) Tahir Shamsi and Aisha Jamal did the conception and design; Tahir Shamsi provided administrative support; Uzma Zaidi, Shafaq Jahanzeb, and Aisha Jamal provided study materials or patients; Ali Salim, Mahjabeen Imam, Qurratul Ain collected and assembled data; Tahir Khan and Aisha Jamal did data analysis and interpretation; Aisha Jamal, Tahir Shamsi, Tahir Khan, Uzma Zaidi, Quratul Ain Rizvi, Shafaq Jahanzeb, Ali Salim, and Mehjabeen Imam wrote the manuscript; Aisha Jamal, Tahir Shamsi, Tahir Khan, Uzma Zaidi, Quratul Ain Rizvi, Shafaq Jahanzeb, Ali Salim, and Mehjabeen Imam gave final approval of the manuscript .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
- National institute of health (NIH). The stem cell information Stem Cell Basics. [Online]. [cited 2018 Oct 3]. Available from: https://stemcells.nih.gov/info/basics.htm.
- Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009 Mar;24(2):98.
- Nadig RR. Stem cell therapy–hype or hope? A review. J Conserv Dent. 2009 Oct;12(4):131.
- Bajada S, Mazakova I, Richardson JB, et al. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008 Jun;2(4):169–183.
- Good RA, Meuwissen HJ, Hong R, et al. Bone marrow transplantation: correction of immune deficit in lymphopenic immunologic deficiency and correction of an immunologically induced pancytopenia. Trans Assoc Am Physicians. 1969;82:278.
- Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011 Sep 1;118(9):2618–2621.
- Bacigalupo A, Socié G, Schrezenmeier H, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica. 2012 Aug 1;97(8):1142–1148.
- de Latour R P, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow transplantation experience. Blood. J Am Soc Hematol. 2013 Dec 19;122(26):4279–4286.
- Abutalib SA. Clinical manual of blood and bone marrow transplantation [Internet]. Abutalib SA, Hari P, editors. Nashville (TN): John Wiley & Sons; 2017. doi:10.1002/9781119095491.
- Peerschke EI, Moung C, Pessin MS, et al. Evaluation of new automated hematopoietic progenitor cell analysis in the clinical management of peripheral blood stem cell collections. Transfusion. 2015;55(8):2001–2009.
- Bender JG, To LB, Williams S, et al. Defining a therapeutic dose of peripheral blood stem cells. J Hematother. 1992;1(4):329–341.
- Hequet O. Hematopoietic stem and progenitor cell harvesting: technical advances and clinical utility. J Blood Med. 2015;18(6):55–67.
- To LB, Haylock DN, Simmons PJ, et al. The biology and clinical uses of blood stem cells. Blood. 1997;89(7):2233–2258.
- D'Rozario J, Parisotto R, Stapleton J, et al. Pre infusion, post thaw CD34 + peripheral blood stem cell enumeration as a predictor of haematopoietic engraftment in autologous haematopoietic cell transplantation. Transfus Apher Sci. 2014;50(3):443–450.
- ChinYee I, Anderson L, Keeney M, et al. Quality assurance of stem cell enumeration by flow cytometry. Cytom: J Int Soc Anal Cytol. 1997;30(6):296–303.
- Fritsch G, Printz D, Stimpfl M, et al. Quantification of CD34 + cells: comparison of methods. Transfusion. 1997;37(8):775–784.
- Gratama JW, Orfao A, Barnett D, et al. Flow cytometric enumeration of CD34 + hematopoietic stem and progenitor cells. Cytometry. 1998;34(3):128–142.
- Elliott C, Samson DM, Armitage S, et al. When to harvest peripheral-blood stem cells after mobilization therapy: prediction of CD34-positive cell yield by preceding day CD34-positive concentration in peripheral blood. J Clin Oncol. 1996;14(3):970–973.
- Fatorova I, Blaha M, Lanska M, et al. Timing of peripheral blood stem cell yield: comparison of alternative methods with the classic method for CD34 cell determination. Bio Med Res Int. 2014;2014:1–13.
- Kikuchi-Taura A, Soma T, Matsuyama T, et al. A new protocol for quantifying CD34 + cells in peripheral blood of patients with cardiovascular disease. Tex Heart Inst J. 2006;33(4):427–429.
- Keeney M, Chin-Yee I, Weir K, et al. Single platform flow cytometric absolute CD34 + cell counts based on the ISHAGE guidelines. Cytom: J Int Soc Anal Cytol. 1998 Apr 15;34(2):61–70.
- Sutherland DR, Stewart AK, Keating A. CD34 antigen: molecular features and potential clinical applications. Stem Cells. 1993;11(S3):50–57.
- Sutherland DR, Keeney M, Gratama JW. Enumeration of CD34 + hematopoietic stem and progenitor cells. Curr Protoc Cytom. 2003;25(1):6–4.
- Letestu R, Marzac C, Audat F, et al. Use of hematopoietic progenitor cell count on the Sysmex XE-2100 for peripheral blood stem cell harvest monitoring. Leuk Lymphoma. 2007;48(1):89–96.
- Zulkafli Z, Mustaffa R, Yusoff SM. Hematopoietic progenitor cells as a predictive of CD34 + enumeration prior to peripheral blood stem cells harvesting. Bali Med J. 2014;3(3):112–115.
- Oelschlaegel U, Bornhaeuser M, Thiede C, et al. HPC enumeration with the Sysmex XE-2100 can guide further flow cytometric CD34 + measurements and timing of leukaphereses. Cytotherapy. 2003;5(5):414–419.
- Park KU, Kim SH, Suh C, et al. Correlation of hematopoietic progenitor cell count determined by the SE-9000™ automated hematology analyzer with CD34 + cell count by flow cytometry in leukapheresis products. Am J Hematol. 2001;67(1):42–47.
- Steussy BW, Capper M, Krasowski MD, et al. Algorithms utilizing peripheral blood hematopoietic progenitor cell counts in lieu of some CD34 + cell counts predict successful peripheral blood stem cell collections with substantial time and cost savings. ISBT Sci Ser. 2016;11(3):153–162.
- Yamane T, Takekawa K, Tatsumi N. Possibility of identification of hematopoietic stem cells using a conventional blood cell counter. Eur J Haematol. 1995;55(3):207–208.
- Takekawa K Y, Suzuki K, Hino M, et al. Identification of hematopoietic stem cells by the SE-9000™ automated hematology analyzer in peripheral blood stem cell harvest samples. Acta Haematol. 1997;98:54–55..
- Mishra S, Kulkarni U, Mathews N, et al. PB2388 a study to compare hpc determined on the sysmex xn-9000 with flowcytometric cd34 count in peripheral blood and in the harvested peripheral blood stem cell graft from autologous and allogenic donors. HemaSphere. 2019 Jun 1;3(S1):1062.
- Dima FB, Midolo M, Benedetti F, et al. Assessment of haematopoietic progenitor cell counting with the sysmex® XN-1000 to guide timing of apheresis of peripheral blood stem cells. Blood Transfus. 2019 Jul 25;1:1–9.
- Peerschke EI, Moung C, Pessin MS, et al. Evaluation of new automated hematopoietic progenitor cell analysis in the clinical management of peripheral blood stem cell collections. Transfusion. 2015 Aug;55(8):2001–2009.
- Jamal A, Khan MT, Parveen S, et al. Peripheral blood stem cell harvest HPC count Is an effective surrogate marker for CD34+ cell count in allogeneic stem cell transplant setting. Transl Oncol. 2020 Jul 1;13(7):100788.
- Shaw BE, Logan BR, Spellman SR, et al. Development of an unrelated donor selection score predictive of survival after HCT: donor age matters most. Biol Blood Marrow Transplant. 2018 May 1;24(5):1049–1056.
- Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood, J Am Soc Hematol. 2016 Jan 14;127(2):260–267.
- Ayuk F, Zabelina T, Wortmann F, et al. Donor choice according to age for allo-SCT for AML in complete remission. Bone Marrow Transplant. 2013 Aug;48(8):1028–1032.
- Anderlini P. Sixty as the new forty: considerations on older related stem cell donors. Bone Marrow Transplant. 2017 Jan;52(1):15–19.
- Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014 Dec 25;371(26):2488–2498.