?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives: To study the effect of TET on the reversal of drug resistance in the bone marrow microenvironment, and to further promote the research on drug reversal.Methods: We established a co-culture system of bone marrow mesenchymal stem cells (BM-MSC) and K562 cell lines, and compared the cell inhibition rate of K562 cells between the co-culture group and K562 singleculture group by daunorubicin (DNR) single-drug intervention with CCK-8 and also compared K562 proliferation in the co-culture group and K562 single-culture group after combined intervention with DNR and TET, then used Western blot and RT-qPCR to verify the expression of P-gp of K562 cells at protein and mRNA levels, confirmed the concentration of DNR in K562 of different experimental groups by HPLC-MS.Results: According to the results of CCK-8, after co-culture with bone marrow mesenchymal stem cells (BM-MSCs), the inhibition rate of DNR on K562 decreased significantly. When TET (1μmol/L) combined with daunorubicin (DNR) treated on the co-culture group, the inhibition rate increased significantly. Then, the results of RT-qPCR and western blot showed a remarkable difference of the expression of P-glycoprotein (P-gp). After co-culture with BM-MSCs, the protein expression of P-gp showed a significant upward trend. After adding TET intervention, the expression of P-gp decreased both in mRNA and protein levels. Also, the DNR concentration in K562 also performed the correspondent trend.Conclusion: The bone marrow microenvironment can promote the MDR of acute leukemia. TET can reverse the MDR mediated by the bone marrow microenvironment by inhibiting the expression of P-glycoprotein.
1. Introduction
Leukemia is a malignant clonal disease of the hematopoietic system, which accounts for 4% of all tumors [Citation1]. At present, chemotherapy is still the most important option for the treatment of leukemia, but it is undeniable that a large proportion of patients have a poor prognosis or even death due to chemotherapy failure. Many factors affect chemotherapy, among them, the emergence of multidrug resistance (MDR) greatly limits the effect of chemotherapy drugs. Therefore, the research on multidrug resistance of leukemia cells has always been a research hotspot in hematology. In recent years, studies have found that the bone marrow microenvironment not only plays an important role in the hematopoietic process, but is also closely related to the occurrence and development of tumor resistance. Among them, Mesenchymal stem cells (MSCs) are an important part of the bone marrow microenvironment, which promotes the proliferation and differentiation of hematopoietic stem cells, and affects the development of tumors [Citation2]. Tetrandrine (TET) is a calcium channel blocker. Many studies have proved the resistance reversal effect of tetrandrine without obvious side effects [Citation3, Citation4]. TET may significantly increase the intracellular chemotherapeutic drug concentration by changing the expression of MDR1 gene and P-glycoprotein (P-gp), and reverse the resistance of tumor cells to chemotherapeutic drugs [Citation5]. However, although the resistance reversal effect of TET has been confirmed in experiments, its clinical effect is not satisfactory. We speculate whether the influence of bone marrow microenvironment has been ignored in previous experiments. Therefore, this study will introduce the concept of bone marrow microenvironment to observe the resistance reversal effect and mechanism of TET.
2. Methods and materials
2.1. Cell culture
K562 cells are preserved in our laboratory. The K562 cell line was inoculated into T25 culture flasks with RPMI Medium 1640 (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA), cultured in a constant temperature incubator at 37°C and 5% CO2, and the medium was changed every other day and passaged once every 2 days. Take logarithmic growth phase cells for experimental research.
BM-MSC cells (Zhongqiao Xinzhou Company, China) were inoculated into T25 culture flasks with Mesenchymal Stem Cell Medium (Zhongqiao Xinzhou Company, China), and placed in a T25 culture flask. Culture in a constant temperature incubator with %CO2, change the medium once every 2–3 days, and pass once when the cells grow 90% of the culture flask. Use the 4-6th generation cells for follow-up experimental research.
To establish the co-culture system, BM-MSCs in the logarithmic growth phase were inoculated into 96 well plates, 2 × 103 per well, and cultured overnight in a constant temperature incubator at 37°C and 5% CO2 until the BM-MSCs adhered to the wall. The next day K562 cells were inoculated with 1 × 104 K562 cells per well. The number of cells in the co-culture group was inoculated with K562:BM-MSC = 5:1.
2.2. Cell counting kit-8 (CCK-8)
In the co-culture group, BM-MSCs in logarithmic growth phase were inoculated into 96 well plates, 2 × 103 per well, and cultured overnight in a constant temperature incubator at 37°C and 5% CO2 until the BM-MSCs adhered to the wall. The next day K562 cells were inoculated with 1 × 104 K562 cells per well and the co-culture system was established. The number of cells in the co-culture group was inoculated with K562:BM-MSC = 5:1. DNR and TET were respectively inoculated according to the different working concentration, and each group had three multiple wells. The final volume of each well was 200μl. Place it in a 37°C, 5% CO2 constant temperature incubator, take it out after 48 h. Since MSC grows adherently and K562 grows in suspension, we can easily separate K562 for subsequent experiments. Then we added 10% volume of CCK-8 solution to separate K562, incubate for 3 h in the dark, and measure the absorbance value of each well at a wavelength of 490 nm (OD490). The calculation method of cell inhibition rate is the same as follows:
where S: The OD490 of the experimental group (including K562 cells, medium, DNR and CCK-8); C: The OD490 of the control group (including K562 cells, medium, CCK-8); B: The OD490 of the blank group (including medium and CCK-8).
2.3. Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted using TRIzol (Ambion, USA), and total RNA (100 ng) was reverse transcribed by using PrimeScriptTM RT reagent Kit (TAKARA, Japan) to generate. Real-time PCR was performed using a SYBR Premix Ex Taq™ Kit (TAKARA, Japan) with each primer. GAPDH was used as an internal control. Each primer was designed according to the Gene Bank. The sequences were as follows: GAPDH forward 5′-TGACTTCAACAGCGACACCCA-3′, reverse 5′-CACCCTGTTGCTGTAGCCAAA-3′; P-gp forward 5′-TGGTTTGATGTGCACGATCTTGGG-3′, reverse 5′- AGATCAGCAGGAAAGCAGCACCTA-3′.
2.4. Western blot analysis
Cells were lysed in RIPA buffer (Solarbio, China), and treated with 1% protease inhibitor and 1% phosphatase inhibitor cocktail (Solarbio, China). 30µg total protein from each cell-lysate was separated by SDS-PAGE (Yamei, China), and then electrotransfer onto a PVDF membrane (Biosharp, China). Rabbit monoclonal antibody against P-gp (1:1000 dilution) (Abcam, USA) was used as primary antibodies. Anti-β-actin (1:3000 dilution) (Proteintech, China) was used as a control. Horseradish peroxidase-conjugated anti-rabbit/mouse IgG antibody (Proteintech, China) was used as a secondary antibody.
2.5. High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS)
Daunorubicin hydrochloride (Macklin, China) was dissolved, prepared into 2.0 mg/mL pregnant liquor, centrifuged, filtered with a microporous membrane and stored in the dark. Then the pregnant liquor was diluted with 100%methanol to a working solution of 2000ng/mL for further experiments. According to the results of preliminary spectrometry, experimental conditions were determined: Mass spectrometer: TSQ Quantum triple quadrupole mass spectrometer (Thermo, USA); Chromatograph: UltiMate 3000 RS (Thermo, USA); Chromatographic Column: ThermoHypersilGOLD (100 × 2.1 mm, 1.9 µm); mobile phase: methanol, 0.1%formic acid water; flow rate: 0.45 mL/min.
2.6. Statistical analysis
All of the statistical data are presented as the mean ± SD. Statistical significance of the differences was determined using one-way analysis of variance (ANVOA). Significance was defined as P < 0.05. SPSS26.0 was used for statistical treatment of CCK-8 detection data, the histogram was used to show the results. The results of RT-qPCR were analyzed using the comparative domain value method in Graphpad prism8.0. ImageJ image analysis software was used to process and analyze Western blot results. The grayscale values of each group were also displayed as mean ± SD, SPSS26.0 was used to process the data.
3. Results
3.1. Morphological characteristics of BM-MSCs and co-culture system
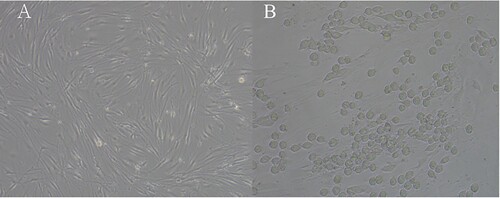
After inoculating the newly passaged BM-MSCs in a culture flask for about 12 h, the cells can grow adherently, and proliferate to 90% confluence in about 5–7 days. Under the inverted microscope, the BM-MSCs can be observed to be long spindle-shaped with a vortex shape in the growth direction (A). BM-MSCs and K562 were inoculated at a ratio of 1:5. After 48 h, it was seen that K562 aggregated to BM-MSCs, and some merged with BM-MSCs as a shape of spindle (B).
3.2. Influence of BM-MSCs on K562
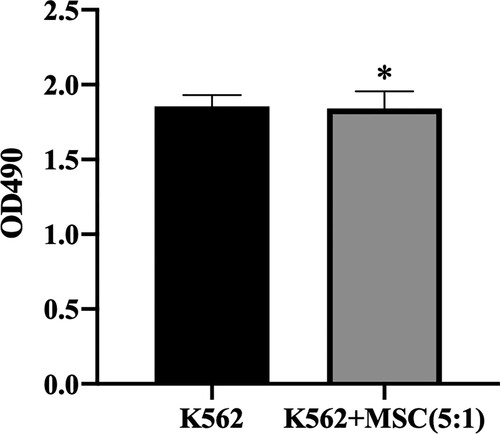
CCK-8 was used to determine the influence of BM-MSCs on K562. It was found that after co-culture with BM-MSCs, there was no significant difference in the number of K562 cells compared with the blank control group () (P > 0.05). The result showed that BM-MSCs have no remarkable influence on the proliferation of K562.
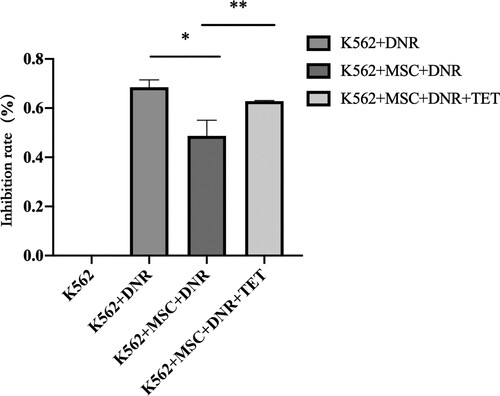
3.3. Inhibition rate of DNR on K562 between co-culture group and K562 culture group after TET treatment
CCK-8 was used to determine the effect of TET (1μmol/l) on the inhibition rate of K562 cells after DNR administration. It was found that after MSC co-culture, the inhibition rate of DNR on K562 cells decreased significantly (P < 0.05). TET (1μmol/l) combined with DNR acted on K562 cells in a co-culture system, and the inhibition rate increased significantly (P < 0.05) ().
Figure 3. Inhibition rate of DNR on K562 with different treatments. Data are presented as mean ± SEM and analyzed by one-way ANOVA compared to the vehicle control (*)with statistical significance at P < 0.05, between K562 + BM-MSC + DNR and K562 + BM-MSC + DNR + TET (**) with statistical significance at P < 0.05.
3.4. The level of P-gp with different treatments
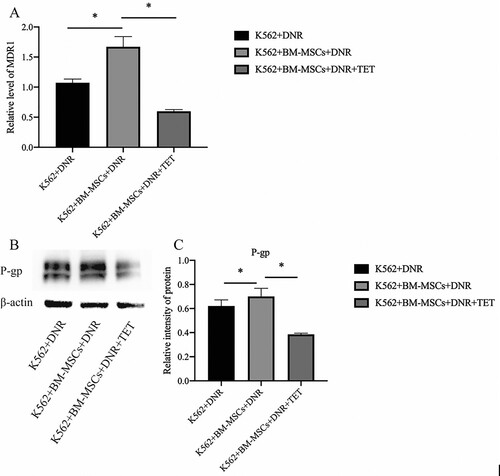
With the K562 single-drug intervention of DNR as the vehicle control group, the gene expression level of MDR1 was detected by RT-qPCR. From the results, we can find that after co-cultivation with BM-MSCs, the relative expression of MDR1 increased significantly (P < 0.05), and after the intervention of TET (1μmol/l), the relative expression level of MDR1 mRNA decreased significantly (P < 0.05) (A). Since P-gp is the expression product of MDR1, the western blot is used to detect the expression of P-gp. From the western blot results, it can be seen that after co-cultivation with BM-MSCs, the protein expression of P-gp showed a significant upward trend (P < 0.05), and after the intervention of TET (1μmol/l), the protein expression of P-gp decreased significantly (P < 0.05) (B, C).
3.5. The concentration of DNR in K562 of different experimental groups
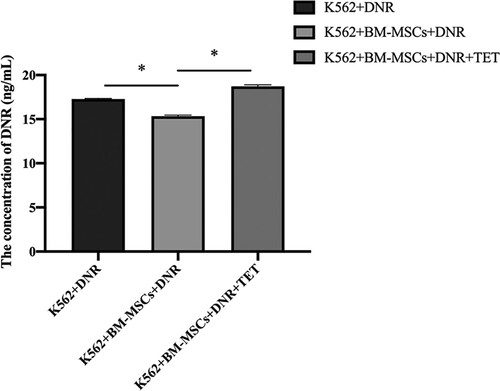
With the K562 single-drug intervention of DNR as the vehicle control group, the concentration of DNR in K562 cells was detected by HPLC-MS. From the results, we can find that after co-cultivation with BM-MSCs, the DNR concentration in K562 decreased significantly (P < 0.05), and after the intervention of TET (1 µmol/L), the concentration in K562 increased significantly (P < 0.05) ().
4. Discussion
Chemotherapy is the main clinical treatment for leukemia. However, the emergence of multidrug resistance (MDR) has greatly affected the effectiveness of chemotherapy. At present, we understand that many factors are affecting multidrug resistance. Among them, the role of bone marrow microenvironment in the drug resistance of tumor cells has been paid more and more attention by the scientific community, which may be one of the breakthroughs on the reversal of tumor resistance.
TET is a bisbenzyl isoquinoline alkaloid. In recent years, the anti-tumor effect of TET and the drug resistance reversal effect of tumor cells have been fully confirmed in vivo and in vitro experiments, and there are no obvious side effects [Citation3, Citation4]. However, the effect of TET is not satisfactory in its application. We doubt whether the effect of bone marrow microenvironment on leukemia cells has been ignored in previous studies. The bone marrow microenvironment is also called the bone marrow niche, which can function through the many types of cells contained in it [Citation6]. Mesenchymal stem cells (MSCs) are one of the important cell types. According to recent studies, we know that the bone marrow microenvironment may mediate the occurrence and development of multidrug resistance in leukemia by secreting cytokines, changing the adhesion of leukemia cells, and regulating the expression of resistance-related genes [Citation7,Citation8]. Therefore, this project established a leukemia cell line K562 and BM-MSCs co-culture system. After the intervention of the anthracycline chemotherapeutic drug daunorubicin (DNR), it was found that the inhibition rate of K562 cells was significantly reduced in the co-culture system, which confirmed that BM-MSCs do have the effect of promoting drug resistance; subsequent intervention with TET found that the DNR inhibition rate of K562 cells rebounded, indicating that TET also has the effect of reversing the drug resistance of leukemia cell lines in the bone marrow microenvironment.
P-glycoprotein (P-gp) is a complex drug efflux pump. Because it is overexpressed in a variety of tumor cells and is closely related to tumor multidrug resistance, it has been extensively studied [Citation9]. The main P-gp inhibitors are calcium channel blockers, calmodulin inhibitors, cyclosporin A and its derivatives, antimalarials, estrogen and progesterone and antihormonal compounds, anti- Arrhythmia drugs and certain Chinese herbal medicines [Citation10]. As a calcium ion channel blocker, TET has little effect on tumor suppression, but it can be used in combination with chemotherapy drugs to significantly inhibit P-gp expression [Citation11]. In this study, we used RT-qPCR to find that the relative expression of P-gp in the K562 in the co-culture group was significantly increased compared with the single culture group; after TET intervention, the P-gp level in the K562 cell line decreased significantly. It was verified by western blot that compared with the single culture group, the P-gp protein expression of the K562 in the co-culture group was significantly increased, and after the intervention of TET, the P-gp protein expression of the K562 cell line was obvious again decline. It shows that BM-MSCs may promote the expression of P-glycoprotein in leukemia cells, and the intervention of TET can inhibit the expression of P-gp, and thus play a role in reversing the drug resistance of leukemia cells. Next, we applied HPLC-MS to determine the DNR concentration in K562 cells under different treatments. The results showed that after co-culture with BM-MSCs, the drug concentration in K562 cells decreased significantly, and with the use of TET, the DNR concentration increased significantly. Studies have found that calcium channel blockers such as verapamil can bind to P-gp on the surface of drug-resistant cell membranes, increase the degree of phosphorylation of P-gp, and thereby inhibit the transport of P-gp of chemotherapeutic drugs outside the tumor cells. Since TET and verapamil are also calcium channel blockers, combined with our experimental results, we consider that TET may also reverse drug resistance by regulating intracellular calcium. The specific mechanism has not been well understood, and we will conduct more in-depth research in the future.
5. Conclusion
In summary, our research shows that the bone marrow microenvironment can promote the emergence of acute leukemia drug resistance, and TET can influence this drug resistance by regulating the expression or function of P-gp. The results of this study provide a scientific theoretical basis for TET combined with chemotherapy to treat relapsed and refractory acute leukemia.
Authors’ contributions
XYZ, NJ and BAC were responsible for confirming the topic and analyzing article. XYZ designed and wrote the paper, edited the figures, legends and tables. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834.
- Dai CL, et al. Tetrandrine achieved plasma concentrations capable of reversing MDR in vitro and had no apparent effect on doxorubicin pharmacokinetics in mice. Cancer Chemother Pharmacol. 2007;60(5):741–750.
- Xu WL, et al. Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leuk Res. 2006;30(4):407–413.
- Chambers SK, et al. Pharmacokinetic and phase I trial of intraperitoneal carboplatin and cyclosporine in refractory ovarian cancer patients. J Clin Oncol. 1997;15(5):1945–1952.
- Shafat MS, et al. The bone marrow microenvironment - home of the leukemic blasts. Blood Rev. 2017;31(5):277–286.
- Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988.
- Dillmann F, et al. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk Lymphoma. 2009;50(10):1676–1686.
- Zhou L, Wang H, Li Y. Stimuli-Responsive nanomedicines for overcoming Cancer multidrug resistance. Theranostics. 2018;8(4):1059–1074.
- Mollazadeh S, et al. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018;214:118–123.
- Xu W, et al. Bisbenzylisoquinoline alkaloids and P-glycoprotein function: a structure activity relationship study. Bioorg Med Chem. 2020;28(12):115553.