ABSTRACT
Objectives
To explore whether the addition of HMA to GO could bring additional clinical benefits to AML patients.
Background
Several studies have shown the HMA plus GO combination showed exciting results. However, the comparison of the clinical efficacy and safety profile of this combination with GO monotherapy has not been witnessed.
Methods
We performed a systematic review and meta-analysis of 18 clinical trials regarding the mono-GO therapy alone or the HMA + GO combination. The random-effect model or fixed-effect model was applied to the study based on heterogeneity.
Results
Pooled data of HMA + GO combination are more effective than mono-GO therapy among the unfit acute myeloid leukemia (AML) patients, based on the evaluation of overall response rate (ORR) (HMA + GO vs. mono-GO, 48% vs. 25%), composite complete remission (CRc) (HMA + GO vs. mono-GO, 42% vs. 25%). But pooled data of CRc and ORR showed no notable difference among R/R AML patients. Safety profiles have demonstrated that the HMA + GO combination was tolerable, considering the relatively low toxicities.
Conclusion
HMA + GO combination, more effective and better tolerated than mono-GO therapy should be recommended to treat unfit AML patients instead of R/R AML patients.
Introduction
Acute myeloid leukemia(AML) is a kind of myeloid malignancy characterized by increased self-renewal and abnormal proliferation of myeloid blasts [Citation1–3]. Recently there have been tremendous advances in the treatment of AML. However, refractory/relapsed AML or unfit AML eligible for receiving intensive chemotherapy remains a therapeutic challenge. According to recent studies, demethylation agents (5-azacitidine (AZA) and decitabine (DAC)) could bring substantial benefits for patients with AML [Citation4–7]. Thus, HMA-based regimen became an attractive therapeutic option in the hematological research, offering more choices to the R/R AML and unfit AML.
Hypomethylating agents have been approved for the treatment of myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) [Citation8,Citation9]. Considering the critical role in the treatment of myeloid neoplasms, hypomethylation agent (HMA)-based regimens, especially the combinations with novel agents nowadays, constantly emerge, spurred by the new insights into the targeted drug (Bcl-2 inhibitor, lenalidomide, HDACi, PD-1 inhibitor, APR-246, FLT3 inhibitor, etc.). Indeed, it’s critical to explore the optimizing combination from these targeted drugs, considering the reality that not all AML patients could earn survival benefits from these regimens, and in clinical practice, not all AML patients are responsive to HMA treatment [Citation10].
To date, two hypomethylation agents (5-azacitidine and decitabine ) have been approved by the FDA for treating myeloid neoplasms,, which are analogs of cytidine [Citation11]. Treating myeloid leukemia cells with hypomethylation agents enhances the activity of GO (Gemtuzumab ozogamicin) by increasing the expression of Syk in vitro [Citation12]. Meanwhile, the exposure of AML cells to the hypomethylation agents increases their CD33 expression [Citation13]. Thus, the combination of HMA plus GO became an alternative option for treating AML patients.
New strategies to improve clinical outcomes involve new agents and combinations with reduced toxicity. CD33 is a novel and attractive target for the treatment of AML that is overexpressed on the surface of healthy myeloid cells and myeloid leukemia cells in over 80% of AML patients [Citation14]. Gemtuzumab ozogamicin(GO) has a high affinity to the CD33 receptor without intrinsic anti-leukemic activity as a recombinant IgG4 kappa antibody [Citation15]. Binding to the CD33, GO could be trafficked from endosomes to lysosomes following the internalization of ADC [Citation16]. Then, the free calicheamicin derivative is generated and moved to the nucleus, resulting in cell cycle arrest and apoptosis after binding to DNA [Citation17,Citation18].
As an antibody–drug conjugate, consisting of a monoclonal antibody targeting CD33 [Citation19], the Gemtuzumab ozogamicin(GO) was first approved in 2000 by the FDA for the treatment of acute myeloid leukemia(AML). Despite the known clinical efficacy, GO was withdrawn from the market for the increased early death rates in de novo AML patients. In 2017, a fractionated-dosing schedule led to its re-approval for AML based on its efficacy and safety profile [Citation20]. Adverse effects could be mitigated using a fractionated dosing schedule, effectively reducing the Cmax level by 75% [Citation21].
In vitro study, the azacitidine plus GO demonstrated a profound synergistic effect [Citation22]. Several latest clinical trials have shown the tolerability and early efficiency of this combination. Herein, this systematic meta-analysis assesses whether the addition of HMA to mono-GO therapy could bring more clinical benefit to AML patients.
Materials and methods
Search methods and selection of studies
We searched from MEDLINE, PubMed, EMBASE, and google scholar from the public databases. The searching strategy included key terms as follows: acute myeloid leukemia or AML, hypomethylation agents or HMA, azacitidine, decitabine, GO or gemtuzumab ozogamicin. We screened the titles and abstracts to identify the eligible studies.
The primary aim of clinical outcome and measures
The primary aim of this meta-analysis was to evaluate the clinical response of the GO with or without the hypomethylation agent, including composite CR rate (CRc) and overall response rate (ORR). The CRc was referred to as the proportion of patients who achieved CR, CRi, or CRp after receiving therapy; meanwhile, the ORR was defined as the proportion of patients who achieved CR, CRi, CRp, or partial remission after receiving the treatment. CR was defined as the bone marrow blast count <5%, with the full recovery of a peripheral blood cell. CRi was defined as meeting the CR criteria except that the peripheral blood counts (ANC or platelets) were not fully restored. While the CRp was referred to as meeting the CR criteria only without restoring the platelets. Partial remission was defined as a decrease of at least 50% in the percentage of blasts to 5% to 25% in the bone marrow aspirate. The results would be shown in the randomeffect model, if there was high heterogeneity, or else in the fixed-effect model.
Survival information includes relapse-free survival (RFS) and overall survival (OS). The RFS was referred to as the time point of received CR to the time point of relapse or death with any cause. Overall survival was referred to as the time point of diagnosis to death, outcome of any reason.
Study selection and inclusion/exclusion criteria
The publications selected from MEDLINE, PubMed, EMBASE, and google scholar were carefully screened classified frontline GO, frontline HMA + GO, relapsed/refractory GO, relapsed/refractory HMA + GO. The included studies should meet the following criteria: (1) included AML patients who received mono-GO or HMA (decitabine or azacitidine) + GO regimen; (2) reported complete data included the clinical response (CR, CRi, ORR) or survival data; (3) reported the clinical outcomes of grade 3–4 hematological or non-hematological adverse events (AEs); (4) full paper available. Preclinical studies, case reports, and trials only reporting the pediatric patients were not included in this study. Besides, clinical trials of GO used in the consolidation stage were not included.
Risk of bias
Evaluation of the bias for the included studies was described in the Cochrane Handbook. The funnel plot and Egger test were used to check publication bias. If any disagreement regarding bias was present, then the joint discussion was held to attain consensus according to the Cochrane Handbook of Bias.
Statistical analysis
The statistical analysis of the efficiency and toxicity data in this study was performed on the R platform (meta package). Heterogeneity of all the included studies was analyzed with the chi-squared test and I2 statistic to use a fixed- or random-effect model. Less than 25% of the heterogeneity meant of no heterogeneity; then, a fixed- effect model will be applied; otherwise, a subgroup analysis will be used to find the sources of the heterogeneity. Before calculating the estimated proportions of each group, the Sharpiro-wilk normality test will be used to decide which data transformation form (logarithmic conversion, logistic conversion, arcsine conversion, Free-man Tukey conversion) to be used to make the data normalized.
Results
Search results
The 152 related articles were initially identified by searching in PubMed, EMBASE and Cochrane Library. After omitting the duplicated data, 53 articles were further reviewed after the careful selection of the titles and the abstracts. Finally, 18 articles were involved in this research, containing azacitidine/decitabine plus GO regimen [Citation13,Citation23–30] and GO monotherapy [Citation31–39] (). The flow diagram of the literature search and the trial selection process is shown in .
Figure 1. Flow diagram of the literature search and trial selection process. Illustration of the number of articles identified in the literature search and reasons for exclusion.
Table 1. The general characteristics of selected studies.
Study characteristics and the risk of bias
The risk bias for the included studies (n ≥ 3) was conducted by the linear regression method on the R platform; the exact regression value and P value were presented with each forest plot (samples less than 2 will not present the bias value and P value). No obvious publication bias was found during this study.
Efficacy
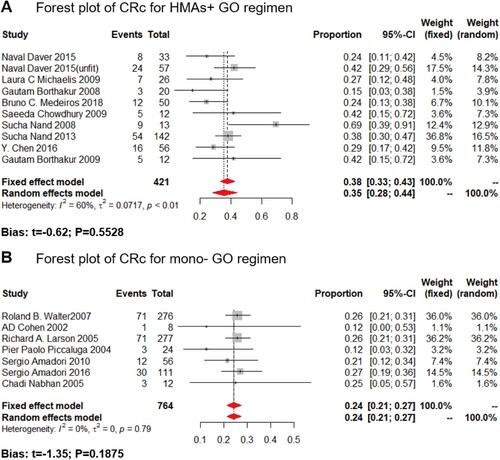
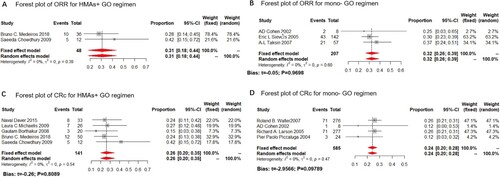
In this study, we analyzed both CRc and ORR as the clinical outcome for the monotherapy or HMA plus GO combination. GO monotherapy was all administered at 9 mg/m2. While in the HMA + GO regimen, GO was administered at 3 mg/m2 at 8 studies; only one was administered at 6 mg/m2. Data available for the CRc rate were derived from 17 trials, including 1520 patients, ranging from 12% to 69%, with the lowest in the mono-GO therapy, and the highest in the HMA + GO regimen ().
Figure 3. Overall response rate (ORR) of the HMA + GO and GO regimen. Forest plot of estimated ORR of the HMA + GO(A) and mono-GO(B) regimen among AML patients.
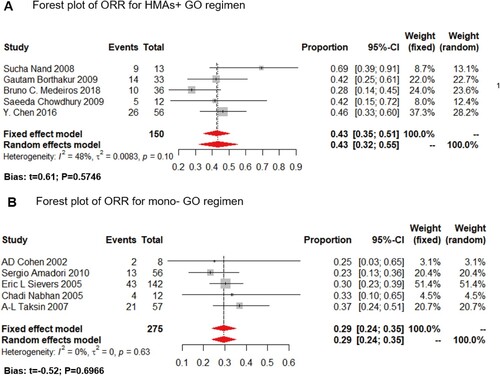
Meanwhile, data available for reporting ORR ranged from 23% to 69%, with the lowest one in the mono-GO cohort, and the highest in the HMA + GO regimen ().
Figure 2. Composite complete remission rate (CRc) of the HMA + GO and GO regimen. Forest plot of estimated CRc rate of the HMA + GO(A) and mono-GO(B) regimen among AML patients.
The OS of AML patients was reported in 8 trials and documented in , the median OS of HMA + GO and mono GO ranged from 9 to 11 months and 1 to 11.4 months, respectively. The most prolonged OS was observed in the HMA + GO cohort.
Considering the heterogeneity of the unfit and R/R AML, we constructed a subgroup analysis according to different types of disease.
Frontline AML
The GO dosage of all included studies for frontline AML patients was administered at 3 mg/m2, compared to the 9 mg/m2 of GO monotherapy. Only 3 and 2 studies reported the ORR for HMA + GO and mono-GO regimen for frontline treatment, respectively. The estimated proportions of ORR were 48% (fixed effect model, 95%CI: 38%–58%) in the HMA + GO cohort, and 25% (fixed effect model, 95%CI: 14%–35%) in the mono-GO cohort ((A,B)). As for the CRc rate, 5 and 3 studies reported the CRc rate for HMA + GO and mono-GO regimen, respectively. The result showed a merged result of 42% (random-effect model, 95%CI: 32%–55%) in the HMA + GO therapy, and 25% (fixed-effect model, 95%CI: 18%–31%) in the mono-GO combination ((C,D)). Besides, longer OS and RFS were observed in the HMA + GO cohort, compared to the mono-GO cohort.
This finding denoted that the patients receiving the HMA + GO regimen was more effective among AML patients in frontline over the mono-GO regimen.
Relapsed and refractory AML
The GO dosage of included studies for R/R AML was administered at 3 mg/m2 (study of B.C Medeiros was administered at 6 mg/m2), compared to the 9 mg/m2 of GO monotherapy. Only 2 and 3 studies reported the ORR for the HMA + GO and mono-GO regimen for R/R AML patients, respectively. The result showed that the estimated proportions of ORR for the HMA + GO regimen in the R/R AML subgroup was 31% (fixed-effect model, 95%CI: 18%–44%), while that of mono-GO therapy was 32% (fixed-effect model, 95%CI: 26%–39%) ((A,B)). Meanwhile, 5 and 4 studies reported the CRc rate for the HMA + GO and mono-GO regimen, respectively. The estimated proportion of CRc for the HMA + GO regimen in the R/R AML subgroup was 26% (fixed-effect model, 95%CI: 20%–35%), while that of mono-GO therapy was 24% (fixed-effect model, 95%CI: 20%–28%) ((C,D)).
Figure 4. ORR and CRc rate of the HMA + GO and GO regimen among frontline AML patients. Forest plot of estimated proportions of overall response rate (ORR) of the HMA + GO (A) and mono-GO (B) regimen among frontline AML patients; Forest plot of estimated proportions of composite complete remission rate (CRc) of the HMA + GO (C) and mono- GO (D) regimen among frontline patients.
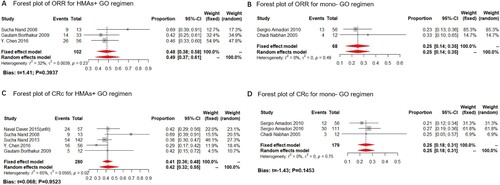
The above findings suggested that the HMA + GO therapy had a relatively minor clinical benefit over the mono-GO treatment in R/R AML. Moreover, the difference between the two is minimal, making it hard to decide which regimen is more suitable for R/R AML patients.
Safety
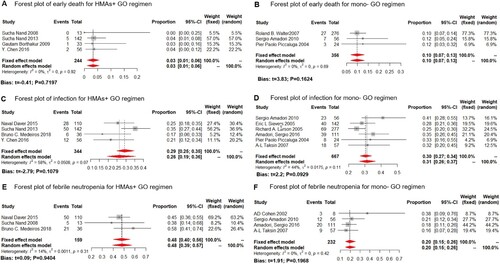
Fourteen trials reported on the safety profiles. As for the early death (ED) (GO dosage of HMA + GO was administered at 3 mg/m2, for GO monotherapy, it was 9 mg/m2), the overall early death rate was 3% (fixed-effect model, 95%CI: 1%–6%) and 10% (fixed-effect model, 95%CI: 7%–13%) for the HMA + GO and mono-GO, respectively ((A,B)). The ED rate was lower in the HMA + GO cohort than the mono-GO therapy.
Figure 5. ORR and CRc rate of the HMA + GO and GO regimen among R/R AML patients. Forest plot of estimated proportions of overall response rate (ORR) of the HMA + GO (A) and mono-GO (B) regimen among R/R AML patients; Forest plot of estimated proportions of composite complete remission rate (CRc) of the HMA + GO (C) and mono-GO (D) regimen among R/R patients.
The most common grade 3 and 4 adverse events included hematological toxicity, and almost all patients experienced peripheral cytopenia. As for the non-hematological AEs, the grade 3/4 infection and febrile neutropenia were rated the top 2 (GO dosage of HMA + GO was administered at 3 mg/m2, with one administered at 6 mg/m2, compared to the 9 mg/m2 of the GO monotherapy). Grade 3/4 infection occurred in 26% (random-effect model, 95%CI: 19%–36%) and 31% (random-effect model, 95%CI: 26%–37%) of patients receiving the HMA + GO and mono-GO regimen, respectively ((C,D)). While febrile neutropenia occurred in 48% (fixed-effect model, 95%CI: 40%–56%) and 20% (fixed-effect model, 95%CI: 15%–26%) in the HMA + GO and mono-GO regimen, respectively ((E,F)).
Veno-occlusive disease (VOD) of the liver is a common side effect of the GO. So, here we sort out the relevant data of the VOD. Only 4 studies in the Aza + GO cohort reported the incidence rate of the VOD; no case was reported to be diagnosed (0/13, 0/142, 0/36, 0/53). While in the mono-GO cohort, R. A Larson reported 16 cases of VOD (5.8%, 16/277) [Citation32], and Pier Paolo Piccaluga reported 1 case (4.2%, 1/24) [Citation33].
Figure 6. Safety profile of the HMA + GO and GO regimen. Forest plot of early death (ED) rate for the HMA + GO(A) and GO(B) regimen; Forest plot of grade 3/4 infection rate for the HMA + GO(C) and GO(D) regimen; Forest plot of grade 3/4 febrile neutropenia rate for the HMA + GO(E) and GO(F) regimen.
Discussion
The clinical outcome of unfit and R/R AML patients remains unsatisfactory primarily, with a relatively low estimated survival rate below 10% at five years [Citation40]. The reasons may include a high incidence of cytogenetics abnormalities and comorbid conditions, secondary AML derived from MDS/MPN/CMML [Citation41,Citation42].
Here, we report that the HMA + GO combination earns more clinical benefit over mono-GO therapy among unfit AML patients, offering evidence of optimizing for the treatment of AML patients.
Firstly, we compared the clinical efficiency of the mono-GO and HMA + GO regimens in treating frontline AML patients. The result showed that the CRc and ORR of the HMA + GO regimen was relatively higher, compared to the mono-GO therapy, without increasing the rates of early death. Secondly, although with insufficient survival data, a longer OS and RFS trend was observed in the HMA + GO cohort (), compared to the mono-GO regimen among frontline AML patients.
However, to the refractory or relapsed AML patients, the addition of hypomethylation agents didn’t bring more clinical benefit in terms of both ORR and CRc. This finding also denoted that the GO monotherapy would be better to build off in relapsed refractory since it has the same activity as HMA plus GO combination. Moreover, for those unfit AML patients who were refractory for the HMA regimen, GO could be used as the backbone of combination with venetoclax or IDH1/2 inhibitors, FLT-2 inhibitors, anti-CD47, etc.
Besides, a low incidence rate of infection and ED rate was observed in the HMA cohort, except for the percentage of febrile neutropenia. The Aza + GO combination had a relatively low incidence rate of the VOD (no case was reported), which is a common side effect of GO. This result denoted that hypomethylation agents did not further increase the VOD risk of GO, waiting for further experimental confirmation.
Overall, the result suggested that the unfit AML patients seem to earn more clinical benefits from the HMA + GO regimen. While the ORR and CRc of relapsed and refractory AML patients were very similar between the two regimens, making it difficult to decide which was more suitable.
We compared the clinical data with Aza + venetoclax, a novel combination that has made remarkable progress in unfit AML. In the VIALE-A study [Citation43], the composite CR rate for unfit AML was 66.4%, higher than our meta-analysis result of the HMA + GO (unfit AML, 42%) offering an optional choice for unfit AML patients. However, considering the reality that the clinical outcome of unfit AML patients is still poor, the HMA + GO regimen could be potential options in the future, given clinicians and patients more options. With the rapid development of precise diagnosis in myeloid malignancies, future studies will answer of which population is more suitable for the HMA + GO regimen. In addition, we could see a phase 3, multi-center, randomly designed clinical trial, explore triplet combinations of HMA/GO/VEN as the frontline therapy vs. HMA + VEN alone or alternating HMA + VEN (2 cycles) with 2 cycles of HMA/VEN/GO, as this may lead to more MRD negative remissions.
Novel anti-CD33 agents have emerged in recent days, including Vadastuximab talirine [Citation44–46] and lintuzumab [Citation47,Citation48], IMGN779 [Citation49,Citation50], all have witnessed hopeful clinical outcomes, highlighting the combination of anti-CD33 agent plus HMA, which might bring more treatment options for the AML patients.
The limitation of our study is that most included studies were single-center, phase II clinical trials, and the sample size is small. Another limitation is that over half of the included studies lack the survival data (OS and RFS). Hence, a multi-center, phase III, randomized clinical trial for the HMA plus GO regimen is needed in the future.
Conclusion
This study is a preliminary system evaluation of the HMA plus GO combination, and the results highlighted this promising therapeutic choice to the AML patients, especially the frontline AML patients, who are ineligible for receiving intensive chemotherapy. The HMA + GO combination could be one of the alternative options for the unfit AML patients without increasing the rate of ED and infection. The findings above need random controlled trials in the future to validate. Furthermore, novel hypomethylation agents and novel anti-CD33 agents might bring more hope to the patients, waiting to be seen in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Döhner H, Weisdorf DJ, Bloomfield C. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152.
- Henderson E. Treatment of acute leukemia. in Seminars Hematol, 6: 271–319 (July 1969). 1969. National Cancer Inst., Bethesda, Md.
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062.
- Aldoss I. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404.
- Flotho C. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia. 2009;23(6):1019–1028.
- Hollenbach PW. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5:2.
- Bohl SR, Bullinger L, Rücker F. Epigenetic therapy: azacytidine and decitabine in acute myeloid leukemia. Expert Rev Hematol. 2018;11(5):361–371.
- Issa J-PJ. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103(5):1635–1640.
- Silverman LR. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the cancer and leukemia group B. J Clin Oncol. 2006;24(24):3895–3903.
- Gardin C, Dombret H. Hypomethylating agents as a therapy for AML. Curr Hematol Malig Rep. 2017;12(1):1–10.
- Montalban-Bravo G, Garcia-Manero G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol. 2018;93(1):129–147.
- Balaian L, Ball EJL. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia. 2006;20(12):2093–2101.
- Nand S. Hydroxyurea, azacitidine and gemtuzumab ozogamicin therapy in patients with previously untreated non-M3 acute myeloid leukemia and high-risk myelodysplastic syndromes in the elderly: results from a pilot trial. Leuk Lymphoma. 2008;49(11):2141–2147.
- Sievers E. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate: presented in part at the 1997 Annual Meeting of the American Society of Clinical Oncology, Denver, CO; the 1997 European Cancer Conference, Hamburg, Germany; and the 1997 Annual Meeting of the American Society of Hematology, San Diego, CA. Blood, The Journal of the American Society of Hematology. 1999;93(11):3678–3684.
- Sievers EL. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–3254.
- Jedema I. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18(2):316–325.
- McGavin JK, Spencer CM. Gemtuzumab ozogamicin. Drugs. 2001;61(9):1317–1322.
- van der Velden VH. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood J Am Soc Hematol. 2001;97(10):3197–3204.
- Damle NK, Frost P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol. 2003;3(4):386–390.
- Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011;17(20):6417–6427.
- Food I. Drug administration %J safety concerns associated with Aranesp Amgen, and L. Procrit Ortho Biotech, for the treatment of anemia associated with cancer chemotherapy, Oncologic drugs advisory committee briefing document. 2004.
- Ishikawa T. Novel therapeutic strategies using hypomethylating agents in the treatment of myelodysplastic syndrome. Int J Clin Oncol. 2014;19(1):10–15.
- Michaelis LC. Azacitadine and low-dose gemtuzumab ozogamicin for the treatment of poor-risk acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), including relapsed, refractory disease. Blood. 2009;114(22):1034.
- Borthakur G. Decitabine and gemtuzumab ozogamicin in acute myelogenous leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112(11):2985–2985.
- Chen Y. Phase II study of decitabine and gemtuzumab ozogamicin (GO) in acute myelogenous leukemia (AML) and high-risk myelodysplastic syndrome (MDS). J Clin Oncol. 2010;28(Suppl. 15):6566–6566.
- Chowdhury S, Seropian S, Marks PW. Decitabine combined with fractionated gemtuzumab ozogamicin therapy in patients with relapsed or refractory acute myeloid leukemia. Am J Hematol. 2009;84(9):599–600.
- Nand S. A phase 2 trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood. 2013;122(20):3432–3439.
- Fathi AT. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132(11):1125–1133.
- Roland BW. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: a phase I/II study. Haematologica. 2014;99(1):54–59.
- Medeiros BC. A phase I/II trial of the combination of azacitidine and gemtuzumab ozogamicin for treatment of relapsed acute myeloid leukemia. Clin Lymph Myeloma Leuk. 2018;18(5):346–352.
- Amadori S. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972–979.
- Larson RA. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104(7):1442–1452.
- Piccaluga PP. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymph. 2004;45(9):1791–1795.
- Taksin AL. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66–71.
- Walter RB. Gemtuzumab ozogamicin in combination with Vorinostat and Azacitidine in older patients with relapsed or refractory Acute Myeloid Leukemia (AML): final results from a phase 1/2 study. Blood. 2013;122(21):3936.
- Cohen A. Gemtuzumab ozogamicin (Mylotarg) monotherapy for relapsed AML after hematopoietic stem cell transplant: efficacy and incidence of hepatic veno-occlusive disease. Bone Marrow Transplant. 2002;30(1):23–28.
- Nabhan C. Phase II pilot trial of gemtuzumab ozogamicin (GO) as first line therapy in acute myeloid leukemia patients age 65 or older. Leuk Res. 2005;29(1):53–57.
- Sievers EL. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–3254.
- Amadori S. Randomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leukaemia groups (AML-19). Br J Haematol. 2010;149(3):376–382.
- Stone RM. The difficult problem of acute myeloid leukemia in the older adult. Cancer J Clin. 2002;52(6):363–371.
- Hiddemann W. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17(11):3569–3576.
- Sekeres MA, Stone R. Older adults with acute myeloid leukemia. Curr Oncol Rep. 2002;4(5):403–409.
- DiNardo CD. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Stein EM. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood J Am Soc Hematol. 2018;131(4):387–396.
- Fathi AT. Vadastuximab talirine plus hypomethylating agents: a well-tolerated regimen with high remission rate in frontline older patients with acute myeloid leukemia (AML). Washington (DC): American Society of Hematology. 2016.
- Bixby DL. Vadastuximab talirine monotherapy in older patients with treatment naive CD33-positive acute myeloid leukemia (AML)Washington (DC): American Society of Hematology. 2016.
- Feldman EJ. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23(18):4110–4116.
- Raza A. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial. Leuk Lymph. 2009;50(8):1336–1344.
- Whiteman KR. The antibody-drug conjugate (ADC) IMGN779 is highly active in vitro and in vivo against acute myeloid leukemia (AML) with FLT3-ITD mutationsWashington (DC): American Society of Hematology. 2014.
- Kovtun Y. IMGN779, a novel CD33-targeting antibody–drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol Cancer Ther. 2018;17(6):1271–1279.
