ABSTRACT
Objectives
: Nucleoporin 210 (NUP210) is a membrane-spanning nuclear protein known to be involved in the development of solid tumours; however, its role in haematological cancers has not been investigated. This study aimed to assess the expression and prognostic potential of NUP210 gene expression in patients with acute myeloid leukaemia (AML).
Methods
: In this study, we assessed the expression and prognostic potential of NUP210 gene expression in patients with AML through bioinformatics analysis of The Cancer Genome Atlas and Genotype-Tissue Expression databases.
Results
:The expression of NUP210 mRNA in bone marrow was significantly increased in patients with AML compared to that in healthy individuals and was correlated with AML subtypes according to French-American-British classification as well as with bone marrow blast counts and patient sex (P < 0.05). The high NUP210 expression level was an independent biomarker of poor prognosis in the total AML population (P < 0.05) and separately in female but not male patients.
Conclusion
: Our results of NUP210 mRNA analyses revealed, for the first time, that NUP210 transcription was upregulated in patients with AML and positively associated with unfavourable AML prognosis, suggesting that NUP210 expression can be used as guidance in patient stratification for targeted therapy.
| Abbreviations: | ||
| NUP210 | = | Nucleoporin 210 |
| AML | = | acute myeloid leukaemia |
| IDH1/2 | = | isocitrate dehydrogenase isoforms ½ |
| BCL2 | = | B-cell lymphoma 2 |
| FLT3 | = | FMS-like tyrosine kinase 3 |
| NPCs | = | Nuclear pore complexes |
| TCGA | = | The Cancer Genome Atlas |
| FAB | = | French-American-British |
| ROC | = | receiver operating characteristic |
| AUC | = | area under the curve |
| GTEx | = | genotype-tissue expression |
Introduction
Acute myeloid leukaemia (AML) is a common haematological malignancy caused by genetic alterations in precursor cells or hematopoietic stem cells, which disrupt normal myeloid differentiation and result in uncontrolled proliferation of immature myeloid cells in bone marrow and peripheral blood. The pathogenesis of AML is complex; therefore, traditional chemotherapy, which is the most effective treatment approach, nevertheless results in low rates of complete remission and progression-free survival. Studies have shown that many genetic abnormalities detected in patients with AML are significantly related to disease prognosis. The progress of molecular targeted therapy has led to the development of inhibitors targeting specific oncogenes or signalling molecules associated with AML, such as isocitrate dehydrogenase isoforms 1/2 (IDH1/2), B-cell lymphoma 2 (BCL2), and FMS-like tyrosine kinase 3 (FLT3), which show favourable clinical efficacy, thus providing additional treatment options for patients with AML. Therefore, the identification of novel molecular prognostic biomarkers and potential therapeutic targets in AML may significantly improve the clinical outcome of the affected patients.
Nuclear pore complexes (NPCs) are large multiprotein structures, which act as gatekeepers regulating the flow of macromolecules through the nuclear envelope. Nucleoporins (Nups) are the basic structural and functional units of NPCs, which, in addition to nucleocytoplasmic transport, regulate other cellular functions such as gene expression, DNA damage repair, mitosis, and differentiation in a transport-independent manner [Citation1,Citation2]. Accumulating evidence indicates that Nups play an important role in the occurrence and development of numerous types of cancer. Thus, high expression of NUP88 is associated with tumour invasion in breast cancer, colorectal cancer, and melanoma [Citation3–5], whereas NUP62 may promote the progression of ovarian, prostate, and squamous cell carcinomas [Citation6–8]. Furthermore, rearrangements of NUP98 and NUP214 genes have been reported to be associated with various types of haematological malignancies [Citation9].
NUP210 is a membrane-spanning glycoprotein representing a major component of the NPC, which provides a starting point for NPC assembly. NUP210 is known to be involved in the development of lung adenocarcinoma, cervical cancer, and liver cancer [Citation10–12]; however, its role in haematological malignancies has not been reported. The objective of this study was to investigate the expression of NUP210 and its clinical significance in patients with AML using bioinformatics analysis of the data from The Cancer Genome Atlas (TCGA) database [Citation13]. Our findings revealed the correlation between NUP210 expression and patient outcome in AML, suggesting NUP210 as a novel candidate prognostic biomarker and treatment target in AML.
Materials and methods
Gene expression profiles of normal human bone marrow and bone marrow of patients with AML were downloaded from the genotype-tissue expression (GTEx) [Citation14,Citation15] and TCGA databases, respectively, using the UCSC Xena browser [Citation16]. The clinical characteristics of patients with AML were also obtained from TCGA; patients with incomplete clinical information were excluded. The expression of the NUP210 gene was compared between patients with AML and normal controls by edgeR software package, and the association of NUP210 expression with AML outcome was analysed by survival software package.
The Kaplan-Meier method was used to estimate the overall survival rate and prognosis of patients with different NUP210 expression levels. Receiver operating characteristic (ROC) curve analysis was used to assess the value of NUP210 as a potential predictive biomarker and multivariate Cox regression analysis was used to compare the prognostic relevance of NUP210 expression and other clinical variables. Statistical analysis was performed with R software version 3.4.1.
Results
NUP210 mRNA expression in patients with AML
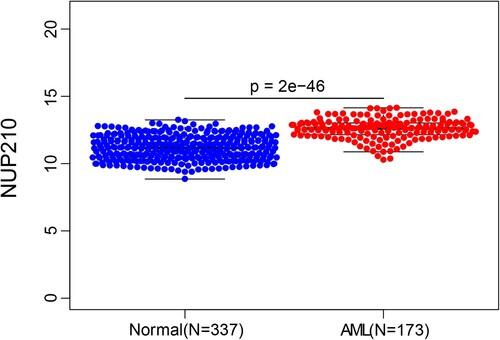
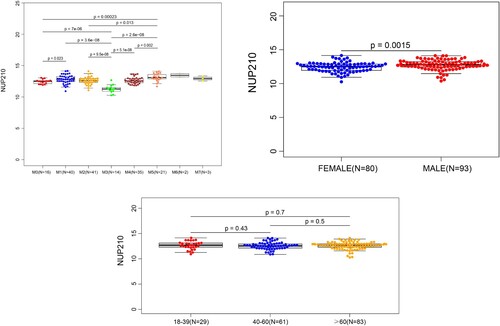
Comparison of NUP210 expression between the AML group (n = 173) and normal control group (n = 337) revealed that NUP210 mRNA level was significantly higher in the bone marrow of patients with AML than in that of healthy individuals (). NUP210 expression was further subjected to subgroup analysis according to AML subtypes by French-American-British (FAB) classification, age at diagnosis, and sex. As shown in , NUP210 mRNA level was reduced in patients with subtype M3 and increased in those with subtype M5 compared to the other subgroups, and the difference was statistically significant (except for that between M5 and M1). Furthermore, NUP210 expression was stronger in men than in women (P = 0.0015; B), but there was no correlation with patient age (C).
Relationship between NUP210 expression and clinical features
Since patients with subtype M3 had the lowest NUP210 gene expression and better survival outcomes, we excluded subtype M3 from further analysis. Consequently, 146 non-M3 patients with complete gene expression data and basic clinical information were evaluated; among them, 80 were men and 66 women, with an average age of 56.18 ± 15.97 years (range, 18–88 years) and mean peripheral blood leukocyte counts of (38.13 ± 47.36) ×109/L. These patients were divided into high and low expression groups according to the median NUP210 mRNA levels. The NUP210-high expression group contained more men (P = 0.01262) and was characterised by significantly decreased bone marrow blast counts (P = 0.007447) compared to the NUP210-low expression group (). Furthermore, there were more patients with subtype M5 in the NUP210-high expression group than in the NUP210-low expression group (). However, there was no difference in age at diagnosis, white blood cell counts, karyotype and risk stratification between the two groups ().
Table 1. Clinical characteristics of patients with AML in the NUP210-high and -low expression groups
NUP210 expression is correlated with AML prognosis
To evaluate the association between NUP210 expression and the outcome of non-M3 AML, we performed Kaplan-Meier survival analysis. The results indicated that the overall survival rate in the NUP210-low expression group was higher than that in the NUP210-high expression group (P < 0.001; A). Interestingly, NUP210 mRNA levels were significantly correlated with AML prognosis in female (P < 0.001) but not in male (P = 0.246) patients (B and C). NUP210 expression, age, sex, white blood cell counts, peripheral blood and bone marrow primitive cell counts, and FAB classification were selected to construct the Cox regression model for risk stratification. Cox regression analysis showed that NUP210 mRNA level was an independent risk factor for unfavourable outcomes in AML (HR = 1.7707, P = 0.0018; ), further confirming the prognostic value of NUP210 expression.
Figure 3. Probability of survival for patients with AML in NUP210-high and NUP210-low expression groups. (A) All patients; (B) women; (C) men.
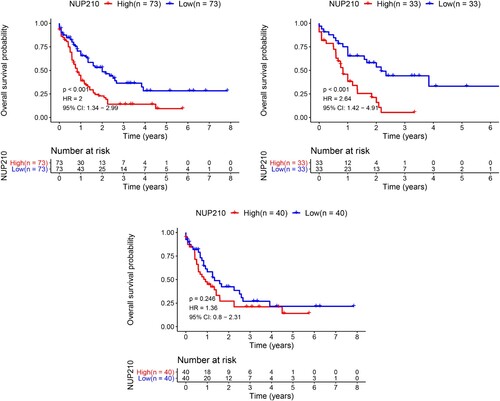
Table 2. Multivariate analyses of factors potentially affecting the overall survival of patients with AML.
NUP210 mRNA expression is a potential prognostic biomarker in AML
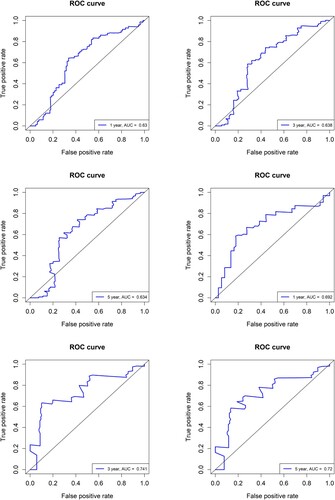
The ability of NUP210 expression to predict AML prognosis was validated by ROC curve analysis. For all patients with AML, the area under the curve (AUC) values corresponding to the expected survival times of 1, 3, and 5 years were 0.630, 0.638, and 0.634, respectively (A–C), whereas for female patients these values were 0.692, 0.741 and 0.720, respectively (D–F). These results indicate that the NUP210 expression level is a candidate prognostic biomarker in AML, especially for female patients.
Discussion
Nups are the main constituents of NPCs, which are the main channels mediating the flow of molecules between the cytoplasm and nucleus. In addition to their main function as nucleocytoplasmic transport regulators, Nups can control gene expression and regulate DNA damage repair and mitosis, and there is ample evidence that structural changes and abnormal expression of Nups are closely related to the occurrence and progression of cancers.
NUP214 is the first Nup shown to be associated with haematological malignancies through t(6;9)(p23;q34) chromosomal translocation which results in fusion protein DEK-NUP214 [Citation17]. NUP98, which plays an important role in the inter- and intra-cellular molecular transport, is involved in the development of myeloid haematopoietic cells [Citation18]. In patients with haematopoietic malignancies, NUP98 fusion with over 20 different partner genes has been detected, resulting in the deregulation of cell mitosis, gene transcription, nucleocytoplasmic transport, and other processes involved in carcinogenesis. The prognosis of Nup98 fusion-positive haematological cancers is very poor [Citation19,Citation20].
In addition to acting as fusion partners, Nups can regulate a number of cell processes involved in oncogenesis, such as gene transcription, epigenetic modifications [Citation10], epithelial–mesenchymal transformation [Citation21], and cell cycle [Citation22]. The expression of NUP88 is associated with tumour invasion in breast cancer, colorectal cancer, and hepatocellular carcinoma, whereas NUP62 plays a tumour-promoting role in ovarian cancer, prostate cancer, and squamous cell carcinoma. NUP210 has been shown to be strongly expressed in 26 lung adenocarcinoma cell lines, where its promoter region is modified through histone acetylation (H3K27ac) and methylation (H3K4me3); therefore, it is considered a very promising epigenetic marker for lung adenocarcinoma [Citation10]. Furthermore, NUP210 overexpression in cervical cancer results in the inhibition of Fas-mediated apoptosis, whereas its knockdown can restore apoptosis and normal cell proliferation [Citation11]. NUP210 also functions as a synergistic regulator of SMARCB1-dependent chromatin remodelling in liver cancer; it has been shown that SMARCB targeting of the NUP210 enhancer region alters H3K27 acetylation and downstream gene expression and that NUP210 serves as a chromatin scaffold for SMARCB1 and P300, promoting the formation of hepatocellular carcinoma [Citation12]. However, there have been no reports on NUP210 activity in haematological cancers.
In this study, we compared NUP210 mRNA expression data of patients with AML and healthy individuals extracted from TCGA and GTEx databases respectively. The results indicated that NUP210 expression was significantly increased in the bone marrow of patients with AML compared to that of healthy controls and was correlated with FAB-classified AML subtypes and sex of the patients. Furthermore, the expression level of NUP210 mRNA was significantly lower in patients with subtype M3 and significantly higher in those with subtype M3 compared to the other AML subtype groups. Thus, future studies should focus on the association between NUP210 expression and non-M3 AML, especially the M5 subtype.
According to Kaplan-Meier analysis, the overall survival rate of patients with low NUP210 expression was higher than that of patients with high expression. The prognostic value of NUP210 expression was confirmed by multivariate analysis. However, it should be noted that NUP210 expression did not show significant prognostic ability in male patients. ROC curve estimation further supported the excellent performance of NUP210 expression as an AML prognostic biomarker, especially in female patients. Studies on larger samples are required to confirm the dissimilarity in the NUP210 prognostic potential between men and women. If there is indeed a sex-related difference, it suggests the existence of distinct sex-specific genetic and biochemical mechanisms underlying NUP210 activity in AML.
In conclusion, in this study we used public databases to analyse, for the first time, the expression of the NUP210 gene and its relevance to AML prognosis. Our findings characterise NUP210 as a potential prognostic biomarker that could be used to predict the outcome and guide therapeutic strategy for patients with AML.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13(11):687–699.
- Chatel G, Fahrenkrog B. Dynamics and diverse functions of nuclear pore complex proteins. Nucleus. 2012;3(2):162–171.
- Agudo D, Gómez-Esquer F, Martínez-Arribas F, et al.Nup88mRNA overexpression is associated with high aggressiveness of breast cancer. Int J Cancer. 2004;109(5):717–720.
- Zhao ZR, Zhang LJ, Wang YY, et al. Increased serum level of Nup88 protein is associated with the development of colorectal cancer. Medical Oncology. 2012;29(3):1789–1795.
- Takahashi N, van Kilsdonk JW, Ostendorf B, et al. Tumor marker nucleoporin 88kDa regulates nucleocytoplasmic transport of NF-κB. Biochem Biophys Res Commun. 2008;374(3):424–430.
- Karacosta LG, Kuroski LA, Hofmann WA, et al. Nucleoporin 62 and Ca2+/calmodulin dependent kinase kinase 2 regulate androgen receptor activity in castrate resistant prostate cancer cells. Prostate. 2016;76(3):294–306.
- Hazawa M, Lin DC, Kobayashi A, et al. ROCK-dependent phosphorylation ofNUP62 regulates p63 nuclear transport and squamous cell carcinoma proliferation. EMBO Rep. 2018;19(1):73–88.
- Kinoshita Y, Kalir T, Rahaman J, et al. Alterations in nuclear pore architecture allow cancer cell entry into or exit from drug-resistant dormancy. Am J Pathol. 2012;180(1):375–389.
- Takeda A, Yaseen NR. Nucleoporins and nucleocytoplasmic transport in hematologic malignancies. Semin Cancer Biol. 2014;27:3–10.
- Kikutake C, Yahara K. Identification of epigenetic biomarkers of lung adenocarcinoma through multi-omics data analysis. PLoS One. 2016;11(4):e0152918.
- Gu Q, Hou W, Liu H, et al. Nup210 and MicroRNA-22 modulate Fas to elicit HeLa cell cycle arrest. Yonsei Med J. 2020;61(5):371–381.
- Hong SH, Son KH, Ha SY, et al. Nucleoporin 210 serves a key scaffold for SMARCB1 in liver cancer. Cancer Res. 2021;81(2):356–370.
- Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074.
- GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45(6):580–585.
- GTEx Consortium. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348(6235):648–660.
- Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the xena platform. Nat Biotechnol. 2020;38(6):675–678.
- Kraemer D, Wozniak RW, Blobel G, et al. The human CAN protein, a putative oncogene productassociated with myeloid leukemogenesis, is a nuclear pore complex protein thatfaces the cytoplasm.. Proc Natl Acad Sci USA. 1994;91(4):1519–1523.
- Franks TM, McCloskey A, Shokirev MN, et al. Nup98 recruits the Wdr82-Set1A/COMPASS complex to promoters to regulate H3K4 trimethylation in hematopoietic progenitor cells. Genes Dev. 2017;31(22):2222–2234.
- Petit A, Ragu C, Della-Valle V, et al. NUP98-HMGB3: a novel oncogenic fusion. Leukemia. 2010;24(3):654–658.
- Kaltenbach S, Soler G, Barin C, et al. NUP98-MLL fusion in human acute myeloblastic leukemia. Blood. 2010;116(13):2332–2335.
- Makise M, Nakamura H, Kuniyasu A. The role of vimentin in the tumor marker Nup88-dependent multinucleated phenotype. BMC Cancer. 2018;18(1):519.
- Naylor RM, Jeganathan KB, Cao X, et al. Nuclear pore protein NUP88 activates anaphase-promoting complex to promote aneuploidy. J Clin Invest. 2016;126(2):543–559.