ABSTRACT
Objectives
Bendamustine is a standard treatment for low-grade B-cell lymphomas, and considered safe in clinical trials. Its safety in routine practice might be different.
Methods
We retrospectively analyzed the infection complications in an unselected cohort of patients treated with bendamustine over a nine-year period. Patients were regularly monitored for blood counts and cytomegalovirus (CMV) reactivation by antigen assay and polymerase chain reaction. They received granulocyte colony stimulating factor for neutropenia, and routine anti-pneumocystis and optional anti-fungal prophylaxis.
Results
There were 179 men and 127 women at a median age of 61.5 (20–90) years, 52% receiving bendamustine for relapsed/refractory disease. Malignancies included low-grade B-cell lymphomas (54%), myeloma (10%), T-cell lymphomas (11%), Hodgkin lymphoma (2%) and other lymphoid neoplasms (23%). Most patients had good performance status (Eastern Cooperative Oncology Group score: 0–1, 72%). CMV reactivation occurred in 58 patients (19%) at a median age of 68 (39–85) years. Univariate analysis showed CMV reactivation to be significantly associated with elevated lactate dehydrogenase (P = 0.045), decreased albumin (P = 0.003) and older age (reactivation versus no reactivation: 66.3 ± 11.4 versus 59.4 ± 14.5 years, P = 0.0016). Age remained the only significant risk on multivariate analysis. CMV reactivation resulted in retinitis (N = 4), ependymitis/ventriculitis (N = 1) and duodenitis/colitis (N = 1). Invasive fungal disease occurred in five patients (candidemia, N = 2; aspergillosis N = 1; cryptococcemia, N = 1; scedosporiosis, N–1). Nineteen patients had culture positive septicaemia.
Conclusion
Our observations showed that even with a vigorous anti-infective strategy, bendamustine treatment was still associated with significant risks of bacterial and opportunistic viral and fungal infections.
Introduction
Bendamustine is an alkylating agent and purine analogue hybrid used predominantly in B-cell lymphomas and chronic lymphocytic leukaemia [Citation1], and in selected haematological malignancies, including T-cell lymphoma [Citation2] and myeloma [Citation3]. Despite these diverse applications, bendamustine is primarily used to treat indolent B-cell lymphomas [Citation1]. In comparison with more conventional strategies, including fludarabine-containing regimens, CVP (cyclophosphamide, vincristine, prednisolone) and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone), bendamustine use was associated with a superior overall survival [Citation1]. Because of a relative simplicity of administration, bendamustine has become increasingly popular in the management of indolent B-cell malignancies [Citation1].
The safety profile of bendamustine has been examined. In a meta-analysis of datasets from nine clinical trials, the rates of neutropenia and infections of bendamustine treatment were found to be statistically comparable with those of fludarabine and alkylating agents [Citation4]. In a more recent analysis, grade 3–4 adverse events of bendamustine were more frequent than those of chlorambucil, but comparable with those of regimens containing fludarabine, anthracyclines and alkylating agents [Citation1]. These analyses employed data from clinical trials, which often recruited relatively low-risk patients, who had normal organ functions, no active infections and good performance status.
Analysis of patients treated with bendamustine in actual clinical practice, however, revealed a different safety profile. Data from the National Cancer Institute surveillance, epidemiology, and end results (SEER) showed that bendamustine-treated patients with indolent lymphomas had significantly increased risks of common and opportunistic infections, as compared with patients treated with other non-bendamustine regimens [Citation5].
Bendamustine suppresses T-cells, owing to its structural similarity to purine analogues [Citation6]. As it is often combined with an anti-CD20 antibody in B-cell lymphomas, there is also B-cell suppression. Hence, bendamustine-treated patients have profound and prolonged depletion of T-cells and B-cells, to the extent that irradiated blood products are recommended indefinitely for them [Citation7]. Hence, increases in infective risks might accordingly be expected.
Experience of the real-world use of bendamustine in Asia is limited, due to its relatively late approval (2016 in Japan and 2019 in China). Hence, its safety profile in routine practice in these populations is not well-defined.
In this study, we conducted a retrospective analysis of the infective complications in a cohort of unselected patients with haematological malignancies, who were treated with bendamustine in a non-trial setting over a nine-year period.
Materials and methods
Patients
The pharmacy prescription records of the study institute, a quaternary referral academic centre, from June 2010 to April 2019 were reviewed, and files of patients who had received at least one cycle of bendamustine were retrieved. Data collected included demographics, diagnoses, prior therapies, cycles of bendamustine used, and infective (bacterial, viral and fungal) episodes. The study was approved by the institute review board, and patients gave informed consent to treatment.
Treatment and monitoring
Bendamustine was used at a dose of 90 mg/m2 for two days in a 28-day cycle. Accompanying drugs depended on the indications. Blood counts were obtained weekly to biweekly after treatment, with granulocyte colony stimulating factor (G-CSF) administered for a neutrophil count of <1 × 109/L. All patients receiving bendamustine were given anti-pneumocystis prophylaxis with cotrimoxazole (960 mg twice daily, two days/week) or pentamidine inhalation (300 mg every four weeks). Anti-fungal prophylaxis with oral itraconazole (syrup, 200 mg/day) for outpatients/inpatients or intravenous echinocandin (micafungin or anidulafungin, 100 mg/day) for inpatients was optional but recommended, decision being made based on interaction with concurrent medications, liver function, and the assessment of the attending physician regarding risks of invasive fungal disease (IFD). Cytomegalovirus (CMV) monitoring comprised CMV pp65 antigen assay (the entire study period), qualitative polymerase chain reaction (PCR, from 2011 to 2013) and quantitative PCR (Q-PCR) for plasma CMV DNA (from 2013 onwards) [Citation8]. It was performed every two to four weeks for out-patients. For in-patients, CMV monitoring was performed once weekly, increased to twice weekly if CMV reactivation was detected. Anti-CMV drugs (ganciclovir/valganciclovir/forscarnet) were commenced once plasma CMV DNA was detectable, and continued until two consecutive negative results were obtained. Blood products if required were all irradiated [Citation7].
Disease definitions
CMV virologic reactivation was defined as positive pp65 antigenic assay, PCR or Q-PCR. CMV disease was diagnosed based on clinical or histological features of organ damage due to CMV, supported by positive virologic data, and divided into proven, probable or possible as previously described [Citation9]. Proven, probable and possible IFDs were defined as reported [Citation10]. Because febrile episodes were common in bendamustine-treated patients, only cases with positive blood cultures and hence septicaemia were included in the analysis of bacterial infections.
Statistical analysis
Categorical variables were analysed with chi-squared test and continuous variables with non-parametric tests. The following parameters were analysed for their impacts on CMV reactivation: sex, age, underlying diagnoses, lactate dehydrogenase (LDH, normal versus elevated), concomitant use of an anti-CD20 antibody (rituximab or obinutuzumab) (yes versus no), albumin (normal versus decreased), bone marrow infiltration (present versus absent), and disease status (newly-diagnosed versus relapsed/refractory). Factors with P < 0.10 on univariate analysis were then examined by multivariate analyses. Two-tailed P values of < 0.05 were regarded as significant. All statistical analyses were performed with the SPSS version 26.0 (Chicago, IL, USA).
Results
Patients
During the study period, 312 patients were treated with bendamustine. Data from six patients could not be interpreted and were excluded. There were 179 men and 127 women at a median age of 61.5 (20–90) years, 52% of whom received bendamustine for relapsed/refractory disease (). B-cell malignancies predominated (87%; low-grade lymphomas: 54%; plasma cell myeloma: 10%). In patients with B-cell lymphomas and lymphoproliferative diseases, bendamustine was used together with an anti-CD20 antibody (rituximab or obinutuzumab). A minority of patients had T-cell lymphomas (11%) and classical Hodgkin lymphoma (2%). Most patients had good performance status (Eastern Cooperative Oncology Group, ECOG, score: 0–1, 72%).
Table 1. Demographic and clinicopathologic features of 306 patients receiving bendamustine treatment for lymphoid neoplasms.
CMV virologic reactivation
CMV reactivation occurred in 32 men and 26 women at a median age of 68 (39–85) years; 62% of whom had stage IV disease (). Half of the patients had newly-diagnosed disease receiving bendamustine as first-line treatment. Univariate analysis showed that patients with CMV reactivation, compared to those without, were older (mean age ± standard deviation: 66.3 ± 11.4 versus 59.4 ± 14.5 years, P = 0.001), did not have concomitant use of an anti-CD20 antibody (P = 0.045), and were more likely to have decreased albumin (P = 0.003) and elevated LDH (P = 0.045) (Supplemental file 1). Multivariate analysis showed that older age was the only significant risk associated with CMV reactivation.
Table 2. Demographic and clinicopathologic features of 58 patients with cytomegalovirus virologic reactivation and disease during bendamustine treatment for lymphoid neoplasms.
Clinicopathologic features of CMV disease
CMV disease occurred in five patients (1.6%) (). Four patients had heavily pre-treated refractory lymphoid neoplasms, and one patient received bendamustine as first-line treatment. Three patients received single-agent treatment with bendamustine, whereas in two patients, bendamustine was combined with an anti-CD20 antibody. Before the development of CMV disease, all patients had detectable CMV virologic reactivation, and had received anti-viral treatment with ganciclovir/valganciclovir. Three patients (cases 1, 2 and 4) had very high CMV pp65 antigen levels (peak: 240, 243 and >700 per 2 × 105 leucocytes respectively) during the course of CMV disease. CMV retinitis developed in four patients (case 1–3, 5). In patients 1 and 3, vitreal aspirates were available, showing CMV DNA on PCR. In patients 2 and 5, vitreal aspirate was not available, but ophthalmologic features were typical of CMV retinitis. All four patients therefore had proven CMV disease [Citation9]. Patient 4 developed CMV virologic reactivation not responding to ganciclovir and forscarnet, with CMV pp65 exceeding the upper detection range (> 700 per 2 × 105 leucocytes). This was accompanied by progressive mental deterioration, with magnetic resonance imaging showing features of viral ependymitis/ventriculitis ((A)). Patient 5 developed intractable diarrhoea, with upper/lower endoscopy showing duodenitis and colitis ((B)). Biopsy showed duodenitis with intranuclear CMV inclusion bodies, confirming CMV gut infection. She later developed visual impairment, and ophthalmological examination showed bilateral retinitis.
Figure 1. Infective complications of bendamustine-treated patients. (A). Patient 4 of the cytomegalovirus (CMV) disease cohort. Magnetic resonance imaging showed periventricular hyperintensities (arrows) indicative of ependymitis/ventriculitis. (B). Patient 5 of the CMV disease cohort. Upper endoscopy showing duodenal inflammation (left panel), and colonoscopy showing colitis (right panel). Histopathology of duodenal biopsy showed intranuclear CMV inclusion bodies, confirming CMV disease. (C). Patient 4 of the invasive fungal disease (IFD) cohort. Chest X ray showing bilateral consolidation. The patient was subsequently found to have cryptococcemia. (D). Patient 1 of the IFD cohort. Computed tomography (CT) scan of chest, showing wedge-shaped segmental lobar involvement (arrow) and consolidation (open arrow). Sputum grew Aspergillus niger. (E). Patient 3 of the IFD cohort. CT scan showed dense, well-circumscribed lesions (arrows). Bronchoalveolar aspirate grew Scedosporium apiospermum.
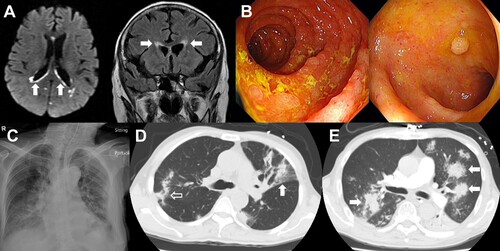
Table 3. Cytomegalovirus (CMV) disease in five patients treated with bendamustine.
Treatment outcome of CMV disease
Patients 1–3 responded with suppression of CMV DNA/pp65 to undetectable levels. Vision was significantly improved in patients 2 and 3, but not in patient 1. Patient 4 received cidofovir as a salvage, resulting in undetectable CMV pp65, although CMV DNA remained detectable. She had mental state improvement, but died of sepsis before PCR became negative. Patient 5 achieved negative CMV PCR/pp65, resulting in cessation of diarrhoea and some improvement in vision. All patients finally died of complications of their underlying malignancies or concomitant sepsis, with no death directly attributable to uncontrolled CMV disease.
Clinicopathologic features of IFD
Five patients developed IFD (1.6%) (). Bendamustine was used as the first-line agent in four patients (cases 2–5). Patient 1 had received multiple regimens, including an allogeneic haematopoietic stem cell transplantation (HSCT). In this patient, the neutrophil count was normal at the time of positive sputum culture for Aspergillus niger. However, in the ensuing three weeks grade 3 neutropenia developed, which might account for the subsequent pulmonary IFD. Bendamustine was combined with an anti-CD20 antibody in four patients, and used as the sole agent in one patient who had angioimmunoblastic T-cell lymphoma. The lung was the primary site of infection in three patients (cases 1, 3 and 4). IFD was proven in patients 2, 4 and 5 (positive fungal culture from blood) ((C)), and probable in patients 1 and 3 (positive fungal culture from sputum and bronchoalveolar lavage) ((D, E)). All patients had neutropenia and features of sepsis at the time of IFD, with three of them receiving anti-fungal prophylaxis. Patient 5 (corresponding to patient 5 in ) had concomitant CMV colitis, and Candida parapsilosis fungemia was probably secondary to fungal translocation because of mucosal damage. For three patients on antifungal prophylaxis, the involved fungi might be inherently resistant to the drugs used (Aspergillus niger, micafungin; Scedosporium apiospermum, anidulafungin; Candida parapsilosis, itraconazole), although antifungal drug sensitivity had not been performed for these isolates.
Table 4. Invasive fungal diseases (IFD) in five patients treated with bendamustine.
Treatment outcome of IFD
Patients 1 and 2 responded to antifungal treatment, and both died subsequently of their underlying malignancies. Patients 3 and 4 had IFD with concomitant features of systemic bacterial infection. They both died from sepsis despite vigorous antifungal and antibacterial treatment. Patient 5 had concomitant CMV duodenitis and colitis, and died despite antifungal and antibacterial treatment. Hence, death might be considered related to IFD in patients 3–5.
Bacterial infections
Nineteen patients had blood culture-positive bacterial infections (6.2%) (Supplemental file 2), of whom ten patients (53%) had grade 3/4 neutropenia at the time. There was a predominance of relapsed/refractory patients (68%) with advanced disease (stage IV: 63%) and poor performance (ECOG score ≥ 2: 63%). Septicaemia was mostly due to gram-negative bacteria (68%) or a mixture of gram-negative and gram-positive bacteria (21%). In 16 patients these documented septic episodes were successfully treated. However, in three patients, death was directly attributable to these septic episodes as terminal events.
Discussion
In this cohort, we adopted an infection control programme comparable with that for HSCT. Measures included routine CMV monitoring, weekly/biweekly blood counts and G-CSF support if neutropenia (<1 × 109/L) occurred. Patients received anti-pneumocystis and antifungal prophylaxes. Our vigorous anti-infective strategy, not used in previous studies of bendamustine, led to some unique observations.
We observed CMV virologic reactivation at a high frequency of 19%. This was due to our meticulous monitoring, allowing identification of virologic reactivation that might not be clinically evident. Interestingly, previous review and meta-analysis of bendamustine-treated patients, in the absence of regular monitoring, had not described CMV reactivation as an infective risk [Citation4,Citation6,Citation9,Citation11]. However, CMV reactivation had been observed in small series of Japanese patients [Citation12], being reported in 12/30 patients (40%) in one series [Citation13], and 3/33 patients (9%) in another series [Citation14]. The small patient numbers, different monitoring/diagnostic methods and selection bias accounted for these disparate Japanese observations.
The high CMV virologic reactivation rate in our cohort was clinically consequential, in that CMV disease occurred in five cases. In four patients, CMV retinitis developed. CMV retinitis reflects profoundly immunosuppression, characteristically found in patients with human immunodeficiency virus (HIV) infection, being an acquired immunodeficiency syndrome defining illness [Citation15]. In non-HIV patients, CMV retinitis typically occurs in organ allograft recipients [Citation15]. In lymphoma patients, CMV retinitis has been described after treatment with lympho-depleting drugs, including fludarabine [Citation16], alemtuzumab [Citation17], and 2-chlorodeoxyadenosine [Citation17]. CMV retinitis is exceptionally rare with conventional chemotherapy. The occurrence of CMV retinitis after bendamustine treatment therefore indicated an extent of lympho-depletion comparable with fludarabine, alemtuzumab and 2-chlorodeoxyadenosine. Likewise, we observed one case of CMV ependymitis/ventriculitis, a disease also classically associated with HIV infection [Citation18]. Finally, we found one case of CMV duodenitis/colitis, a condition reported previously in bendamustine-treated patients [Citation12,Citation19,Citation20]. It must be noted that the frequency of CMV disease in this study would probably be much higher had we not treated every case of virologic reactivation pre-emptively, an approach that would have prevented CMV disease in many patients with virologic reactivation.
One reason our study showed such a high frequency of CMV reactivation, not observed in previous studies, was that we adopted regular antigenic and molecular CMV monitoring. However, even if virologic reactivation was missed in previous studies without regular monitoring, CMV disease was unlikely to be overlooked. Hence, the absence of significant CMV disease in previous studies [Citation5] suggested that other factors might be involved. CMV sero-positive rates were different in various countries. In our population, CMV sero-positive rates exceed 95% for patients older than 45 years [Citation8]. On the other hand, in European and North American countries, the sero-positive rates were only 50–60% [Citation21,Citation22]. Without regular monitoring and with a low sero-positive rate, CMV infection was reported to be very low at 1 per 100 patient-years in American patients treated with bendamustine [Citation5]. Therefore, the high CMV sero-positive rate could have contributed to more frequent CMV disease in our cohort. Indeed, the CMV sero-positive rates in the Japanese people were about 85% [Citation23], potentially explaining the frequent observation of CMV reactivation and disease in bendamustine-treated patients in that population [Citation12–14, Citation19].
IFD was found in five patients. Notably, four patients received bendamustine as first-line treatment. IFD is unusual in patients with lymphoid malignancies undergoing first-line chemotherapy, so that anti-fungal prophylaxis is considered unnecessary [Citation24]. However, bendamustine appears to have increased the risk of IFD. In two patients not receiving anti-fungal prophylaxis, yeast fungemia occurred. One patient had cryptococcemia, a condition typically found only in immunocompromised patients [Citation25]. Arguably, this case might have been prevented with a simple azole such as fluconazole. In the three cases receiving anti-fungal prophylaxis, breakthrough IFDs might be caused by inherently resistant fungi. Two patients receiving echinocandin prophylaxis developed pulmonary mould infection. The rate of breakthrough invasive mould infections including invasive aspergillosis was about 5–7% in patients receiving echinocandins [Citation26]. The third patient receiving itraconazole prophylaxis developed Candida parapsilosis fungemia. In in vitro studies, the sensitivities of Candida parapsiolosis isolates to posaconazole and voriconazole were 100% and 99%, whereas that to itraconazole was only 89% [Citation27]. As this was the patient with CMV duodenitis/colitis where a breach of mucosal defence and multiple risk factors for IFD were found, in retrospect a more potent azole such as posaconazole or isavuconazole ought to have been used in this case.
Nineteen patients developed blood culture-positive bacterial infections. Because we excluded septic episodes without positive blood cultures, the actual number of patients with bacterial sepsis would be far greater.
This was a retrospective analysis with some limitations. Infections due to herpes simplex and varicella zoster viruses were not included, partly because data were incomplete. More importantly, these viral infections are common, and might be related to previous chemotherapy or concurrent anti-CD20 antibodies. Hence, the role played by bendamustine could not be clarified. Although bendamustine is lympho-ablative, it is also myelosuppressive. Hence, the degree and duration of neutropenia would affect risks of opportunistic infections. Such data were not complete enough to be analysed. It should be noted that our routine use of G-CSF would have substantially ameliorated the impact of neutropenia. We adopted universal cotrimoxazole prophylaxis, so that the risk of pneumocystis pneumonia, which had been reported in bendamustine-treated patients [Citation28], could not be determined. Finally, we did not have a control cohort of non-bendamustine treated patients to estimate the relative risks of these infective complications. However, CMV virologic reactivation, CMV disease and IFD are all uncommon even in lymphoma patients heavily treated with conventional chemotherapy, so that our data clearly identified bendamustine treatment as an important risk.
Our observations show that bendamustine treatment is associated with opportunistic viral and fungal infections. In our population where the seropositivity rate for CMV could be as high as 98% [Citation29], a programme of regular monitoring for CMV virologic reactivation during bendamustine treatment is advisable. This is especially so in older patients, who were found in this study to have a higher risk of CMV reactivation. IFD might also be increased in these patients, and the use of appropriate antifungal prophylaxis is pertinent.
Ethical approval
This study was approved by the institute review board.
Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download MS Word (15.8 KB)Acknowledgements
Tony K.Y. Wu: treated the patients, analyzed the data, wrote and approved the manuscript. Karen H.K. Tang: treated the patients, analyzed the data, wrote and approved the manuscript. Yu-Yan Hwang: treated the patients, analyzed the data, wrote and approved the manuscript. Thomas S.Y. Chan: treated the patients, analyzed the data, wrote and approved the manuscript. Eric Tse: treated the patients, analyzed the data, wrote and approved the manuscript. Yok-Lam Kwong: treated the patients, analyzed the data, wrote and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vidal L, Gurion R, Shargian L, et al. Bendamustine for patients with indolent B cell lymphoproliferative malignancies including chronic lymphocytic leukaemia - an updated meta-analysis. Br J Haematol. 2019;186(2):234–242.
- Broccoli A, Argnani L, Zinzani PL. Peripheral T-cell lymphomas: focusing on novel agents in relapsed and refractory disease. Cancer Treat Rev. 2017;60:120–129.
- Gentile M, Vigna E, Recchia AG, et al. Bendamustine in multiple myeloma. Eur J Haematol. 2015;95(5):377–388.
- Gafter-Gvili A, Gurion R, Raanani P, et al. Bendamustine-associated infections-systematic review and meta-analysis of randomized controlled trials. Hematol Oncol. 2017;35(4):424–431.
- Fung M, Jacobsen E, Freedman A, et al. Increased risk of infectious complications in older patients with indolent non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis. 2019;68(2):247–255.
- Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016;57(3):512–519.
- Foukaneli T, Kerr P, Bolton-Maggs PHB, et al. Guidelines on the use of irradiated blood components. Br J Haematol. 2020;191(5):704–724.
- Chan TS, Cheng SS, Chen WT, et al. Cost-effectiveness of letermovir as cytomegalovirus prophylaxis in adult recipients of allogeneic hematopoietic stem cell transplantation in Hong Kong. J Med Econ. 2020;23(12):1485–1492.
- Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus infection and disease in transplant patients for Use in clinical trials. Clin Infect Dis. 2017;64(1):87–91.
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and treatment of Cancer and the Mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376.
- Gafter-Gvili A, Ribakovsky E, Mizrahi N, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma. 2016;57(1):63–69.
- Hosoda T, Yokoyama A, Yoneda M, et al. Bendamustine can severely impair T-cell immunity against cytomegalovirus. Leuk Lymphoma. 2013;54(6):1327–1328.
- Hasegawa T, Aisa Y, Shimazaki K, et al. Cytomegalovirus reactivation with bendamustine in patients with low-grade B-cell lymphoma. Ann Hematol. 2015;94(3):515–517.
- Isono N, Imai Y, Watanabe A, et al. Cytomegalovirus reactivation in low-grade B-cell lymphoma patients treated with bendamustine. Leuk Lymphoma. 2016;57(9):2204–2207.
- Port AD, Orlin A, Kiss S, et al. Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther. 2017;33(4):224–234.
- Chan TS, Cheung CY, Yeung IY, et al. Cytomegalovirus retinitis complicating combination therapy with rituximab and fludarabine. Ann Hematol. 2015;94(6):1043–1047.
- Iu LP, Fan MC, Lau JK, et al. Long-term follow-up of Cytomegalovirus retinitis in non-HIV immunocompromised patients: clinical features and visual prognosis. Am J Ophthalmol. 2016;165:145–153.
- Vinters HV, Kwok MK, Ho HW, et al. Cytomegalovirus in the nervous system of patients with the acquired immune deficiency syndrome. Brain. 1989;112(Pt 1):245–268.
- Chiba A, Nakamura F, Nakazaki K, et al. Cytomegalovirus antigenemia and end-organ disease in Japanese patients treated with bendamustine. Leuk Lymphoma. 2018;59(3):749–751.
- Cona A, Tesoro D, Chiamenti M, et al. Disseminated cytomegalovirus disease after bendamustine: a case report and analysis of circulating B- and T-cell subsets. BMC Infect Dis. 2019;19(1):881.
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–1447.
- Marshall GS, Stout GG. Cytomegalovirus seroprevalence among women of childbearing age during a 10-year period. Am J Perinatol. 2005;22(7):371–376.
- Nishimura N, Kimura H, Yabuta Y, et al. Prevalence of maternal cytomegalovirus (CMV) antibody and detection of CMV DNA in amniotic fluid. Microbiol Immunol. 1999;43(8):781–784.
- Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36(30):3043–3054.
- Chen R, Zhang Y, Zhou P, et al. Cryptococcemia according to immune status: An analysis of 65 critical cases. Infect Dis Ther. 2021;10(1):363–371.
- Lionakis MS, Lewis RE, Kontoyiannis DP. Breakthrough invasive mold infections in the hematology patient: current concepts and future directions. Clin Infect Dis. 2018;67(10):1621–1630.
- Khodavaisy S, Badali H, Meis JF, et al. Comparative in vitro activities of seven antifungal drugs against clinical isolates of Candida parapsilosis complex. J Mycol Med. 2020;30(3):100968.
- Sarlo KM, Dixon BN, Ni A, et al. Incidence of infectious complications with the combination of bendamustine and an anti-CD20 monoclonal antibody. Leuk Lymphoma. 2020;61(2):364–369.
- Huang Y, Li T, Yu H, et al. Maternal CMV seroprevalence rate in early gestation and congenital cytomegalovirus infection in a Chinese population. Emerg Microbes Infect. 2021;10(1):1824–1831.
