ABSTRACT
Objectives
Patients with haematologic malignancies are at high risk of developing invasive fungal infections (IFIs). Current guidelines recommend the use of azoles for IFI prophylaxis; however, in many clinical situations, antifungal prophylaxis is used off-label. We conducted a systematic literature review to provide haematologists with the available evidence on the effectiveness and safety of isavuconazole in IFI prophylaxis in interventional and real-world, observational studies.
Methods
Embase, MEDLINE and Cochrane Library databases, and relevant conference proceedings and clinical trial registries, were searched for studies on the effectiveness and safety of isavuconazole prophylaxis in adults at high risk of IFIs. Studies were assessed for inclusion and risk of bias.
Results
Nine studies were eligible for inclusion in the review, eight of which were in haematologic populations (patients undergoing haematopoietic stem cell transplantation or with acute myeloid leukaemia or myelodysplastic syndromes; n = 5) or included haematologic populations (n = 3). Evidence from these studies suggests isavuconazole is effective for IFI prophylaxis in the haematologic setting. However, the studies frequently lacked safety data, most were based on small patient populations from single centres and risk of bias could not be assessed for five studies.
Discussion
These findings provide evidence for isavuconazole as an alternative azole for prophylaxis in high-risk populations. Limitations include lack of applicability of risk of bias assessment tools, level of filtering applied in the search strategy and focus on English-language publications.
Conclusion
Isavuconazole may be an effective azole for IFI prophylaxis in high-risk haematologic populations, although further studies are needed.
1. Introduction
Invasive fungal infections (IFIs) are associated with high mortality rates [Citation1, Citation2]. Guidelines recommend antifungal prophylaxis for patients at high risk of developing IFIs, including those with graft-versus-host disease (GvHD) following allogenic haematopoietic stem cell transplantation (HSCT), patients receiving remission-induction and reinduction chemotherapy for acute myeloid leukaemia (AML) or myelodysplastic syndromes (MDS) and those with prolonged neutropenia [Citation3–7]. Guideline recommendations mention azole antifungals, such as fluconazole, posaconazole and voriconazole, as well as amphotericin B formulations for prophylaxis [Citation3–7]. A network meta-analysis of randomized controlled trials (RCTs) supports the efficacy of these azoles for IFI prophylaxis in high-risk patients [Citation8].
Despite guideline recommendations and available trial data, posaconazole and voriconazole are the only azoles approved for prophylaxis in the EU and the US (posaconazole) [Citation9, Citation10] or the EU only (voriconazole) [Citation11], with approvals limited to specific patient populations. Posaconazole prophylaxis is indicated for patients at high risk of IFIs, namely HSCT recipients undergoing high-dose immunosuppressive therapy for GvHD or patients receiving remission-induction chemotherapy for AML or MDS expected to result in prolonged neutropenia; voriconazole is indicated for IFI prophylaxis in high-risk allogeneic HSCT recipients [Citation10, Citation11]. In the US, posaconazole prophylaxis is indicated for invasive Aspergillus and Candida infections in patients at high risk of these infections due to being severely immunocompromised, such as HSCT recipients with GvHD or those with haematologic malignancies with prolonged neutropenia from chemotherapy [Citation9]. Prophylactic treatments are not approved in other haematologic indications associated with a high risk of IFIs [Citation3–5, Citation12]; therefore, antifungal prophylaxis is frequently administered off-label. Furthermore, in situations where the approved azoles cause adverse drug–drug interactions, alternative therapies are lacking. Posaconazole poses a risk of overexposure of midostaurin, which is prescribed in patients with advanced systemic mastocytosis or FMS-like tyrosine kinase-3-mutated AML. Isavuconazole may be an alternative option but is not formally recommended [Citation13]. Since isavuconazole is not associated with QTc prolongation, it may provide value in patients using QT-prolonging medications [Citation14]. Given these challenges, collecting data is essential to inform the appropriate use of azoles.
Isavuconazole is an intravenous or oral azole antifungal that was approved in 2015 to treat invasive aspergillosis or mucormycosis in the US and the EU [Citation14, Citation15]. Isavuconazole had not been approved when the current antifungal prophylaxis guidelines were developed, and there was limited clinical evidence for its effectiveness and safety in IFI prophylaxis [Citation3–7].
Isavuconazole has favourable pharmacokinetics [Citation16] and fewer drug–drug interactions than posaconazole and voriconazole [Citation9, Citation13, Citation15, Citation17]. Some evidence suggests that isavuconazole may also have less hepatic toxicity than posaconazole and voriconazole [Citation18–20].
European Medicines Agency guidance [Citation21] states that ‘studies that assess the use of an antifungal agent for prophylaxis of invasive fungal diseases would be conducted only after an agent has demonstrated satisfactory clinical efficacy in the treatment of several types of invasive fungal diseases.’ Isavuconazole has demonstrated non-inferiority to voriconazole for treating aspergillosis [Citation19] and to amphotericin B for treating mucormycosis [Citation22].
While isavuconazole is not currently approved for prophylaxis, interventional [Citation23–25] and real-world observational studies [Citation20, Citation26–31] support its effectiveness and safety for this purpose. In a large retrospective study of isavuconazole or voriconazole prophylaxis following lung transplantation [Citation28], isavuconazole was comparable with voriconazole in preventing breakthrough (b)IFIs and mortality at 1-year post-lung transplantation. Furthermore, discontinuation rates due to adverse events (AEs), including hepatic toxicity, were significantly lower with isavuconazole [Citation28]. To understand how these findings translate to haematologic settings, we conducted a systematic literature review (SLR) to identify all currently available evidence from interventional and real-world studies on the effectiveness and safety of isavuconazole for IFI prophylaxis.
2. Materials and methods
To identify evidence on the prophylactic use of isavuconazole and other antifungals for IFIs in high-risk populations, Embase, MEDLINE and Cochrane Library databases (Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews) were searched on 23 September 2020 (Table S1–S3) and relevant conference proceedings (2018–2020), clinical trial registries and bibliographic reference lists of included studies and relevant SLRs/network meta-analyses were also hand-searched.
Eligibility criteria, including patient populations, are displayed in . Studies were initially identified by including five interventions, then narrowed down to only studies including isavuconazole. Patient populations were determined in each study.
Table 1. Eligibility criteria for the systematic literature review (PICOS).
Results from database searches were downloaded in Covidence® (Melbourne, VIC, Australia) and duplicates were removed. Titles and abstracts were screened for relevance by a single reviewer and confirmed by a second reviewer if required. Full papers were evaluated by a single reviewer to confirm relevance and verified by a second reviewer; disputes regarding eligibility were referred to a third reviewer. Data from relevant publications were extracted into standardized data-extraction tables and validated through an independent internal data check. For bIFIs, proven or probable IFIs were extracted.
Risk of bias was assessed using the seven-criteria checklist provided in the National Institute for Health and Care Excellence (NICE) single technology appraisal user guide [Citation32] and the Newcastle-Ottawa Scale [Citation33] for RCTs and observational studies, respectively.
No ethical approval was required as this review article has no original research data.
3. Results
The initial search identified 2792 records, of which 152 studies were eligible for inclusion (). This was narrowed down to nine studies that included isavuconazole (). Efficacy results are summarized in . Key findings by patient population, including safety results, are presented in the following sections.
Figure 1. PRISMA flow diagram of the literature search. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
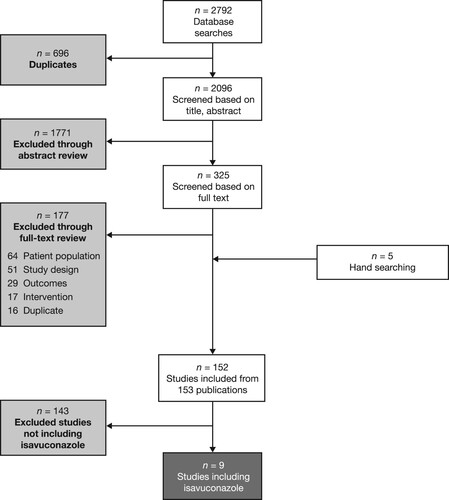
Table 2. Characteristics of prophylactic isavuconazole studies.
Table 3. bIFI and mortality rates in isavuconazole studies.
3.1. HSCT
Two studies investigated isavuconazole in patients undergoing HSCT [Citation25, Citation26]. Cupri et al [Citation26] conducted a study, published as a conference abstract, comparing isavuconazole prophylaxis with historical fluconazole prophylaxis. Isavuconazole and fluconazole groups had similar bIFI rates. Mucositis (grade 3–4) was reported in 62% and 61% of patients in the isavuconazole and fluconazole groups, respectively. No other safety outcomes were reported.
Stern et al [Citation25] reported a single-arm study in which micafungin was administered followed by isavuconazole. Three of 95 patients (3.2%) developed bIFIs and six patients (6.3%) died during the study, including two deaths due to Candida infection. Seven patients (7.4%) discontinued isavuconazole due to toxicity, most commonly liver function abnormalities (n = 5). No other safety outcomes were reported.
3.2. AML/MDS
Three studies in patients with AML/MDS included isavuconazole [Citation20, Citation23, Citation27]. Bose et al [Citation23] reported an open-label, Phase 2 study of isavuconazole prophylaxis in patients with AML/MDS [Citation23]. Four of 65 patients (6.2%) had bIFIs: one proven and three probable. The survival rate was 92%. Three AEs were reported, possibly attributable to isavuconazole: grade 1 transaminitis (n = 2) and elevated bilirubin (n = 1). Two patients discontinued due to deaths (leukaemia progression, n = 1; cardiac arrest, n = 1) and three due to mild-to-moderate elevations of aminotransferases or total bilirubin. No other safety outcomes were reported.
Aldoss et al [Citation27] conducted a study to explore risk factors for IFIs in patients with AML treated with venetoclax with hypomethylating agents. bIFI rates varied between prophylaxis groups (isavuconazole [20%], fluconazole [25%], posaconazole [12%], voriconazole [0%], micafungin [13%]) and no prophylaxis [8%]). Mortality rates and safety outcomes were not reported.
DiPippo et al [Citation20] reported a study of isavuconazole in patients with leukaemia with prior toxicity to posaconazole. Only a minority of patients received isavuconazole prophylaxis, and data were not reported separately for this group.
3.3. Mixed study populations
Three studies in mixed adult populations included isavuconazole [Citation29-31]. Vu et al [Citation31] reported a study at a single centre of hospitalized patients with autologous and allogeneic HSCT, AML, myeloma, B-cell acute lymphoblastic leukaemia, acute promyelocytic leukaemia, natural killer/T-cell lymphoma with MDS, solid organ transplants, chronic granulomatous disease, systemic lupus erythematous, and no underlying immunocompromised condition. Among patients who received primary or secondary isavuconazole prophylaxis, there were two bIFIs (7.7%) during primary prophylaxis: one probable bIFI in a patient with AML and one proven bIFI in a patient who had undergone allogeneic HSCT. Both patients died. Mortality rates and safety outcomes were not reported.
Bowen et al [Citation29] studied hospitalized patients at a single centre who had received isavuconazole prophylaxis. Underlying conditions included AML, GvHD, MDS, other types of leukaemia and aplastic anaemia. There were six bIFIs (6.1%; three proven and three probable). While discontinuation rates due to AEs were not reported, five patients discontinued due to a suspected drug-induced liver injury and one due to a suspected drug rash. Mortality rates and other safety outcomes were not reported.
Fontana et al [Citation30] reported a study at a single centre of patients with a haematologic malignancy or undergoing HSCT, who received primary isavuconazole prophylaxis. In the main analysis, the rate of bIFIs of invasive pulmonary aspergillosis exceeded expectations based on experience: seven patients (4.8%) died within a median of 12 days from bIFI onset. Subsequently, bIFIs were retrospectively analyzed among patients who received isavuconazole, posaconazole or voriconazole prophylaxis during chemotherapy for AML. In patients with de novo AML, bIFIs occurred during courses of isavuconazole (8%), posaconazole (3%; p = 0.6 vs isavuconazole) and voriconazole (0%; p = 0.04 vs isavuconazole). In patients with relapsed/refractory AML, bIFIs occurred at similar rates with no significant differences between isavuconazole, posaconazole and voriconazole. Mortality rates and safety outcomes were not reported.
3.4. Solid organ transplant
Samanta et al [Citation28] reported a study comparing isavuconazole versus voriconazole prophylaxis following lung transplantation [Citation28]. Within 1 year of transplant, a total of 14 (9.7%) and 18 (11.5%) patients had died in the isavuconazole and voriconazole groups, respectively (p = 0.54). Fifteen (10.9%) and 54 (35.5%) patients, respectively, discontinued due to AEs.
3.5. Assessment of bias
Assessment of bias was possible for four studies following PRISMA guidelines [Citation20, Citation27, Citation28, Citation30]; there was a low risk of bias from their inclusion (Table S4). As all studies were observational, the NICE checklist was not used. The assessment tools were not applicable to the other studies, as they were non-comparative or did not provide enough published data.
4. Discussion
This SLR identified evidence on the effectiveness and safety of isavuconazole for IFI prophylaxis in haematologic populations, including real-world evidence.
Overall, nine studies were identified that included isavuconazole prophylaxis for IFIs, eight of which were in the haematologic setting [Citation20, Citation23, Citation25–31]. The majority were observational and provide insights into the real-world use of isavuconazole [Citation20, Citation26–31]. All nine studies were published in 2019–2020; therefore, these data have become available since the publication of major guidelines on antifungal prophylaxis for IFIs in high-risk patients [Citation3–7].
4.1. HSCT
One isavuconazole prophylaxis study reported bIFI rates; 1-year survival rates and AE rates were similar to those with fluconazole prophylaxis [Citation26]. Furthermore, the single-arm study by Stern et al [Citation25] concluded that the data supported isavuconazole for antifungal prophylaxis after HSCT [Citation25]. A follow-up to this study was published after the SLR was conducted, in which the efficacy and safety of isavuconazole versus voriconazole as the primary antifungal prophylaxis were evaluated in a frequency-matched cohort analysis in allogeneic HSCT recipients (voriconazole, n = 210; isavuconazole, n = 95) [Citation34]. Patients discontinued voriconazole significantly earlier and more frequently than isavuconazole, most commonly because of biochemical hepatotoxicity (voriconazole, n = 48/210 [22.9%] and isavuconazole, n = 5/95 [5.3%]; p = 0.0002). None developed bIFIs in the voriconazole group, while three patients (3.2%) developed candidaemia in the isavuconazole group at a median of 48 days post-HSCT. There were no significant differences between the voriconazole and isavuconazole groups in overall survival or the number of probable or proven IFIs at 180 days post-HSCT. Primary antifungal prophylaxis with isavuconazole showed a more favourable safety profile and comparable efficacy versus voriconazole in HSCT recipients; however, this was an unblinded, non-randomized, observational study.
A 2021 single-centre case report, published after the SLR was conducted, reported on five patients who received isavuconazole prophylaxis following allogenic HSCT [Citation12]. The five patients had malignancies at various remission stages: ALK-positive anaplastic lymphoma, AML, Hodgkin lymphoma, diffuse large B-cell lymphoma and acute lymphoblastic leukaemia [Citation12]. One patient on voriconazole prophylaxis changed to isavuconazole due to hepatic toxicity [Citation12]. Following the change to isavuconazole, toxicities resolved [Citation12]. Another patient remained on isavuconazole prophylaxis at the time the paper was written (December 2020), and no IFIs were reported in any of the patients [Citation12].
4.2. AML/MDS
An open-label, Phase 2 study concluded that isavuconazole is effective for antifungal prophylaxis in patients with newly diagnosed AML/MDS undergoing remission-induction chemotherapy [Citation23]. These findings are supported by a retrospective study that explored risk factors for IFIs in patients with AML treated with venetoclax with hypomethylating agents [Citation27]. In this study, the type of antifungal prophylaxis did not influence the development of IFIs; however, group sample sizes were small and safety outcomes were not reported [Citation27]. In a retrospective study that treated patients with prior posaconazole toxicity prophylactically with isavuconazole [Citation20], data were also not provided separately for these patients, limiting interpretation [Citation20].
Finally, a Phase 2 study was not included in the SLR as it was not identified by the search strategy but rather during a hand-search of the reference lists of citations identified by the SLR; this study explored the safety of low- and high-dose isavuconazole prophylaxis in a small group of patients with AML, and concluded that isavuconazole was well tolerated at both doses [Citation24].
4.3. Mixed study populations
All three observational studies in mixed populations were retrospective and conducted in a single centre. Vu et al [Citation31] and Bowen et al [Citation29] provided limited dosing information and, in all studies, follow-up duration was unclear. Two of the studies were supportive of isavuconazole prophylaxis [Citation29, Citation31], but one noted a possible increased incidence of bIFIs, particularly invasive pulmonary aspergillosis [Citation30]. All three studies noted that further data are required to assess isavuconazole for antifungal prophylaxis in these populations.
Findings from this SLR in mixed study populations have provided evidence regarding isavuconazole prophylaxis in indications, namely autologous HSCT [Citation31] and haematologic malignancies such as other leukaemias [Citation29]: acute promyelocytic leukaemia, natural killer/T-cell lymphoma and myeloma [Citation31], that currently have no approved prophylactic azole.
This is noteworthy as isavuconazole has fewer drug–drug interactions, a weaker cytochrome P450 3A4 inhibitory effect and potentially lower risk of hepatic and renal toxicity than posaconazole and voriconazole [Citation9, Citation10, Citation13, Citation15, Citation17–20, Citation35, Citation36]. A risk–benefit analysis is recommended where intravenous posaconazole or voriconazole are indicated in patients with moderate-to-severe renal impairment; however, no dose adjustment of intravenous isavuconazole is required, as <1% is renally excreted [Citation36].
4.4. Strengths and limitations
Our SLR followed a robust design. Multiple databases were searched, and bias was assessed using checklists specific to RCTs and observational studies. Nonetheless, the methodology was subject to limitations. The risk of bias assessment tools were unsuitable for non-comparative studies. Additionally, Cupri et al [Citation26], published as a conference abstract, was not assessed for bias due to a lack of information reported. The search strategy included validated filters for publication type, which meant one potentially relevant study was not identified by the database searches [Citation24].
The focus on English-language publications may have excluded relevant non-English records. Conference proceedings were hand-searched for the period 2018–2020 on the basis that older records would have been published as manuscripts by the time the SLR was conducted, which may have excluded studies. However, no date restriction was applied to conference abstracts indexed in Embase, reducing the likelihood of excluding older records.
Results were not directly compared across studies due to variations in study designs, patient populations, additional interventions, prophylaxis and follow-up durations, and outcomes. Within-study comparisons were possible in some cases, but many studies did not include a comparator arm [Citation26–28, Citation30].
4.5. Conclusions
Several interventional and real-world observational studies have looked at isavuconazole prophylaxis for IFIs. These include investigations in haematologic settings, where isavuconazole prophylaxis was generally effective, but safety data were often lacking. Although the limited evidence available supports the use of isavuconazole as a prophylactic treatment, further studies are needed, particularly to provide insight into its potential safety value. This review may assist treatment decisions for patients at high risk of IFIs with no approved prophylactic azole or those requiring an alternative option.
Authorship contributions
CB, JAA and MI conceptualized the study design; NW and TM acquired the data; and all authors analyzed the data; substantially revised and critically reviewed the article; reviewed and agreed on all versions of the article before submission, including the final version accepted for publication and any significant changes introduced at the proofing stage; agreed on the journal to which to submit to; agreed to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Supplemental Material
Download MS Word (65.4 KB)Acknowledgements
Source Health Economics extracted the data for the systematic literature review and medical writing support, under the guidance of the authors, was provided by Robyn Wilson, BSc, CMC Connect, McCann Health Medical Communications, both of which were funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163(6):461-464).
Disclosure statement
YP and JH report no conflicts of interest. CB is an employee of Pfizer Pharma GmbH. MI and JAA are employees of Pfizer Inc. TM and NW are employees of Source Health Economics. OP has previously received honoraria from Pfizer Inc; however, OP did not receive an honorarium for his work on the current manuscript. OP has received honoraria or travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech, Novartis and Therakos. He has received research support from Gilead, Incyte, Jazz, Neovii Biotech and Takeda. He is a member of advisory boards for Gilead, Jazz, MSD, Omeros, Priothera, Shionogi and SOBI.
Additional information
Funding
Notes on contributors
Yang Ping
Yang Ping is Deputy Chief Physician and Doctor of Medicine in the Hematology Department at Peking University Third Hospital, Beijing, China. She has been engaged in the clinical diagnosis and treatment of haematological diseases for a long time and has experience in the diagnosis and treatment of various benign and malignant diseases of the blood system, particularly haematological malignancies such as lymphoma and multiple myeloma. At the same time, she has rich clinical experience in immunotherapy and haematopoietic stem cell transplantation. She has participated in several national and international clinical studies on lymphoma. Her academic appointments are as follows: Standing Committee Member of the Lymphoma Branch of the Beijing Cancer Pathology Precision Diagnosis Research Association, Standing Committee Member of the Youth Committee of the China Medical Education Association, Member of the Lymphoma Special Committee of the China Elderly Health Care Association, Member of the Lymphoma Immunotherapy Special Committee of the Beijing Cancer Prevention Society and Member of the Red Blood Cell Special Committee of the Beijing Cancer Prevention Society. She was awarded Outstanding Young Physician of Peking University Third Hospital.
Jing Hongmei
Jing Hongmei is a Professor and Doctoral Supervisor as well as the Chief Physician and Director of the Hematology Department at Peking University Third Hospital, Beijing, China. She is also the Director of the CAR T-Cell Therapy Joint Research and Development Center of Peking University Third Hospital. Her academic appointments are as follows: Chairman of the Professional Committee of Hematology and Oncology of the Beijing Society of Integrated Medicine, Vice Chairman of the Oncology Expert Committee of the China Women Doctors Association, Vice Chairman of the Lymphoma Special Committee of the China Medical Education Association, Vice Chairman of the Youth Committee of the Blood Branch of the China Medical Education Association, Member of the Standing Committee of the Geriatric Oncology Committee of the Chinese Geriatric Society, Member of the Diagnostic Group of the Blood Branch of the Chinese Medical Association and Member of the Blood Branch of the Chinese Medical Association. Her speciality lies in lymphatic system tumours and lymphomas.
Christian Bellmann
Christian Bellmann holds a PhD from the Freie Universität Berlin and the Leibniz-Institute for Molecular Pharmacology in Berlin, Germany, where he completed a post-doctoral fellowship on the Campus Berlin-Buch, Berlin, Germany. He is certified in medicine development (post-nominal degree “CMD”) by King’s College London and the IFAPP Academy, London, UK. Currently he is working as a Senior Medical Advisor at Pfizer.
Mónica Inês
Mónica Inês holds a PhD in Science and Health Technologies from the Faculty of Medicine, an MSc in Applied Econometrics and a BSc in Applied Mathematics to Economics and Management from the School of Economics & Management, all awarded by the University of Lisbon, Lisbon, Portugal. She has worked previously in the Portuguese National Authority of Medicines and Health Products and in a health economics consultancy company and is currently working as Global Health Economics and Outcomes Research Director, Rare Disease at Pfizer. She is also a Professor in Pharmacoeconomics (Lusófona University of Humanities and Technologies, Lisbon, Portugal) and Health Economics Applied Econometrics (University of Lisbon). Mónica serves as Elected President of the International Society for Pharmacoeconomics and Outcomes Research Portugal Regional Chapter. Mónica's research interests are related to the application of health economics, namely health technology assessment and outcomes research studies.
Tom Macmillan
Tom Macmillan joined Source Health Economics in 2020 having previously worked as an Information Specialist and Health Technology Assessor at King’s College London, London, UK. He has worked in the health economics and outcomes research field since 2013 and has experience delivering a wide variety of health technology assessment (HTA) projects, including several years working on the NICE medical technologies evaluation programme. He has previously worked on systematic reviews for the Royal College of Surgeons at the National Surgical Commissioning Centre and current awareness bulletins for surgical specialities. His disease experience comprises vascular access, diabetes, oncology, neurodegenerative disease, wound healing and surgery and he has worked extensively on HTAs for medical devices. He completed an MSc in Information Management in 2014 and holds a BA in English Literature and Linguistics.
Neil Webb
Neil Webb joined Source Health Economics in 2018. He was previously a Senior Consultant at DRG Abacus from 2012 to 2018, working as a Specialist Team Lead for their systematic review and NMA Centre of Excellence. He has a BSc in Medical Genetics from Swansea University, Swansea, UK, which included a year in industry at GlaxoSmithKline.
Jalal A. Aram
Jalal (Jay) A. Aram is a Doctor of Medicine with a focus on internal medicine and clinical research and has over 25 years of experience. Research interests include clinical mycology, treatment and diagnosis as well as microbiology and fungal diagnostics. He joined Pfizer Inc in 2007, with increasing clinical development and medical affairs responsibilities in creating a global anti-infective strategy, data and evidence generation, publication plans and grant administration.
Olaf Penack
Olaf Penack obtained his MD from the University in Göttingen, Göttingen, Germany, in 1999 and joined Charité – Universitätsmedizin Berlin, Berlin, Germany, as a Clinical Fellow and Scientist in 2002. In 2009, he completed a post-doctoral fellowship at the Memorial Sloan Kettering Cancer Center in New York City, USA. Currently, he is Director of the Advanced Cellular Therapy Programme in the Department of Hematology, Oncology and Tumorimmunology at Charité – Universitätsmedizin Berlin, Campus Virchow Clinic. His aim is to improve the outcome of cellular therapies by optimized clinical management as well as by development of novel therapeutic approaches. One specific focus of Olaf Penack’s work is the management of infectious complications in immunosuppressed patients.
References
- Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014;33(1):7–21.
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm. 2009;66(19):1711–1717.
- Fleming S, Yannakou CK, Haeusler GM, et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 2014;44(12b):1283–1297.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–93.
- Maertens JA, Girmenia C, Brüggemann RJ, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221–3230.
- Mellinghoff SC, Panse J, Alakel N, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol. 2018;97(2):197–207.
- Ullmann AJ, Schmidt-Hieber M, Bertz H, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95(9):1435–1455.
- Zhao YJ, Khoo AL, Tan G, et al. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob Agents Chemother. 2016;60(1):376–386.
- US Food and Drug Administration. Noxafil (posaconazole) prescribing information. 2015.
- European Medicines Agency Noxafil (posaconazole): summary of product characteristics. 2014.
- European Medicines Agency Vfend (voriconazole): summary of product characteristics. 2021.
- Salas MQ, Mussetti A, Muñóz C, et al. Isavuconazole prophylaxis against invasive fungal infections in allogeneic stem cell transplantation: a single-center experience [published online ahead of print February 8, 2021]. Hematol Transfus Cell Ther; doi:10.1016/j.htct.2021.01.002.
- Menna P, Salvatorelli E, Del Principe MI, et al. Choosing antifungals for the midostaurin-treated patient: does CYP3A4 outweigh recommendations? A brief insight from real life [published online ahead of print March 5, 2021]. Chemotherapy: doi:10.1159/000513989.
- European Medicines Agency Cresemba (isavuconazole): summary of product characteristics. 2019.
- US Food and Drug Administration Cresemba (isavuconazonium sulfate) prescribing information. 2019.
- Schmitt-Hoffmann A, Roos B, Heep M, et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother. 2006;50(1):279–285.
- US Food and Drug Administration Vfend (voriconazole) prescribing information. 2015.
- Xing Y, Chen L, Feng Y, et al. Meta-analysis of the safety of voriconazole in definitive, empirical, and prophylactic therapies for invasive fungal infections. BMC Infect Dis. 2017;17(1):798.
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–769.
- DiPippo AJ, Rausch CR, Kontoyiannis DP. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses. 2019;62(1):81–86.
- European Medicines Agency Guideline on the clinical evaluation of antifungal agents for the treatment and prophylaxis of invasive fungal disease. 2010.
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837.
- Bose P, McCue D, Wurster S, et al. Isavuconazole as primary anti-fungal prophylaxis in patients with acute myeloid leukemia or myelodysplastic syndrome: an open-label, prospective, phase II study. Clin Infect Dis. 2021;72(10):1755–1763.
- Cornely OA, Böhme A, Schmitt-Hoffmann A, et al. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother. 2015;59(4):2078–2085.
- Stern A, Su Y, Lee YJ, et al. A single-center, open-label trial of isavuconazole prophylaxis against invasive fungal infection in patients undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26(6):1195–1202.
- Cupri A, Leotta S, Markovic U, et al. Isavuconazole prophylaxis during early phases of allogeneic HSC transplantation is not associated to an increase need of cyclosporin-a dose modification. Blood. 2019;134(Suppl 1):3271.
- Aldoss I, Dadwal S, Zhang J, et al. Invasive fungal infections in acute myeloid leukemia treated with venetoclax and hypomethylating agents. Blood Adv. 2019;3(23):4043–4049.
- Samanta P, Clancy CJ, Marini RV, et al. Isavuconazole is as effective as and better tolerated than voriconazole for antifungal prophylaxis in lung transplant recipients. Clin Infect Dis. 2021;73(3):416–426.
- Bowen CD, Tallman GB, Hakki M, et al. Isavuconazole to prevent invasive fungal infection in immunocompromised adults: initial experience at an academic medical centre. Mycoses. 2019;62(8):665–672.
- Fontana L, Perlin DS, Zhao Y, et al. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin Infect Dis. 2020;70(5):723–730.
- Vu CA, Rana MM, Jacobs SE, et al. Isavuconazole for the prophylaxis and treatment of invasive fungal disease: a single-center experience. Transpl Infect Dis. 2020;23(2):e13469.
- National Institute for Health and Care Excellence Single technology appraisal: user guide for company evidence submission template. 2015.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021.
- Bogler Y, Stern A, Su Y, et al. Efficacy and safety of isavuconazole compared with voriconazole as primary antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients [published online ahead of print May 24, 2021]. Med Mycol; doi:10.1093/mmy/myab025.
- Klatt ME, Eschenauer GA. Review of pharmacologic considerations in the use of azole antifungals in lung transplant recipients. J Fungi (Basel). 2021;7(2):76.
- Tragiannidis A, Gkampeta A, Vousvouki M, et al. Antifungal agents and the kidney: pharmacokinetics, clinical nephrotoxicity, and interactions [published online ahead of print June 1, 2021]. Expert Opin Drug Saf; doi:10.1080/14740338.2021.
