ABSTRACT
Objective
Acute lymphoblastic leukemia is the most common malignant disease in children. CD34 and CD38 are expressed in both normal and leukemia cells, but studies of their prognostic associations in childhood acute lymphoblastic leukemia are limited. The aim of this study was to investigate the prognostic effect of CD34 + CD38− leukemia cells in this childhood cancer.
Methods
From January 2014 to January 2019, children with newly diagnosed acute lymphoblastic leukemia were included in this study and followed up until July 2020. The participants were divided into CD34+ and CD34− groups according to CD34 expression level at diagnosis, and the CD34+ group was further divided into CD34 + CD38− and CD34 + CD38+ subgroups based on CD38 expression level. We tracked clinical biological features, therapeutic outcomes, and other patient data for comparisons.
Results
The OS and EFS did not differ significantly between the CD34+ and CD34− groups (both P > 0.05). CD34+CD38- group and CD34+CD38+ group were further compared. OS differed significantly between these two groups (χ2 = 3.89, P = 0.048), as did the recurrence rate (χ2 = 5.04, P = 0.025), but EFS did not (χ2 = 1.45, P > 0.05). Survival analysis in patients with recurrence showed a significantly higher OS for the CD34 + CD38+ group compared with the CD34 + CD38− group (χ2 = 5.08, P = 0.024). The CD34+CD38- group and CD34+CD38+ group were matched for propensity scores. When recurrence was compared in the two groups after matching, the difference was statistically significant (P < 0.001).
Conclusion
CD34+ and CD34− expression does not differ by prognosis in children with acute lymphoblastic leukemia, but CD34 + CD38− may indicate a poor prognosis.
Introduction
In acute lymphoblastic leukemia (ALL), a major form of childhood leukemia, lymphoblastic stem cells undergo differentiation block, apoptosis disorder, and malignant proliferation during the differentiation process [Citation1]. With the continuous development of minimal residual disease (MRD) detection and other technologies, risk stratification in pediatric ALL has become more reliable, and individualized treatment based on risk stratification is increasingly successful. The long-term survival rate has reached more than 90% in some medical centers [Citation2]. Nevertheless, progressive leukemia, especially recurrence, remains a leading cause of childhood disease-related death and treatment-related deaths, along with secondary tumors and other unwanted outcomes [Citation3,Citation4].
Recurrence risk is based on multiple factors, including cytogenetics, genomic characteristics, and MRD [Citation5,Citation6]. In recent years, leukemic stem cells (LSCs) as the hypothesized origin of recurrence have become a research hotspot [Citation7]. Like normal hematopoietic stem cells, LSCs are organized hierarchically, with unlimited self-renewal and the ability to continuously produce immature cells, so that disease transmission ability is unlimited [Citation8]. The phenotype and hierarchy of LSCs have not been clearly defined, but they are mainly believed to exist as CD34 + CD38+, CD34 + CD38−, and CD34− cell populations. Among these, CD34 + CD38− has important predictive significance for acute myeloid leukemia [Citation9]. In the study of chronic myeloid leukemia, LSCs also are believed to be present in the cloned CD34 + CD38− fragment [Citation10].
For the current work, we conducted a retrospective cohort study of children newly diagnosed with ALL. The study aims were to further explore the prognostic significance of CD34 + CD38− leukemia cells in childhood ALL and to accurately stratify children with ALL and provide evidence for treatment.
Patients and methods
Study population and treatment protocol
From 1 January 2014 to 1 January 2019, children newly diagnosed with ALL were enrolled in the pediatric blood group of Huai'an First Hospital affiliated with Nanjing Medical University and the pediatric blood group of Xuzhou Medical University Affiliated Hospital. Inclusion criteria were diagnosis with evidence of bone marrow cell morphology, immunological typing, cytogenetics, and molecular biology and age under 14 years at first diagnosis. Exclusion criteria were ceasing treatment or not following the Chinese Children’s Leukemia Group (CCLG)-2008 regimen for chemotherapy, trisomy 21 or mature B-cell ALL (B-ALL), central nervous system leukemia (CNSL) or testicular leukemia (TL) at the first diagnosis, and a history of bone marrow transplantation or immunotherapy. According to relevant studies, cell and molecular genetics phenotypes were divided into three categories: unfavorable = low diploid with chromosome number <45, t(9;22)(q34;q11.2)/BCR-ABL1, t(4;11)(q21;q23)/MLL-AF4 or other mixed-lineage leukemia (MLL) gene rearrangements, t(1;19)(q23;p13)/E2A-PBX1, iAMP21, IKZF1 deletion, Ph-like fusion gene; favorable = hyperdiploid with chromosome number >50, t(12;21) (p13;q22)/TEL-AML1; and other = unspecified chromosome abnormalities and normal /deletion cytogenetics [Citation5]. All included children received the CCLG-2008 regimen of chemotherapy (). This study was approved by the Ethics Committee of Huai'an First Hospital affiliated with Nanjing Medical University and Xuzhou Medical University Affiliated Hospital.
Table 1. The acute lymphocyte leukocyte CCLG-ALL 2008 program.
Grouping and follow-up
Using flow cytometry, immunotyping was performed at the first diagnosis, and the expression of cell surface antigens CD34 and CD38 was detected. CD45/SSC was used to delineate leukemia cells. Positive expression was considered to be cell antigen CD34 expression ≥20 and cell antigen CD38 expression ≥30% [Citation11,Citation12]. Children were divided into CD34+ and CD34− groups. The CD34+ group was further subdivided into a CD34 + CD38− group and a CD34 + CD38+ group according to cell surface antigen CD38 expression.
All children included in the study were followed up until July 1, 2020, using outpatient or telephone follow-up. Overall survival (OS) was defined as the time from the start of treatment to death or the last follow-up, and event-free survival (EFS) was defined as the time from the start of treatment to the occurrence of the first event or the last follow-up. Outcome events in patients were non-remission, recurrence, death, second tumor, and loss to follow-up. Loss to follow-up referenced leaving treatment after entering the second treatment course or without any form of follow-up recorded within one year after completing chemotherapy. Patients who were lost to follow-up were counted as the last follow-up date in the OS period. Recurrence stage was defined as follows: very early recurrence was the time from diagnosis to recurrence <18 months, early recurrence was recurrence within 18 months after diagnosis to 6 months after the end of treatment, and late recurrence was recurrence after 6 months after the end of treatment [Citation13].
Statistical analysis
All statistical analyses were performed with SPSS 24.0 statistical software. Normally distributed measurement data are presented as means ± standard deviations, non-normally distributed measurement data as medians (lower and upper quartiles), and frequency data as the number of cases and percentages. For comparisons between groups, we used t-tests or the χ2 test. The Kaplan–Meier method was applied for generating survival curves for EFS and OS, with 95% confidence intervals, and the log-rank test was used for comparing survival across groups. The two groups of data with confounding factors were compared for propensity score matching, with a matching ratio of 1:1, and the match tolerance was 0.02. The count data after pairing was compared by McNemar’s chi-square test, and P < 0.05 was considered statistically significant. Curves were drawn using GraphPad 6.0.
Results
General information
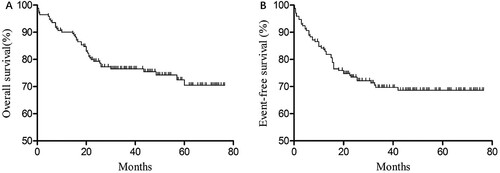
From January 2014 to January 2019, a total of 211 consecutive children were newly diagnosed with ALL. Among them, four were older than 14 years, 21 had ceased treatment or did not undergo CCLG-2008 chemotherapy, eight had received transplantation or immunotherapy, three had trisomy 21 or mature B-ALL, and four had CNSL or TL at the first diagnosis. Finally, 171 children were included in this study, including 99 boys and 72 girls, with a male-to-female ratio of 1.38:1, a median age of 4.2 (0.5–14) years, and an average age of 5.4 years. Of the group, 72 had standard risk, 51 had intermediate risk, and 47 had high risk. Up to the end of follow-up, 37 children experienced recurrence, for a recurrence rate of 21.6%. Two children had second tumors, there were 11 treatment-related deaths, and one child was lost to follow-up. The median follow-up time for the 171 patients was 36 (0.2–76.5) months, the 3-year OS was 77%±3.3%, the expected 5-year OS was 71% ± 4.3% ((A)), the 3-year EFS was 69.7% ± 3.6%, and the expected 5-year EFS is 68.6% ± 3.7% ((B)).
Analysis of clinical biological characteristics of the CD34+ and CD34− groups
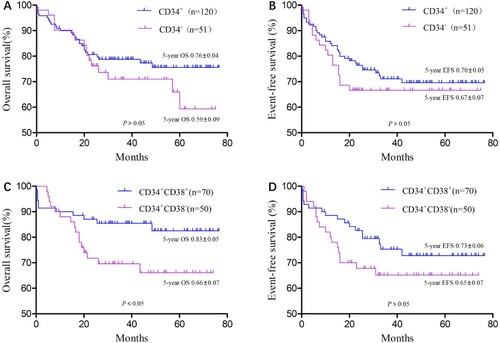
We also divided the 171 children into CD34+ (n = 120) and CD34− (n = 51) groups, according to the CD34 cell antigen expression of leukemia cells. T-cell ALL (T-ALL) was more common in the CD34− group (P < 0.05), but the two groups did not differ in other clinical biological characteristics (P > 0.05; ).
Table 2. Clinical and biological characteristics of the CD34+ and CD34− groups.
Survival analysis
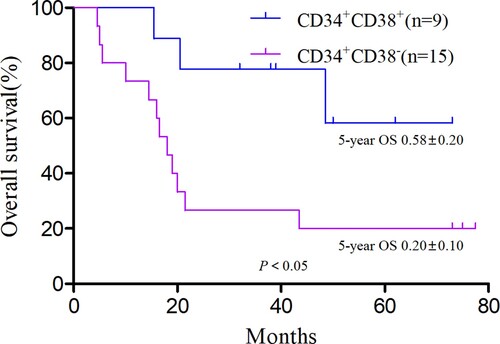
Until 1 July 2020, OS did not differ significantly between the CD34+ and CD34− groups (χ2 = 1.16, P > 0.05; (A)), and neither did EFS t (χ2 = 0.55, P > 0.05; (B)). The CD34+ group was subdivided into the CD34 + CD38+ (n = 70) and CD34 + CD38− (n = 50) groups according to the CD38 cell antigen expression of leukemia cells. In the CD34 + CD38+ group, 14 children were at high risk, 18 were at intermediate risk, and 38 had standard risk. In the CD34 + CD38− group, 16 cases were high risk, 15 were intermediate risk, and 19 were standard risk. Risk level proportions did not differ between the two groups (P > 0.05). OS did differ significantly between the groups (χ2 = 3.89, P = 0.048; (C)), but EFS did not (χ2 = 1.45, P = 0.229; (D)).
Analysis of recurrence in the CD34 + CD38+ and CD34 + CD38− groups
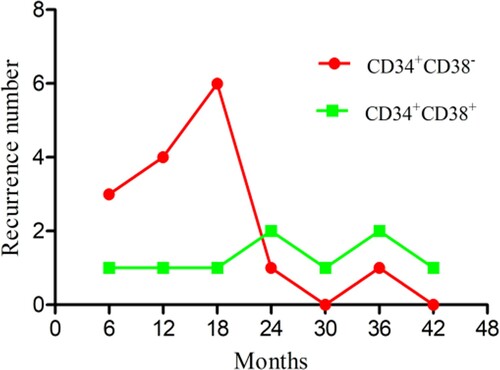
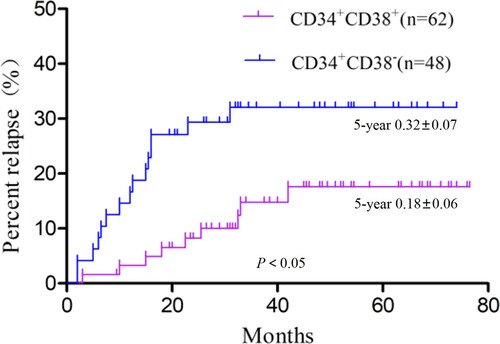
There were nine recurrences in the CD34 + CD38+ group, including four very early recurrences, two early recurrences, and three late recurrences. The CD34 + CD38− group had 15 recurrences, 13 of them very early, one early, and one late. The CD34 + CD38− group had a small peak in the very early recurrence stage, but the CD34 + CD38+ group had no obvious peak in any recurrence stage (). Compared with the CD34 + CD38− group, the recurrence rate in the CD34 + CD38+ group was significantly lower (χ2 = 5.04, P = 0.025; ). The analyses did not include the recurrence rate among those who had treatment-related deaths and loss to follow-up. Further survival analysis of patients with recurrence showed a significantly higher OS in the CD34 + CD38+ group compared with the CD34 + CD38− group (χ2 = 5.08, P = 0.024; ).
Propensity score matching between the CD34 + CD38+ and CD34 + CD38− groups
Based on China’s Recommendations for diagnosis and treatment of acute lymphoblastic leukemia in childhood (4rd revised version), risk factors that are definitely related to the prognosis of childhood ALL include (1) age at diagnosis of <1 year or ≥10 years; (2) peripheral blood white blood cell (WBC) count >50×109/L at diagnosis; (3) CNSL or TL at time of diagnosis; (4) T-ALL immunophenotype; (5) cellular and molecular biological characteristics of a low diploid with chromosome number <45, t(9;22)(q34;q11.2)/BCR-ABL1, t(4;11)(q21;q23)/MLL-AF4 or other MLL gene rearrangements, or t(1;19)(q23;p13)/E2A-PBX1; (6) prednisone treatment ineffective; (7) bone marrow primitive and naive lymphocytes ≥25% on the 15th day of induction remission; (8) at end of induction remission treatment (day 33 of chemotherapy), bone marrow did not show complete remission, with >5% primitive and prolymphocytes; and (9) MRD ≥1×10−4 at the end of induction remission treatment (day 33 of chemotherapy) or ≥1×10−3 before the start of consolidation therapy (week 12).
For propensity score matching, we included the following as variables: age at diagnosis, WBC count in peripheral blood, immunophenotype, molecular genetic characteristics, prednisone response, expression of bone marrow primordial lymphocytes at 15 days of chemotherapy, bone marrow remission at 33 days of chemotherapy, and MRD levels at 33 days and 12 weeks of chemotherapy variables. The baseline comparison before matching is shown in . The analysis yielded a total of 32 cases of exact matching and 4 cases of fuzzy matching. The baseline comparison after matching is shown in . The McNemar paired chi-square test was used to compare the two sets of data after matching, and the results indicated a significant difference (P < 0.001).
Table 3. Baseline comparison between the CD34 + CD38+ and CD34 + CD38− groups (before matching).
Table 4. Baseline comparison between the CD34 + CD38+ and CD34 + CD38− groups (after matching).
Discussion
ALL is the most common malignant disease in children. With more precise risk stratification and continuous improvements in therapy, some advanced medical centers have reached a 5-year EFS of 80%–90%. Even so, recurrence rates are still 10%–20%. Recurrence and refractory disease are serious threats to the lives of children [Citation14].
As cell surface antigens, CD34 and CD38 are closely associated with the origin and differentiation of cells. CD34 is believed to be a marker of early expression of human hematopoietic precursor cells, mainly expressed in hematopoietic progenitor cells and hematopoietic stem cells. Its expression level decreases with cell differentiation [Citation15], whereas the expression of CD38 in hematopoietic cells depends on the differentiation and activation state of cells. CD38 is normally expressed in activated lymphocytes [Citation16]. CD34 and CD38 are expressed in normal and leukemia cells. After the concept of leukemia stem cells based on CD34 and CD38 was proposed, this hypothesized origin of recurrence has been increasingly recognized by the academic community. We collected clinical data on children newly diagnosed with acute lymphocytic leukemia to study the effect of CD34 and CD38 expression on prognosis.
The 171 included children were treated according to the CCLG-2008 protocol. After more than 5 years of follow-up, the recurrence rate was 21.6%, the 5-year OS was 71%±4.3%, and the 5-year EFS was 68.6%±3.7%, similar to reports from most medical centers in China. During the follow-up, two patients died during a prednisone trial, and because we could not rule out that their deaths were unrelated to CD34 and CD38, we did not exclude them from the study.
We found that most of the clinical biological characteristics of the CD34+ and CD34− groups were similar. Only T-ALL was more common in the CD34− group than in the CD34+ group. Previous studies have shown that the proportion of CD34+ cells in B-ALL is significantly higher than in T-ALL, and the prognosis is better than in cancers expressing CD34− [Citation17]. We speculate that one explanation is that CD34− leukemia cells are more prone to T phenotype differentiation. Some studies have shown that CD34 + CD7− leukemia cells are more enriched in T-ALL [Citation18], so this hypothesis needs testing in future studies.
OS and EFS did not differ between the CD34+ and CD34− groups, which is inconsistent with the findings of Wendelien Zeijlemaker et al [Citation19] in a study of acute myeloid leukemia. They found that prognosis with CD34+ acute myeloid leukemia was poor, but some groups have shown that CD34− cells also have strong proliferation and extensive self-renewal capabilities [Citation20]. Studies in China have confirmed that leukemia cells can be found in the CD34− cell population in acute myeloid leukemia, and this cell population may be more primitive [Citation21].
Our results also showed that CD34− and CD34+ cells, as the cells of leukemia origin, were not related to prognosis. Further dividing the patients into a CD34 + CD38+ group and a CD34 + CD38− group, we found a significantly lower OS in the CD34 + CD38− group, suggesting a worse prognosis with CD34 + CD38− leukemia cell expression. Although the groups did not differ significantly in EFS, the recurrence rate was significantly higher in the CD34 + CD38− compared with the CD34 + CD38+ group, and the CD34 + CD38− group was more prone to early recurrence. Early recurrence is an important factor in poor prognosis with ALL [Citation4]. We posit that the early recurrence in the CD34 + CD38− group means a reduced chance for rescue, leading to lower OS but no significant difference in EFS compared with the CD34 + CD38+ group.
We further analyzed survival in the patients with recurrence and found lower OS in the CD34 + CD38− group. This finding seems to confirm that CD34 + CD38− leukemia cells may be more associated with recurrence. Lang et al prospectively isolated three subgroups (CD34 + CD3−, CD34 + CD38+, and CD34−CD38+) and found that although all three had the same potential for leukemia, over time, only CD34 + CD38− cells maintained a stable potential [Citation7]. This evidence supports our hypothesis that low immunogenicity and chemotherapy resistance may be one of the reasons for the recurrence of CD34 + CD38− leukemia cells [Citation9]. Others have reported that STAT5 expressed in CD34 + CD38− stem cells is a potential molecular target for Ph-negative myeloproliferative tumors [Citation22].
Comparing the relative proportions of standard-risk, median-risk, and high-risk patients between the two groups, we found no significant differences. This outcome suggests that compared with traditional risk stratification methods, the predictive value of CD34 + CD38− is only supplemental rather than a substitute.
The current results are based on single-factor analysis and do not address confounding factors. Cox regression analysis is more practical and has been widely used in survival data with confounding factors. For this study, the CD34+ group represented a limited number of cases and involved too many confounding factors, so we expected it to exceed the tolerance of Cox regression analysis and lead to statistical errors. The propensity score matching method reduces bias and can avoid some limitations of the traditional multivariate regression model [Citation23]. Matching two subsets is sufficient to address the confounding bias [Citation24]. For this reason, we conducted propensity score matching analysis for the two treatments with multiple confounding factors. The 36 most comparable groups were screened out. The results showed that recurrence in the CD34 + CD38− group was significantly higher than in the CD34 + CD38+ group, offering strong evidence that CD34 + CD38− is the origin of recurrence.
Our study has several limitations. In the definition of CD34 and CD38 positivity, although we used a qualitative method to establish percentages and have cited supporting literature, adopting a more scientific definition may bring new discoveries [Citation17]. In addition, the number of cases is relatively small, and further studies are needed to validate some of the findings.
In summary, CD34− and CD34+ leukemia cells show no association with prognosis in childhood ALL, but CD34+CD38− leukemia cells are associated with relapse, suggesting a poor prognosis. This information could potentially be used as a supplementary factor in risk assessment in childhood ALL and to guide targeted therapy.
Ethical statement
Informed consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of Huai’an First Hospital Affiliated with Nanjing Medical University and Affiliated Hospital of Xuzhou Medical University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wu C, Li W. Genomics and pharmacogenomics of pediatric acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 2018;126:100–111. doi:10.1016/j.critrevonc.2018.04.002.
- Inaba H, Pui CH. Immunotherapy in pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev. 2019;38(4):595–610. doi:10.1007/s10555-019-09834-0.
- Bhojwani D, Sposto R, Shah NN, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia [published correction appears in Leukemia]. Leukemia. 2019;33(4):884–892. doi:10.1038/s41375-018-0265-z.
- Oskarsson T, Söderhäll S, Arvidson J, et al. Relapsed childhood acute lymphoblastic leukemia in the nordic countries: prognostic factors, treatment and outcome. Haematologica. 2016;101(1):68–76. doi:10.3324/haematol.2015.131680.
- Vrooman LM, Silverman LB. Treatment of childhood acute lymphoblastic leukemia: prognostic factors and clinical advances. Curr Hematol Malig Rep. 2016;11(5):385–394. doi:10.1007/s11899-016-0337-y.
- O'Connor D, Enshaei A, Bartram J, et al. Genotype-Specific minimal residual disease interpretation improves stratification in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2018;36(1):34–43. doi:10.1200/JCO.2017.74.0449.
- Lang F, Wojcik B, Bothur S, et al. Plastic CD34 and CD38 expression in adult B-cell precursor acute lymphoblastic leukemia explains ambiguity of leukemia-initiating stem cell populations. Leukemia. 2017;31(3):731–734. doi:10.1038/leu.2016.315.
- Blatt K, Menzl I, Eisenwort G, et al. Phenotyping and target expression profiling of CD34+/CD38− and CD34+/CD38+ stem- and progenitor cells in acute lymphoblastic leukemia. Neoplasia. 2018;20(6):632–642. doi:10.1016/j.neo.2018.04.004.
- Zeijlemaker W, Grob T, Meijer R, et al. CD34+CD38− leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia. 2019;33(5):1102–1112. doi:10.1038/s41375-018-0326-3.
- Herrmann H, Sadovnik I, Cerny-Reiterer S, et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123(25):3951–3962. doi:10.1182/blood-2013-10-536078.
- Zeng H, Yin LD, Li P, et al. The level of peripheral blood circulating CD34+ cells is higher in acute promyelocytic leukemia patients with adverse prognostic factors. Hematology. 2016;21(9):513–519. doi:10.1080/10245332.2016.1150410.
- Bürgler S, Gimeno A, Parente-Ribes A, et al. Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-γ by a T-bet-dependent mechanism. J Immunol. 2015;194(2):827–835. doi:10.4049/jimmunol.1401350.
- Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009–2017. doi:10.1016/S0140-6736(10)62002-8.
- Yu SL, Zhang H, Ho BC, et al. FPGS relapse-specific mutations in relapsed childhood acute lymphoblastic leukemia. Sci Rep. 2020;10(1):12074, doi:10.1038/s41598-020-69059-y.
- Sidney LE, Branch MJ, Dunphy SE, et al. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–1389. doi:10.1002/stem.1661.
- van de Donk NW, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. doi:10.1111/imr.12389.
- Dakka N, Bellaoui H, Bouzid N, et al. CD10 and CD34 expression in childhood acute lymphoblastic leukemia in Morocco: clinical relevance and outcome. Pediatr Hematol Oncol. 2009;26(4):216–231. doi:10.1080/07357900902897557.
- Gerby B, Clappier E, Armstrong F, et al. Expression of CD34 and CD7 on human T-cell acute lymphoblastic leukemia discriminates functionally heterogeneous cell populations. Leukemia. 2011;25(8):1249–1258. doi:10.1038/leu.2011.93.
- Zeijlemaker W, Kelder A, Wouters R, et al. Absence of leukaemic CD34+ cells in acute myeloid leukaemia is of high prognostic value: a longstanding controversy deciphered. Br J Haematol. 2015;171(2):227–238. doi:10.1111/bjh.13572.
- Sumide K, Matsuoka Y, Kawamura H, et al. A revised road map for the commitment of human cord blood CD34-negative hematopoietic stem cells. Nat Commun. 2018;9(1):2202, doi:10.1038/s41467-018-04441-z.
- Zhang YH, Yang L, Wang YZ, et al. In vitro identification and characteristics of CD34− leukemia stem cell in t(8;21) acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25(5):1289–1294. doi:10.7534/j.issn.1009-2137.2017.05.003.
- Hadzijusufovic E, Keller A, Berger D, et al. STAT5 is expressed in CD34+/CD38− stem cells and serves as a potential molecular target in Ph-negative myeloproliferative neoplasms. Cancers (Basel). 2020;12(4):1021, doi:10.3390/cancers12041021.
- Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–1117. doi:10.1093/ejcts/ezy167.
- Guo J, Zheng P, Wang R, et al. Prognostic relevance of neuroendocrine differentiation in colorectal cancer: a population-based, propensity score matching study. Int J Colorectal Dis. 2020;35(12):2185–2195. doi:10.1007/s00384-020-03708-6.