ABSTRACT
Objectives
There are no real-world data describing infection morbidity in relapsed/refractory myeloma (RRMM) patients treated with anti-CD38 isatuximab in combination with pomalidomide and dexamethasone (IsaPomDex). In this UK-wide retrospective study, we set out to evaluate infections experienced by routine care patients who received this novel therapy across 24 cancer centres during the COVID-19 pandemic.
Methods
The primary endpoint was infection morbidity (incidence, grading, hospitalization) as well as infection-related deaths. Secondary outcomes were clinical predictors of increased incidence of any grade (G2–5) and high grade (≥G3) infections.
Results
In a total cohort of 107 patients who received a median (IQR) of 4 cycles (2–8), 23.4% of patients experienced ≥1 any grade (G2–5) infections (total of 31 episodes) and 18.7% of patients experienced ≥1 high grade (≥G3) infections (total of 22 episodes). Median time (IQR) from start of therapy to first episode was 29 days (16–75). Six patients experienced COVID-19 infection, of whom 5 were not vaccinated and 1 was fully vaccinated. The cumulative duration of infection-related hospitalizations was 159 days. The multivariate (MVA) Poisson Regression analysis demonstrated that a higher co-morbidity burden with Charlson Co-morbidity Index (CCI) score ≥4 (incidence rate ratio (IRR) = 3, p = 0.012) and sub-optimal myeloma response less than a partial response (<PR) (p = 0.048) are independent predictors of ≥ G3 infections.
Conclusion
Our study described initial results of infection burden during IsaPomDex treatment. We recommend close monitoring particularly in elderly patients with co-morbidities, the effective use of an-infective prophylaxis, as well as optimal vaccination strategies, to limit infections.
Introduction
Significant advances in myeloma therapeutics in the last two decades have led to improved survival outcomes, but infections remain a major cause of morbidity and early mortality [Citation1,Citation2]. Infection risk often relates to disease-related immunoparesis, co-morbidities, and systemic therapy. Patterns of infections evolve over time, as the myeloma treatment landscape continues to change, with new therapeutic classes such as proteasome inhibitors (PI), immuno-modulatory drugs (IMiD) [Citation3], and anti-CD38 monoclonal antibodies (Mab). We previously reported a 12-month cumulative infection incidence rate of 33% (all grade) and 22% (≥G3), in transplant-ineligible newly diagnosed patients who received fixed duration therapy [Citation4].
A meta-analysis from 5 phase III randomized trials, demonstrated that adding the anti-CD38 Mab daratumumab to standard MM regimens contributed to higher incidence of all grades of infection (with a risk ratio (RR) of 1.27), and any-grade pneumonia (with a RR of 1.63) [Citation5]. In the relapsed/refractory (RRMM) setting, prior exposure to multiple lines of therapy can further increase susceptibility to infections [Citation6], coupled with the potential additional risk conferred by the Mab drug class [Citation5].
Isatuximab is another anti-CD38 Mab [Citation7], which was recently approved in the UK to treat MM at 3rd relapse, in combination with pomalidomide and dexamethasone (IsaPomDex) [Citation8]. Efficacy of this triplet therapy was demonstrated in ICARIA-MM trial, which reported any grade upper respiratory tract infections and pneumonia in 28% and 20% of patients, respectively [Citation9].
Older patients or those with co-morbidities are under-represented in clinical trials, as demonstrated in the CONNECT-MM registry, which showed that 40% of newly-diagnosed patients were trial-ineligible [Citation10]. Therefore, understanding infection morbidity of this anti-CD38-containing triplet therapy in routine care patients is needed. This would help us identify strategies to maintain therapy, improve tolerability and quality of life (QoL), and minimize the impact of infection-related morbidity on patients’ treatment journey and on healthcare resources.
We performed a UK-wide retrospective study to assess clinical outcomes of real-world patients treated with IsaPomDex across 24 cancer centres. We report a subgroup analysis of infection-related morbidity and mortality in this cohort, and we attempt to identify predictors of infective episodes. This analysis has an added clinical relevance because patients were treated during the peak of the COVID-19 pandemic. To our knowledge, there are no published real-world data describing infection-related events in RRMM setting treated with this novel triplet immuno-chemotherapy during the COVID-19 pandemic.
Materials and methods
Study design, inclusion criteria and baseline data collection
This retrospective study included routine care patients from 24 centres across the UK with a diagnosis of RRMM, who received ≥1 cycles of IsaPomDex, and started therapy between January 2020 and May 2021. It is a 28 day regimen given until disease progression or unacceptable toxicity, as follows: isatuximab intravenous (IV) infusion at 10 mg/kg (weekly on cycle 1, and fortnightly thereafter), pomalidomide 4 mg orally once a day on days 1–21, and dexamethasone weekly at 40 mg (if <75 years old) or 20 mg (if ≥75 years old). All patients consented for retrospective analysis of their records at the point of treatment, and all patient records were anonymized at the point of analysis. Service evaluation approval was obtained prior to starting the study in all participating sites. Data were censored at the end of patient follow up.
Patients’ medical records were used to collect the following baseline patient characteristics: time from diagnosis to start of IsaPomDex, age at start of IsaPomDex, sex, WHO performance status (PS), co-morbidity score as per Charlson co-morbidity index (CCI), anaemia, lymphopenia, hypercalcaemia and renal impairment (according to e-GFR).
Baseline disease characteristics collected included myeloma subtype, lactate dehydrogenase (LDH), known myeloma cytogenetics since diagnosis, myeloma International Staging System (ISS), amyloidosis, plasma cell leukaemia and extramedullary disease. High risk cytogenetics was defined as one or more of the following abnormalities by fluorescence in situ hybridization (FISH): t(4;14), t (14;16), del(17p).
Prior therapies data included prior: transplant, alkylator, PI, IMiD, anti-C38 Mab (daratumumab), and histone deacetylase inhibitor (HDACi) such as panobinostat or vorinostat. IsaPomDex treatment characteristics collected included the number of cycles received, pomalidomide dose reductions, dexamethasone dose reductions, reasons for treatment discontinuation, and the use prophylactic anti-infective medication (antiviral, antifungal and PCP prophylaxis).
Infection data
An infection in this study is defined as a clinically suspected or microbiologically confirmed episode with an intention to treat with an antimicrobial, antifungal or antiviral drug. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5 × 7.pdf) was used to grade infection episodes attributed to myeloma or therapy. Infections data collected for each patient during their period of follow up period included infection diagnosis, grade, number of episodes, hospitalization with infection, number of inpatient days, infection outcome (survival/death), and infections contributing to death events. COVID-19 vaccination status data were collected for patients who experienced SARS-CoV-2 infection in this cohort (i.e. vaccination status at the time of the reported infection).
Study endpoints
The primary endpoint was infection morbidity and mortality during IsaPomDex treatment including nature of infections, incidence rate of any grade (G2–5) infections and high grade (≥G3) infections, infective hospitalizations, and infection-related deaths. Secondary endpoint was clinical predictors of increased incidence rate of any grade, of high-grade infections, and hospitalizations.
Statistical analysis
Percentages were used to summarize categorical variables and median values with range and interquartile range (IQR) to summarize continuous variables. Where there are missing (not known: NK) data for any of the baseline patient/disease/treatment characteristics (e.g. ISS staging, cytogenetics, performance status, anti-infective prophylaxis), the relevant characteristic was annotated with a (*) superscript within the baseline patient/disease/treatment characteristics table, and the number of patients with NK data was described in the legend of the table.
Using Poisson regression, univariate (UVA) and multivariate analyses (MVA) were conducted to assess factors associated with increased incidence rates of: any grade infections, ≥G3 infections, and hospital admissions. Factors investigated were the following: age (<75 vs. ≥75), elevated LDH (Y vs. N), ISS (3 vs. <3), CCI co-morbidity (<4 vs. ≥4), renal impairment (Y vs. N), lymphopenia at baseline (N vs. Y), pomalidomide dose attenuation (Y vs. N), dexamethasone dose attenuation (Y vs. N), and response (≥PR vs. <PR). Covariates with prognostic significance (p ≤ 0.1) in univariate testing were included in the initial multivariate models of the corresponding outcome. Final models were arrived at by backward selection. Variables were retained in the final model if significant at the 5% level. No adjustment has been made for multiple testing. The number of patients with NK baseline data has also been described in the UVA/MVA analysis tables.
A landmark analysis at 3 months after starting therapy was conducted to assess effect of infections within the first 3 months on overall survival (OS) and progression-free survival (PFS) of 3-month survivors. OS was defined as time from initiation of therapy to death from any cause. PFS was evaluated as the time between initiation of therapy and progressive disease or death. Proportional hazards assumption was checked visually (log–log plot of survival).
Results
A total of 107 patients from 24 UK centres were eligible for inclusion, who started therapy between January 2020 and May 2021. Median follow up (interquartile range, IQR) was 3.7 months (0.5–12.4 months). Baseline characteristics of the 2 subgroups (no infection vs. any grade infection) are presented in . Comparing subgroups, median age was (69.5 vs. 67 years), Charlson Co-morbidity Index (CCI) score was ≥4 in (37.8% vs. 52%), and performance status (PS) was ≥2 in (17.1% vs. 24%). Renal presentation (e-GFR < 60 ml/min) was (48.8% vs. 24%), lymphopenia (45.1% vs. 52%), myeloma International Staging System (ISS) III staging (28% vs. 20%), and high-risk cytogenetics (11% vs. 24%). The median number of prior therapies was (3 vs. 3). Median number of IsaPomDex cycles administered to date was (4 vs. 5), with a pomalidomide dose reduction in (40.2% vs. 40%) and a dexamethasone dose reduction in (41.5% vs. 40%). Granulocyte colony-stimulating factor (GCSF) usage rate was numerically higher in those with no infections but without a statistical significance (61% vs. 52%, p = 0.425).
Table 1. Baseline patient, disease and treatment characteristics of sub-groups by infections during IsaPomDex (any grade infections vs. no infections).
Twenty-five patients (23.4%) experienced one more any grade infection (total of 31 episodes) with a median (range) of 1 episode (1–4) per patient. A detailed breakdown of these infections is presented in . Timing of infection was known for 23 episodes in 17 patients. Median time (IQR) from start of therapy to first episode was 29 days (16–75).
Table 2. Infection episodes experienced during IsaPomDex treatment.
By UVA Poisson regression analysis of the incidence of any grade infections, patients achieving an objective response, i.e. partial response or better (≥PR), showed a trend for fewer infections with an incidence rate ratio (IRR) of 0.51, but without a statistical significance (p = 0.115) ().
Table 3. Univariate and multivariate Poisson regression: all grade infections in the total cohort after a median of 4 IsaPomDex cycles.
Twenty patients (18.7%) experienced at least one high grade (≥G3) infection (total of 22 episodes) with a median (range) of 1 episode (1–3) per patient. The nature and number of ≥ G3 infections were: COVID-19 pneumonia (G4 = 2, G5 = 4), neutropenic sepsis (G3 = 1, G4 = 2, G5 = 2), E.coli infection (G4 = 2), urinary tract infection (G3 = 3), lung infection (G3 = 2), Serratia liquifaciens infection (G5 = 1), pseudomonas sepsis (G4 = 1), bacteraemia (G3 = 1), and skin infection (G3 = 1). Vaccination statuses during diagnosis with COVID-19 were: not vaccinated (n = 5) and fully vaccinated (n = 1).
UVA Poisson regression of high-grade infections demonstrated that age ≥75 years (IRR 2.58, p = 0.04) and CCI ≥ 4 (IRR 2.63, p = 0.025) were associated with a statistically higher incidence of ≥G3 infections. Objective myeloma response (≥PR) appeared to have a trend for a protective effect against ≥G3 infections (IRR 0.43), but without statistical significance (p = 0.07) (). In the MVA model (backward selection), higher co-morbidity burden CCI≥4 (IRR = 3, p = 0.012) and sub-optimal myeloma response (<PR) (p = 0.048) remained as independent predictors of ≥ G3 infections ().
Table 4. Univariate and multivariate Poisson regression: ≥G3 infections in the total cohort, after a median of 4 IsaPomDex cycles.
Nineteen patients (17.7%) experienced an infection which led to hospitalization, with a median of 1 episode per patient. The median (range) duration of hospitalization per patient was 8 days (2–17). The cumulative number of hospitalization days related to any AE in this cohort was 207 days, of which 159 days (76.8%) were due to infections. By UVA logistic regression, infection was the only statistically significant variable associated with hospital admissions (odds ratio: 138.2, 95% CI: 28.7–666.1, p < 0.001) ().
Table 5. Univariate logistic regression analysis: hospital admissions in the total cohort, after a median of 4 cycles of IsaPomDex.
Of the 26 death events reported in the total cohort, 10 were at least partly due to infections, which were: COVID-19 pneumonia (n = 4), pneumonia (n = 1), pneumonia with progressive disease (PD) (n = 1), neutropenic sepsis (n = 1), neutropenic sepsis secondary to PD (n = 1), E. Coli septicaemia with PD (n = 1), and Serratia liquefaciens sepsis with a possible PE and PD (n = 1). Causes of the remaining 16 death events were: PD (n = 13), subdural haemorrhage (n = 1), cardiac arrest (n = 1), and not known (n = 1).
After a median follow up of 3.7 months, median progression-free survival (PFS) in the (infection vs. no-infection) subgroups was: (10.2 vs 9 months), while median OS was not reached (NR) in either subgroup. UVA analysis of the occurrence of the first infection episode as a discrete time-varying predictor, showed an association with a higher risk of death (OS HR 3.26, 95% CI 1.34–7.93, p = 0.009) but no detectable effect on PFS was observed (HR 1.27, 95% CI 0.5–3.23, p = 0.617).
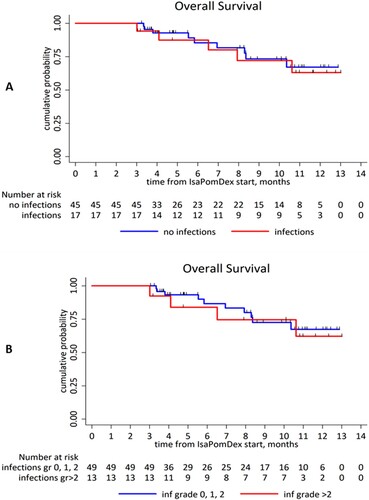
The 3-month landmark analysis demonstrated that early infection history (within 3 months of starting IsaPomDex) did not affect OS among 3-month survivors: for all grades (infection group: NR vs. no-infection: NR, HR 1.18, 95% CI 0.39–3.56, p = 0.763), (A), and ≥G3 infections (infection group: NR vs. no-infection group: NR, HR 1.25, 95% CI 0.39–4.01, p = 0.706), (B).
Discussion
Our study is the first to report an initial detailed account of infections in PI and lenalidomide-refractory RRMM patients treated with IsaPomDex in routine care. We showed an infection morbidity despite the low number of cycles received to date (median of 4 within the short follow up), with a considerable burden on healthcare resources (76.8% of all AE-related hospital inpatient days). ICARIA-MM trial reported G3 upper respiratory tract infections and pneumonia in 3% and 15% of patients, respectively [Citation9]. Our ability to compare trial data with our data is limited by the difference in our reporting method, which included not only respiratory infections, but all body systems; in addition to the difference in the length of follow up.
We reported 10 infected-related deaths in this cohort, out of a total of 26 events. However, 4 of these deaths (40%) were due to COVID-19 in unvaccinated patients, and 4 deaths (40%) were caused by an infection in the context of progressive disease, where it is difficult to ascertain exactly how much these episodes were driven by therapy or by myeloma or by both. Therefore, infection-related mortality during IsaPomDex treatment in our study needs to be interpreted with caution. Our data, however, remains of current clinical interest because this therapy was started within a period of time when the unprecedented COVID-19 pandemic was at its peak in the UK, and when a number of patients were not yet double-vaccinated.
This study included patients who started IsaPomDex between January 2020 and May 2021. Given that the first COVID-19 vaccine was approved in the UK in December 2020, patients who experienced COVID-19 infection between the start of the pandemic and December 2020, whilst on IsaPomDex, would not have been vaccinated because a vaccine was not available (except in the case of participation in a COVID-19 vaccine trial). In addition, December 2020 to May 2021 was a period of time when the UK vaccine rollout was underway. At the start of the pandemic, the UK government advised myeloma patients to shield (stay at home as much as possible and minimize interactions with others), in order to reduce the risk of exposure to the SARS-COV-2 virus, because they fall in the extremely vulnerable clinical group [Citation11]. Shielding, particularly earlier on in the pandemic and prior to vaccine availability, was an important precautionary measure to reduce the risk of COVID-19 in myeloma patients.
Our 3-month landmark analysis showed no difference OS according to infections. The lack of statistical difference may be due to the early time-to-first infection episode (median 29 days), plus the fact that patients who suffered early infection-related deaths (<3 months) were excluded from this landmark analysis. In addition, this analysis is limited by the relatively short survival follow up of this cohort (median of 3.7 months).
We attempted to identify predictors of infective episodes in order to stratify risk of infections, and to inform future clinical practice. We found an independent association between a high co-morbidity burden (CCI≥4) and the incidence of ≥G3 infections. Frailty subgroup analysis of ICARIA-MM trial employed a frailty assessment which takes into account age, CCI score and ECOG PS score [Citation12]. This subgroup analysis demonstrated a higher incidence of high grade (≥G3) infections with IsaPomDex in the frail subgroup compared to the intermediate/fit subgroup (52.1% vs. 39%) [Citation12]. We have also demonstrated a protective effect of an objective myeloma response (≥PR) from ≥G3 infections.
In order to minimize infection-morbidity, we recommend close monitoring of patients [Citation13], and the optimal use of anti-infective prophylaxis throughout the course of IsaPomDex treatment, following a clinician assessment of each individual patient's risk of infections. Anti-infective prophylaxis to be considered can include the preventative use of antivirals in addition to antifungals and pneumocystis pneumonia (PCP) prophylaxis.
Optimal vaccination strategies for myeloma patients on anti-CD38 treatment regimen such as IsaPomDex, are also very important to limit infections. Two recent myeloma studies reported that an initial two-dose COVID-19 vaccination was associated with a significantly weaker responses in patients treated with anti-CD38 Mabs or a B-cell maturation antigen (BCMA)-targeted therapy [Citation14,Citation15]. A significant increase in immune response was observed after a third COVID-19 vaccine dose, in this patient group [Citation16]. Therefore, patients on IsaPomDex should be advised to continue taking precautions to minimize the risk of COVID-19, have a booster vaccine when offered, and seek urgent medical advice if they test positive for SARS-CoV-2, in order to receive early treatment such as with antivirals or monoclonal antibody therapies for COVID-19.
Where appropriate and on a case-by-case basis, a dose-attenuation of pomalidomide and/or dexamethasone in elderly patients with significant co-morbidities may be considered to prevent infections, hospital admissions, and treatment interruptions, particularly in those who have already achieved an optimal or intended myeloma response.
Our study is limited by its retrospective, non-randomized nature with the inherent possibility of unmeasured confounding factors, patient selection bias, the potential for medical chart misinterpretation, lack of complete data (for ISS staging, cytogenetics, LDH and COVID-19 vaccination status), under-reporting of infections, in addition to the relatively short median follow up of 3.7 months, which means that infection burden, may continue to accumulate at a longer follow up of patients who remain on therapy. Despite these limitations, we described in detail the initial UK-wide experience of infection outcomes with this novel triplet therapy during the pandemic.
Conclusion
This is the first real-world study to report an initial detailed account of infection morbidity and mortality in relapsed myeloma patients treated with anti-CD38 Mab-based triplet therapy IsaPomDex during the COVID-19 pandemic. We showed an infection morbidity burden despite the low number of cycles received to date. In order to limit infections in this myeloma setting, close monitoring during IsaPomDex is required, particularly in elderly patients with co-morbidities, in addition to the effective use of an-infective prophylaxis, as well as optimal vaccination strategies.
Ethical approval
The study received NHS service evaluation approval at each participating site.
Acknowledgements
We thank the Clinical Research Network-West Midlands-National Institute for Health Research-UK, for their support in covering article publication charge (APC) for this manuscript.
Disclosure statement
FD (Sanofi: honoraria for education evening for haematologists). SB (Sanofi: honoraria and advisory board). KR (Sanofi: honoraria and advisory board; BMS: research support, honoraria, advisory board, travel support). All other authors have no conflicts of interest to declare.
Data availability statement
All available data relating to this report are presented in the manuscript and the accompanying tables.
References
- Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom medical research council trials between 1980 and 2002 – medical research council adult leukaemia working party. J Clin Oncology. 2005;23(36):9219–9226.
- Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015 Jan;100(1):107–113. DOI:10.3324/haematol.2014.107714. Epub 2014 Oct 24. PMID: 25344526; PMCID: PMC4281323.
- Teh BW, Harrison SJ, Pellegrini M, et al. Changing treatment paradigms for patients with plasma cell myeloma: impact upon immune determinants of infection. Blood Rev. 2014;28(2):75–86.
- Djebbari F, Panitsas F, Eyre TA, et al. Infection-related morbidity in a large study of transplant non-eligible newly diagnosed myeloma patients treated with UK standard of care. Haematologica. 2020 Sep 1;105(9):e474–e479. DOI:10.3324/haematol.2019.240762. PMID: 33054067; PMCID: PMC7556501.
- Htut TW, Tun AM, Sultan A, et al. Updated meta-analysis of randomized controlled trials to evaluate the incidence of infection and pneumonia in patients with multiple myeloma treated with daratumumab. Blood. 2019;134(Suppl. 1):4771–4771. DOI:10.1182/blood-2019-123169.
- Teh BW, Worth LJ, Harrison SJ, et al. Risks and burden of viral respiratory tract infections in patients with multiple myeloma in the era of immunomodulatory drugs and bortezomib: experience at an Australian cancer hospital. Support Care Cancer. 2015 Jul;23(7):1901–1906. DOI:10.1007/s00520-014-2550-3. Epub 2014 Dec 10. PMID: 25487843; PMCID: PMC7087950.
- Richardson PG, Beksaç M, Špička I, et al. Isatuximab for the treatment of relapsed/refractory multiple myeloma. Expert Opin Biol Ther. 2020;20(12):1395–1404. DOI:10.1080/14712598.2021.1841747.
- National Institute for Health and Care Excellence. Isatuximab with pomalidomide and dexamethasone for treating relapsed and refractory multiple myeloma. 2020; [cited 2021 June 22]. Available from: https://www.nice.org.uk/guidance/TA658.
- Attal M, Richardson PG, Vincent Rajkumar S, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. DOI:10.1016/S0140-6736(19)32556-5.
- Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17(9):572–583.
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: delivery of systemic anticancer treatments. 2020a; [cited 2020 Apr 21]. Available from: https://www.nice.org.uk/guidance/ng161/resources/covid19-rapid-guideline-delivery-of-systemic-anticancer-treatments-pdf-66141895710661.
- Schjesvold F, Bringhen S, Richardson G, et al. Isatuximab plus pomalidomide and dexamethasone in frail patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis. Am J Hematol. 2021;96:E423–E427. DOI:10.1002/ajh.26319.
- Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015 Oct;100(10):1254–1266. DOI:10.3324/haematol.2014.117176. PMID: 26432383; PMCID: PMC4591757.
- Aleman A, Upadhyaya B, Tuballes K, et al. PVI/Seronet study group variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39:1442–1444.
- Van Oekelen O, Gleason CR, Agte S, et al. PVI/Seronet team highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028–1030.
- Aleman A, Van Oekelen O, Upadhyaya B, et al. Augmentation of humoral and cellular immune responses after third-dose SARS-CoV-2 vaccination and viral neutralization in myeloma patients. Cancer Cell. 2022;40(5):441–443. Advance online publication. DOI:10.1016/j.ccell.2022.03.013.