ABSTRACT
Objectives
Chronic graft versus host disease (chronic GVHD) still remains the leading cause of late morbidity and mortality for allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients. In this retrospective study, 53 consecutive allo-HSCT patients with chronic GVHD refractory to corticosteroids were treated with extracorporeal photopheresis (ECP).
Methods
This study was performed as a retrospective single-center study. Medical records of a total of 59 patients treated with ECP for chronic GVHD were reviewed.
Results
Best organ responses to ECP were observed in skin, mouth mucosa, eyes and liver. Overall response rate (ORR) to ECP was 81.2% (CR 17% and PR 64.2%). Overall survival (OS) was 84.9% and 36.7%, at 1 and 3 years, respectively. Female sex appears to have an advantage on ORR. Patients achieving ORR were able to maintain their responses with a prolonged continuation of treatments for +6 and +12 months indicating the benefits of longer ECP treatment.
Discussion
We found that patients with chronic GVHD who were treated with ECP for 12 months or longer had a higher response rate. Our findings in line with the data reported previously suggest that patients responding to ECP should continue longer therapy schedules to achieve a better and sustained response. In our cohort, long-term ECP therapy was safe and well-tolerated with no significant adverse effects. Best responses were observed in the patients with skin, eye, liver and oral involvement. The ECP procedure offers the advantage relative to the problems with typical immunosuppressive agents. The female sex appeared to have an advantage based on the cumulative probability of the OR after ECP for chronic GVHD.
Introduction
Chronic GVHD is a major complication of allogeneic hematopoietic cell transplantation (allo-HSCT) initially described by Shulman et al. [Citation1] and Sullivan et al. [Citation2]. It is seen in 30–50% of sibling HLA-matched and 40–70% in recipients of mismatched or unrelated donor transplants. Its incidence depends on many factors such as the transplant source, donor type, age, previous pregnancy, female versus male donor and other factors. Advanced recipient age and the occurrence of acute GVHD have been shown to be the most important risk factors for chronic GVHD [Citation3–5].
Chronic GVHD is a complex multisystem disease leading to chronic damage in many organs including skin, mouth, eyes, liver, lungs, gastrointestinal and musculoskeletal systems. Its features may resemble autoimmune disorders such as scleroderma, primary biliary cirrhosis, wasting syndrome, immune cytopenias, Sjögren’s syndrome and chronic immunodeficiency. Approximately 50% of patients who develop chronic GVHD are diagnosed within 6 months after allo-HSCT. Chronic GVHD is the most important cause of late morbidity and mortality after allo-HSCT occurring in 60–70% of long-term survivors with a significant impact on the long-term quality of life [Citation6–8]. It becomes corticosteroid refractory in approximately 50% of patients.
Chronic GVHD is an immune-mediated inflammatory and fibrotic disease characterized by tissue damage and multisystem organ involvement. Chronic GVHD is thought to be mediated by donor-derived alloreactive T-cells. Alloreactivity is an important requirement because donor T-cells react with host antigen-presenting cells (APCs) and host B-cells. Effector T-cells act on host tissues, inflammatory cytokines stimulate donor alloreactive T cells and in the subsequent stages of thymic injury stimulate the formation of auto- and alloreactive T-cell populations [Citation9]. Recent research studies in the pathophysiology of chronic GVHD indicate a central role for B cell signaling [Citation10, Citation11], thymic injury leading to T- and B-cell dysregulation, aberrant tissue repair and fibrosis [Citation12]. Eventually, loss of central and peripheral tolerance leads to impaired regulatory Treg and Breg cells. Thymic deletion of self-reactive T cells leads to the production of autoreactive T cells, follicular helper T-cells and follicular regulatory T cells and the emergence of fibrosis-promoting factors [Citation5, Citation13–15]. Chronic GVHD can be considered a chronic inflammatory disease of immune dysregulation [Citation5, Citation12].
Signs and symptoms of chronic GVHD have been reported by the NIH Consensus Working Group to standardize criteria for diagnosis and the classification of chronic GVHD [Citation16]. The diagnosis of chronic GVHD can occur at any time after the transplant but requires the presence of at least one diagnostic clinical sign of chronic GVHD, or the presence of at least one distinctive manifestation confirmed by a biopsy or other relevant test in the same or another organ [Citation16–18].
A mild form of chronic GVHD is generally treated by topical steroids. First-line treatment of moderate and severe forms of chronic GVHD includes the use of systemic corticosteroids (1 mg/kg/day) with or without daily calcineurin inhibitor (cyclosporine or tacrolimus). The duration of systemic immunosuppression varies, but requires at least one year of therapy. Approximately, 80% of patients require systemic immunosuppression for two years and 40% require therapy for at least four years [Citation19–21]. The response rate to corticosteroids alone is about 50% with more than half of patients requiring second-line therapy within 2 years [Citation22, Citation23]. If patients fail to respond by three months or show progressive disease, second-line therapies are required.
To date, no standard second-line treatment strategy has been established. There have been various second-line therapies in chronic GVHD including mycophenolate mofetil (MMF), sirolimus, thalidomide, azathioprine, anti-thymocyte globulin, infliximab, imatinib, rituximab, daclizumab, 2’-deoxycoformycin, ibrutinib, ruxolitinib, belumosudil, total body irradiation and extracorporeal photopheresis (ECP) [Citation20–25]. Most reports concerning second-line therapies have a success rate of 25–50%. Treatment choices are empirical and based on factors including ease of use, risk of toxicity and physician experience. For several decades, very little progress has been made in the treatment of chronic GVHD. In recent years, drug researchers have focused their efforts to identify novel therapeutics to target pathways relevant to pathophysiology of chronic GVHD. To date, the only drugs approved by the FDA are ibrutinib [Citation26, Citation27], ruxolitinib [Citation28, Citation29] and belumosudil [Citation30]. Recently, Bleakley et al. reported a very low incidence of severe-acute and any chronic GVHD after depletion of naïve T cells (TN) from peripheral blood stem cell (PBSC) allografts [Citation31]. TN depletion of PBSC grafts resulted in very low incidences of any chronic GVHD without excess risk of relapse or nonrelapse mortality. Among these therapies, MMF and ECP are most frequently used in the second-line treatment of chronic GVHD.
Owisanowski et al. reported the first case report of ECP therapy in chronic GVHD, which has now been used successfully for over twenty-five years [Citation32]. More than 50% of the patients reported have been shown to display significant improvements in skin, liver and oral manifestations of steroid-refractory chronic GVHD after treatment with ECP [Citation33–35].
In this study, we aimed to retrospectively evaluate the efficacy of ECP treatment in patients with chronic GVHD failing corticosteroids and immunosuppressive treatment, as well as the factors affecting responses and outcomes.
Materials and methods
Patient population
The medical records of all patients treated with ECP from May 2003 to September 2015 were reviewed at the University of California at San Diego (UCSD) Division of Nephrology and Apheresis Unit and Moores Cancer Center Division of Blood and Marrow Transplantation. All patients who started on ECP already had received steroids and other systemic immunosuppressant therapy for their treatment of chronic GVHD. Referral for ECP treatment was at the discretion of the primary stem cell transplantation physician and final approval was given by the Apheresis ECP attending physician.
Study design
This is a retrospective single-center study. Medical records of a total of 59 patients treated with ECP for chronic GVHD were reviewed and six patients could not be evaluated because of incomplete medical records. The remaining 53 patients with chronic GVHD treated with ECP were included in the study.
Definitions of response
A complete response (CR) was defined as the resolution of all clinical findings of chronic GVHD. A partial response (PR) was defined as a minimum of 50% improvement of clinical findings of chronic GVHD without a CR and an ability to taper steroids by 50% without relapse of symptoms and clinical findings. No response (NR) was described as no change in symptoms and/or clinical findings or early death due to chronic GVHD. Progressive disease (PD) was defined as a steady increase in symptoms and/or clinical findings of chronic GVHD, as described by Couriel et al. [Citation34]. Steroid refractory chronic GVHD is defined as either failure to improve after at least two months, or progression after one month of standard immunosuppressive therapy including steroids >0.25 mg/kg/day for > 12 weeks and cyclosporine.
ECP treatment schedule
ECP was performed both on outpatient and inpatient basis using the Therakos UVAR XTS and CELLEX closed-circuit systems. All patients initiated ECP therapy with 2 treatments weekly for 4 weeks followed by two consecutive days every two weeks as a maintenance therapy. Tapering and discontinuation of ECP therapy were at the discretion of the treating physician.
Statistical methods
The descriptive statistics are provided as median (range) or count and percentages, for numeric and categorical variables, respectively. Factors associated with overall response (OR) were analyzed using Chi-square, or Fisher’s exact tests, where appropriate. Analysis of censored variables (overall survival (OS), time to OR) were performed using Kaplan-Meier survival analysis, while associated factors were analyzed using the log-rank test. IBM® SPSS® Statistics v23.0 was used for statistical analyses. A type-I error level of less than 5% was used to infer statistical significance.
Results
Demographics and transplant characteristics
summarizes the demographic and transplant characteristics of the 53 patients included in this study. Of the original 59, 6 patients were excluded and the final study cohort, therefore, included 53 patients with chronic GVHD. Thirty-seven patients were male (69.8%) and 16 were female (30.2%). The median age was 45.8 years and 77.4% of patients were Caucasian versus 9.4% African American. Indications for transplantation included non-Hodgkin’s lymphoma (15), acute myeloblastic leukemia (10), acute lymphoblastic leukemia (9), chronic lymphocytic leukemia (5), chronic myeloid leukemia (5), myelodysplastic syndrome (3), multiple myeloma (4), myelofibrosis (1) and adrenoleukodystrophy (1). A total of 50 patients received peripheral blood (94.3%), two patients received cord blood (3.8%) and one patient received bone marrow (1.9%) as the stem cell source. Most patients had a related matched donor graft (n = 25, 48.1%) or unrelated donor graft (n = 25, 48.1%). All (n = 53) patients (100%) had corticosteroid refractory chronic GVHD. Thirty-nine patients had a prior history of acute GVHD. None of these patients received ECP for treatment of acute GVHD in this study.
Table 1. Patient and transplant characteristics.
Chronic GVHD characteristics
Chronic GVHD characteristics are summarized in . The median time from transplantation to chronic GVHD was 10.9 months. Forty-two patients (93.3%) had progressive and 3 patients (6.7%) had limited chronic GVHD. Most patients had involvement of the skin (n = 49, 92.5%) followed by oral (n = 24, 45.3%), ocular (n = 21, 39.6%), liver (n = 12, 22.6%), lungs (n = 13, 24.5%), gastrointestinal (n = 11, 20.8%) and muscle/joints (n = 7, 13.2%). Prior to ECP, all patients (n = 53) received corticosteroids and 39 patients (73.5%) also received a calcineurin inhibitor (cyclosporine or tacrolimus). Concurrent immunosuppressive medications are shown in .
Table 2. Chronic GVHD characteristics (N = 53).
Response to treatment
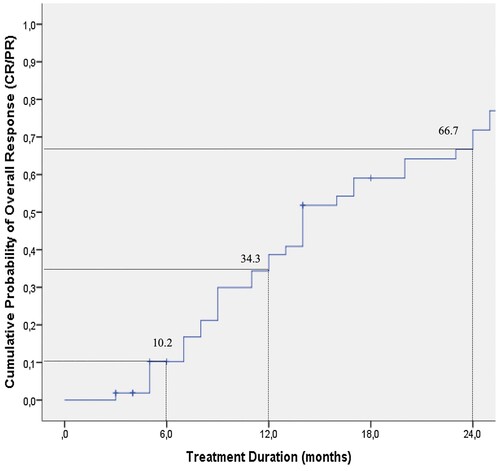
The median duration of ECP was 14 months (3.0–56 months) and complete responses (CR) were seen in 9 (17%) and partial responses (PR) in 34 patients (64.2%) with an OR rate of 81.2%. Of the ten patients who did not achieve a CR or PR, 8 patients showed NR and 2 had PD (). Best improvements were seen in skin, eye, liver and oral involvements (). Duration of ECP was also found to have an impact on responses (). When we analyzed the cumulative probability of the OR to treatment duration of ECP, it was 34.3% and 66.7%, at 12 and 24 months, respectively (). No serious adverse events were observed during ECP treatments. Thirty-eight patients were able to taper or discontinue steroid treatment.
Table 3. Response to ECP treatment in chronic GVHD (N = 53).
Table 4. Best organ responses to ECP treatment.
Table 5. Response to ECP treatment – duration of ECP(months).
Factors affecting response and survival
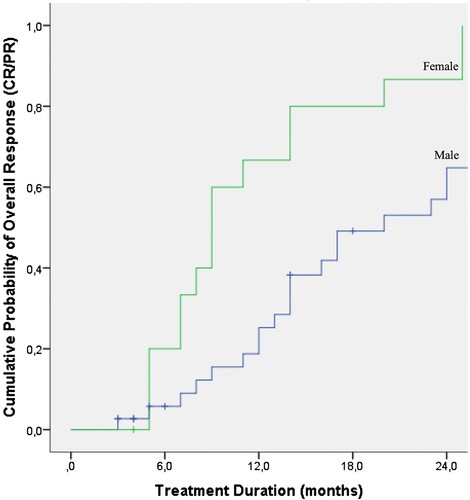
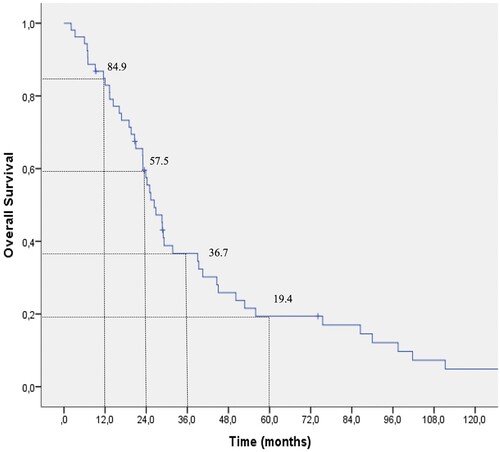
The OR rate was significantly greater in patients who were treated with ECP for 6–12 months (13/13, 100%), and more than 12 months (25/28, 89.3%) than in patients treated for less than 6 months (5/12, 41.7%) (). Several factors affecting the response to ECP are summarized in . Patient’s age, primary disease, donor type and gender were not statistically associated with a difference in OR to ECP. OS was not affected by age, gender, primary disease, donor type, history of acute GVHD or first-line therapy of chronic GVHD (). However, when OR rate was treated as a censored variable, the female sex appeared to have an advantage based on the cumulative probability of the OR after ECP for chronic GVHD (). A Kaplan-Meier analysis for the whole group revealed that the OS of chronic GVHD patients was 57.5% and 19.4% at 24 and 60 months respectively ().
Table 6. Factors affecting response (CR/PR) to ECP.
Table 7. Factors affecting response to ECP (overall survival).
Discussion
ECP has been used successfully to treat severe steroid-refractory chronic GVHD since the late 1990s [Citation19–21, Citation24, Citation34, Citation36]. The objective of our study was to retrospectively evaluate the therapeutic efficacy of ECP in a single center in adult patients with chronic GVHD failing steroids and other common immunosuppressive medications as well as the factors affecting responses and outcomes. In our study, in a cohort of 53 patients with chronic GVHD who were treated with ECP, 43 patients showed significant clinical improvement (CR + PR) and 38 patients were either able to taper or discontinue corticosteroids. Fifty-three patients in our study had all failed primary corticosteroid treatment. The median duration of ECP was 14 months and CR was seen in 17% and a PR in 64.2% of patients, with an OR rate of 81.2%. Best responses were observed in those patients with skin, eye, liver and/or oral involvement. Ten patients did not meet criteria for a PR but had improvement and 8 patients had no change at all. The duration of ECP was found to be an important factor in the responses. The cumulative probability of achieving OR was 34.3 and 66.7% at 12 and 24 months, respectively.
The ECP procedure offers the advantage relative to the problems with typical immunosuppressive agents [Citation37]. The exact mechanism of ECP is not well understood. Immunological effects of ECP include apoptotic cell death of alloreactive lymphocytes, phagocytosis of the apoptotic mononuclear cells by antigen-presenting cells (APCs), secretion of anti-inflammatory cytokines by APCs, increase in antigen-specific Tregs, which in turn suppress the immune response and reduce the secretion of inflammatory cytokines [Citation38–41]. Experimental models suggest however that these effects may be due to the induction of Treg cells [Citation42].
At present, there is no standard approach for patients who are refractory to first-line therapy and there is no standard second-line therapy for steroid refractory chronic GVHD. The Consensus GVHD Conference on Clinical Practice in Chronic GVHD held in 2009 in Regensburg, Germany aimed to summarize the currently available evidence for second-line treatment and provide practical guidelines for the use of therapeutic modalities [Citation43]. When the strength of recommendation of treatment and evidence levels were rated by an expert panel ECP had a recommendation level of C-1 (use in second-line treatment justified) and evidence level of I [Citation43]. Based on the current available literature the American Society of Apheresis classifies ECP for steroid-refractory chronic GVHD as Category II (disorders for which apheresis is accepted as second-line therapy, either as a stand-alone treatment or in conjunction with other modes of treatment) and Grade IB (strong recommendation, moderate-quality evidence) [Citation44].
Couriel et al. retrospectively analyzed 71 patients with chronic GVHD who were treated with ECP and reported a CR + PR of 61%. One year after the initiation of ECP, 22% of patients had discontinued steroids and 10% discontinued all immunosuppressive medications [Citation34]. A meta-analysis by Malik et al. reported clinical experience with ECP in steroid-refractory chronic GVHD showed a 74% response rate for skin disease, 68% for liver involvement, 72% for oral mucosa and 60% for ocular involvement [Citation45]. Apisarnthanarax et al. conducted a retrospective review of ECP in 32 steroid-dependent or steroid refractory chronic GVHD patients and reported CR + PR in 56% after a median of 36 cycles of ECP [Citation46]. Foss et al. in a prospective study of 25 patients with extensive steroid-refractory or steroid-intolerant chronic GVHD patients with progressive onset of chronic GVHD had a higher response rate of 64% compared to those with de novo onset (36%) and 80% reduction or discontinuation of immunosuppressive medication was possible [Citation47]. Greinix et al. treated 29 patients with a 24-week course of ECP who had extensive chronic GVHD refractory to steroids. Complete or partial skin response at week 24 was noted in 9 patients (31%) and extracutaneous chronic GVHD response was highest in oral mucosa with 70% complete and partial resolution after week 24. In addition, they also showed that prolonged ECP to be necessary for optimal therapeutic effects of steroid-refractory chronic GVHD [Citation48].
Flowers et al. reported a randomized controlled prospective trial to evaluate the effects of ECP treatment on the cutaneous and extracutaneous manifestations of chronic GVHD. They reported that the median percentage improvement in total skin score at week 12 was 14.5% for the ECP arm and 8.5% for the control arm and suggested that continued improvement in patients in the ECP arm during 12–24 weeks may require longer treatment periods, particularly in patients with more advanced forms of chronic GVHD [Citation49]. Similarly, we also found that patients with chronic GVHD who were treated with ECP for 12 months or longer had a higher response rate. The fact that our findings are in line with the data from Greinix et al. [Citation48] and Flowers et al. [Citation49] suggest that patients responding to ECP should continue longer therapy schedules to achieve a better and sustained response. In our cohort, long-term ECP therapy was safe and well-tolerated with no significant adverse effects.
Abu-Dalle et al. performed a systematic review of prospective studies to evaluate the treatment outcomes of ECP in corticosteroid refractory acute and chronic GVHD in both pediatric and adult patients. One randomized controlled trial with 95 patients and eight observational studies with 228 patients (acute and chronic GVHD) were included in their review. The authors reported overall response rates of 69% for acute GVHD and 64% for chronic GVHD [Citation50]. They concluded that despite the limited number of available studies, pooled analyses of prospective studies demonstrate encouraging responses to ECP treatment in acute and chronic GVHD after failing steroids and the rate of immunosuppression discontinuation were 23% for chronic GVHD patients. When they analyzed outcomes of ECP therapy in chronic GVHD, organ-specific responses appeared to be higher in skin, followed by gastrointestinal, liver and oral mucosa ECP related mortality rates were extremely low, and no deaths were reported as a result of ECP use.
Inamoto et al. in a study cohort of 312 patients who received second-line systemic treatment of chronic GVHD reported that treatment change was the major cause of treatment failure, failure-free survival was 56% at 6 months after the second-line treatment, and lower steroid doses at 6 months correlated with a subsequent withdrawal of immunosuppressive treatment [Citation51].
We have found that the OR rate was 81.2% with an OS of 85% and 37% at 1 and 3 years, respectively. Of the 43 responders, 14% discontinued steroids, 74% were able to taper steroids and 11.6% were not able to taper corticosteroids. Patients achieving an OR were able to maintain their responses with the continuation of ECP treatments beyond 6+ and 12+ months. Female sex appeared to have an advantage on overall response.
Our study provides evidence that ECP is a second-line effective therapy for chronic GVHD patients with extensive disease, who failed corticosteroids and other immunosuppressive treatments. We have demonstrated that ECP has a therapeutic effect on skin, eye, oral and liver manifestations of chronic GVHD. ECP also allowed many patients with chronic GVHD to taper or discontinue corticosteroids.
Potential limitations of our retrospective study are that first, our cohort is heterogeneous with regard to age, time from GVHD onset to initiation of ECP, and use of different immunosuppressive treatments and conditioning regimens. Secondly, our study consists of only a limited number of patients (n = 53). Thirdly, all patients were receiving systemic immunosuppressive medications before and during ECP therapy, which could have had an additional and/or synergistic effects on the outcomes. None of our patients received ibrutinib, ruxolitinib or belumosudil in the time frame of this study.
In evaluating the available literature we have noticed great heterogeneity of the patients enrolled in the clinical trials, especially criteria used for diagnosis, staging and assessment of response prior to the NIH Chronic GVHD Consensus guidelines. In addition, the concomitant use of immunosuppressive agents with ECP has been an important challenge in the review of the results.
In conclusion, ECP remains an important therapeutic modality as a second-line therapy of chronic GVHD and it was shown to be more effective with better response rates with longer therapy periods. ECP for a period of 24 weeks or longer did not result in any detectable complications and it offered the advantage of tapering or discontinuation of corticosteroids in patients with chronic GVHD. While novel targets are being explored in the treatment of chronic GVHD [Citation52], well-designed basic and clinical research studies on ECP are warranted to provide a better understanding of its mechanism of action and the timing of its use. In addition, research and validation studies are also needed for defining biomarkers to determine which patients will benefit from ECP therapy in chronic GVHD. These studies may also help to identify patients who will need early therapy with ECP. Future multi-center, prospective and randomized clinical studies are warranted to provide a better perspective on the full therapeutic efficacy of ECP and to identify subsets of chronic GVHD patients who may have benefited from more prolonged use of ECP.
Acknowledgements
The authors thank the nurses and administrative staff of the UCSD Apheresis Unit, the patients for their cooperation, the staff at the Moores Cancer Center for their help and Dr. Emre Gedik for his expert technical assistance..
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217.
- Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276.
- Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112, viii–ix.
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233.
- Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–2579.
- Martin PJ, Carpenter PA, Sanders JE, et al. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004;79:221–228.
- Martin PJ, Counts GW, Jr., Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016.
- Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2015;21:266–274.
- Cooke KR, Luznik L, Sarantopoulos S, et al. The biology of chronic graft-versus-host disease: a task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–234.
- Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, et al. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–4927.
- Poe JC, Jia W, Su H, et al. An aberrant NOTCH2-BCR signaling axis in B cells from patients with chronic GVHD. Blood. 2017;130:2131–2145.
- Hamilton BK. Updates in chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2021;2021:648–654.
- Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354–362.
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458.
- MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129:13–21.
- Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956.
- Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30–37.
- Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114:702–708.
- Inamoto Y, Flowers ME. Treatment of chronic graft-versus-host disease in 2011. Curr Opin Hematol. 2011;18:414–420.
- Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133:1191–1200.
- Hill L, Alousi A, Kebriaei P, et al. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9:21–46.
- Martin PJ, Inamoto Y, Carpenter PA, et al. Treatment of chronic graft-versus-host disease: past, present and future. Korean J Hematol. 2011;46:153–163.
- Vogelsang GB. How I treat chronic graft-versus-host disease. Blood. 2001;97:1196–1201.
- Dhir S, Slatter M, Skinner R. Recent advances in the management of graft-versus-host disease. Arch Dis Child. 2014;99:1150–1157.
- Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129:22–29.
- Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–2250.
- Jaglowski SM, Blazar BR. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv. 2018;2:2012–2019.
- Escamilla Gomez V, Garcia-Gutierrez V, Lopez Corral L, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55:641–648.
- Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–238.
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study. Blood. 2021;138:2278–2289.
- Bleakley M, Sehgal A, Seropian S, et al. Naive T-cell depletion to prevent chronic graft-versus-host disease. J Clin Oncol. 2022;40:1174.
- Owsianowski M, Gollnick H, Siegert W, et al. Successful treatment of chronic graft-versus-host disease with extracorporeal photopheresis. Bone Marrow Transplant. 1994;14:845–848.
- Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615.
- Couriel DR, Hosing C, Saliba R, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080.
- Worel N, Leitner G. Clinical results of extracorporeal photopheresis. Transfus Med Hemother. 2012;39:254–262.
- Greinix HT, Worel N, Just U, et al. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfus Apher Sci. 2014;50:349–357.
- Ward DM. Extracorporeal photopheresis: how, when, and why. J Clin Apher. 2011;26:276–285.
- Hart JW, Shiue LH, Shpall EJ, et al. Extracorporeal photopheresis in the treatment of graft-versus-host disease: evidence and opinion. Ther Adv Hematol. 2013;4:320–334.
- Voss CY, Fry TJ, Coppes MJ, et al. Extending the horizon for cell-based immunotherapy by understanding the mechanisms of action of photopheresis. Transfus Med Rev. 2010;24:22–32.
- Bruserud O, Tvedt TH, Paulsen PQ, et al. Extracorporeal photopheresis (photochemotherapy) in the treatment of acute and chronic graft versus host disease: immunological mechanisms and the results from clinical studies. Cancer Immunol Immunother. 2014;63:757–777.
- Biagi E, Di Biaso I, Leoni V, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4 + CD25 + GITR + Foxp3 + CD62L + functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39.
- Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521.
- Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1–17.
- Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31:149–162.
- Malik MI, Litzow M, Hogan W, et al. Extracorporeal photopheresis for chronic graft-versus-host disease: a systematic review and meta-analysis. Blood Res. 2014;49:100–106.
- Apisarnthanarax N, Donato M, Korbling M, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31:459–465.
- Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35:1187–1193.
- Greinix HT, van Besien K, Elmaagacli AH, et al. Progressive improvement in cutaneous and extracutaneous chronic graft-versus-host disease after a 24-week course of extracorporeal photopheresis–results of a crossover randomized study. Biol Blood Marrow Transplant. 2011;17:1775–1782.
- Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674.
- Abu-Dalle I, Reljic T, Nishihori T, et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol Blood Marrow Transplant. 2014;20:1677–1686.
- Inamoto Y, Storer BE, Lee SJ, et al. Failure-free survival after second-line systemic treatment of chronic graft-versus-host disease. Blood. 2013;121:2340–2346.
- Im A, Hakim FT, Pavletic SZ. Novel targets in the treatment of chronic graft-versus-host disease. Leukemia. 2017;31:543–554.