ABSTRACT
Objective
The current diagnostic methods for multiple myeloma (MM) include bone marrow aspiration and biopsy, which are not only complicated but also invasive, causing great pain to patients. Micro ribonucleotides (miRNA) exist stably in circulating plasma and could be easily detected, making them promising specific diagnostic markers for MM.
Methods
The expression of plasma miR-448 in MM patients was detected by real-time quantitative PCR analysis. Spearman correlation was used to analyze correlations between miR-448 expression and various clinic pathological characteristics of MM patients. The ROC and the AUC (95% confidence interval) were exploited to evaluate the potential of miR-448 acting as a diagnostic marker for MM patients.
Results and discussion
In this study, we found that the expression level of miR-448 in patients with MM was significantly higher than that in normal people and showed statistically differential expression in different stages of MM. Besides, we observed that the abundance of miR-448 in the plasma of newly diagnosed MM patients was positively correlated with the proportion of plasma cells (PC) in the bone marrow and the concentration of serum M protein (MP), respectively. In addition, ROC analysis demonstrated the sensitivity and specificity of the diagnostic value of miR-448 for MM are 92.90% and 87.50%, respectively.
Conclusion
Taken together, these results indicated that miR-448 may serve as a potential molecular diagnostic marker for MM.
1. Introduction
MM is a malignant tumor characterized by the monoclonal proliferation of PC in the bone marrow and excessive release of monoclonal protein in the blood, respectively [Citation1]. Clinically, MM is the second most common hematological malignancy around the world, and most of MM patients show symptoms of end-organ damage, such as lytic bone disease, anemia, immune dysfunction and renal impairment [Citation2–5]. Since firstly reported in 1844, researchers have focused on identifying effective and sensitive diagnostic markers for MM [Citation7]. Fortunately, numerous diagnostic methods for MM are being constantly developed and exploited at present [Citation6]; however, most of the operations are invasive and accompanied with unavoidable poor stability and reproducibility. Therefore, it is necessary to explore some noninvasive, sensitive and specific biomarkers for the diagnosis of MM.
miRNAs are endogenous noncoding and single-stranded RNAs composed of 19–22 nucleotides. By specifically binding to the 3'-end untranslated region (3'-UTR) of the target gene, miRNAs possess the capability to catalyze mRNA cleavage or suppress mRNA translation [Citation8], thereby participating in a variety of physiological and pathological processes, including tumor-related diagnosis assistance, molecular therapy selection and prognostic evaluation [Citation9]. Besides, enormous studies have shown that miRNAs are tissue- or differentiation-specific and can stably exist in plasma, making it possible to serve as the potential biomarker for various blood system diseases, including MM [Citation10–12].
In recent years, numerous studies have confirmed that miR-448 was involved in colon cancer [Citation13], pancreatic cancer [Citation14], gastric cancer [Citation15], epithelial ovarian cancer [Citation16] and other cancers [Citation17] by regulating cell proliferation, migration, invasion and immune escape as well as drug resistance, making it being regarded as a promising biomarker. A previous study also found that miR-448 was downregulated in patients with Hodgkin's lymphoma compared with healthy donors [Citation18], indicating the close correlation between miR-448 and the blood system disease. However, the role of miR-448 in the pathogenesis of MM remains unknown and thus is worth exploring.
In this paper, we mainly detected miR-448 expression in MM patients and explored the association between clinical characteristics of MM patients and miR-448 expression. Additionally, we further evaluated the specificity and sensitivity of plasma miR-488 in identifying MM patients.
2. Materials and methods
2.1 Patient samples
30 newly diagnosed, 30 relapsed and 30 remissions (≥VGPR, very good partial remission) MM patients undergoing treatment at the Affiliated Hospital of Chengde Medical College from September 2019 to December 2020 were enrolled. All patients in this study met the diagnostic criteria for MM by IMWG. The study was approved by the Ethics Committee of Affiliated Hospital of Chengde Medical College and both myeloma patients and healthy donors signed informed consent forms.
2.2 Sample preparation
5 ml of venous blood from each participant were collected and kept in an EDTA-anticoagulated tube (BD, Franklin Lakes, NJ, USA), Then, the tube was centrifuged at 3,000 rpm for 15 min. Finally, plasma was transmitted to a fresh tube and stored at –80°C for further use.
2.3 miRNA extraction
Total miRNA in the plasma was extracted using an miRcute miRNA Isolation Kit (Tiangen, Beijing) according to the manufacturer's instructions. In brief, an equal volume of lysis buffer MZ was added to 200 μL of plasma, mixed by a vortex for 30 s and incubated for 5 min at room temperature, followed by centrifugation at 12,000 rpm for 10 min. Then, the supernatant was transferred to a new RNase-free tube and reacted with chloroform. Later, the supernatant was mixed with an appropriate volume of absolute ethanol and transferred to the adsorption column miRspin and the adsorption column miRelute, respectively. Thereafter, 500 μL deproteinization solution MRD and 500 μL rinsing solution RW were applied to wash the adsorption column miRelute. Finally, 15–30 μL RNase-Free water was used to dissolve the RNA in the adsorption column miRelute.
2.4 Real-time quantitative PCR analysis
After reverse transcription of miRNA to cDNA by miRcute Plus miRNA First-Strand cDNA Kit (TIANGEN) according to the manufacturer’s recommendations, miRcute Plus miRNA qPCR Kit (SYBR Green) and the Roche Cobas z480 detection system (Roche Molecular Diagnostics) were used for real-time quantitative PCR analysis. The procedure was set as follows: pre-denaturation at 95°C for 10 min; 94°C for 20 s, 63°C for 34 s, 72°C for 34 s, repeated for five cycles, followed by 94°C for 20 s, 60°C for 34 s, repeated for 40 cycles. CR100 was used as an external control for the normalization of miR-448 expression and 2−ΔΔCt method was used to analyze the results.
2.5 Statistical analysis
Statistical analysis was performed using SPSS25.0 software with the data presented as mean ± standard error (SE). One-way ANOVA test was used to analyze differences among multiple groups. For data that are not normally distributed or data with uneven variance, the Mann–Whitney U test is used for comparison between the two groups, whereas the Kruskal–Wallis H (K) test is used for the comparison between the four groups. The ROC and the AUC (95% confidence interval) were exploited to evaluate the potential of miR-448 as a diagnostic marker. Spearman correlation was used to analyze correlations between various clinicopathological characteristics of MM patients and miR-448 expression. Values of p < 0.05 were considered statistically significant.
3. Results
3.1 General clinical characteristics of MM patients
We firstly analyzed the clinical characteristics of research subjects enrolled in this study. Results presented in showed that there were no statistical differences in age, gender and calcium ion (Ca2+) among the four groups, while the concentration of albumin (Alb), hemoglobin (Hb), serum creatinine (Scr) and lactate dehydrogenase (LD) exhibited great significance (P < 0.001 in each clinical indicators).
Table 1. Basic clinical characteristics of all research subjects.
3.2 Comparison of miRNA-448 expression levels in different groups
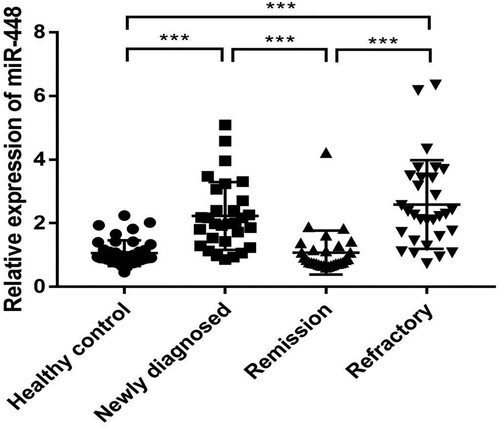
We them performed RT-qPCR to determine the expression levels of miR-448 in plasma samples from 90 MM patients and 40 healthy individuals. Results in showed that miR-448 expression was significantly higher in newly diagnosed group (2.23 ± 1.07 fold) and refractory group (2.59 ± 1.39 fold) than that in the healthy control group (1.06 ± 0.40 fold) (both P < 0.001), respectively. Besides, we observed that the relative expression of plasma miR-448 in the newly diagnosed group (2.23 ± 1.07 fold) and refractory group (2.59 ± 1.39 fold) was remarkably higher compared to the remission group (1.07 ± 0.69 fold). However, no difference was found between the remission group (1.07 ± 0.69 fold) and healthy control group. Collectively, these results indicated that miR-448 was greatly involved in the progression of MM.
3.3 Correlations between miR-448 expression and the clinical-pathological features of MM patients
We subsequently investigated the correlation between the relative plasma expression of miR-448 and the clinical-pathological features of 30 newly diagnosed patients with MM. Results in demonstrated that the relative expression of miR-448 was not correlated to age, gender, ISS stage, type of heavy and light chain, or renal damage. However, we determined that miR-448 expression was markedly higher in Durie–Salmon stage III patients (2.40 ± 1.03 fold) than in stage I/II patients (2.03 ± 1.11 fold) (P < 0.05). These results suggested that the expression profiles of miR-448 in plasma may be related to the development of clinical symptoms in MM patients.
Table 2. Association of miR-448 expression and clinical characteristics in newly diagnosed MM patients.
3.4 Correlation of miR-448 expression with tumor burden indicators of MM
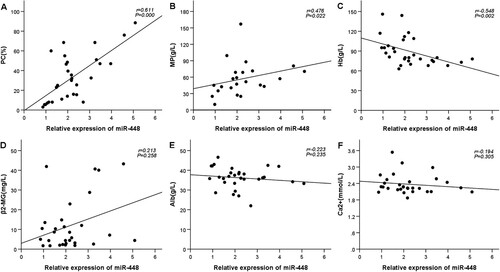
To assess the relationship between plasma expression of miR-448 and serum tumor burden indicators of MM, we further exploited Spearman correlation analysis. Results in showed that miR-448 was positively correlated with the proportion of PC in the bone marrow (r = 0.611, P < 0.05), MP (r = 0.476, P < 0.05), whereas negatively correlated with the content of hemoglobin (Hb) (r = −0.548, P < 0.05). However, no correlation was observed between miR-448 expression and β2-microglobulin (β2-MG) (r = 0.213, P > 0.05), albumin (Alb) (r = −0.223, P > 0.05), as well as calcium ion (Ca2+) (r = −0.194, P > 0.05).
3.5 Analysis of sensitivity and specificity of miRNA-448 as a diagnostic marker.
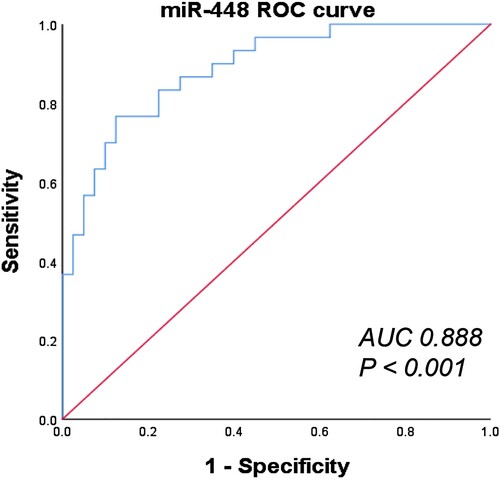
To explore the diagnostic value of miR-448 in MM, we then conducted ROC analysis to validate the specificity and sensitivity of plasma miR-488 in identifying MM patients. As shown in , the area under the ROC curve (AUC) is 0.888, and the 95% CI is 0.812∼0.963. Besides, we observed that the sensitivity and specificity of diagnosing MM even reached 76.70% and 87.50% when the expression level of plasma miR-448 in patients with MM is 1.455 fold, respectively. Taken together, miR-448 was reliable in distinguishing newly diagnosed MM patients from healthy controls and thus could be regarded as a more accurate molecular marker for MM.
4. Discussion
In the past two decades, a large number of studies have confirmed that miRNA dysregulation is widely involved in the pathophysiological process of human malignant tumors. Meanwhile, miRNA plays the role of proto-oncogene or tumor suppressor gene by regulating the corresponding carcinogenic pathway or tumor suppressor pathway, respectively [Citation19,Citation20]. On the other hand, the rich content and stable presence of miRNAs in body fluids, such as blood, urine and saliva, making them possible to serve as new diagnostic markers and at the same time, be detected with portability and noninvasiveness [Citation21].
Given the fact that the available diagnostic methods of MM are usually nonspecific (such as β2 microglobulin) and specific but invasive (such as bone marrow aspiration and biopsy), some sensitive, specific and noninvasive biomarkers for MM diagnostic are really needed. To this point, miRNAs can provide solutions to the dilemma of the limitations that existed in MM diagnosis [Citation22,Citation23]. Besides, miRNAs may also ameliorate the accuracy of MM diagnosis and evaluate the changes in the early stage of onset as well as to improve the prognosis of MM patients.
In view of these, we initially collected plasma samples from MM patients (30 newly diagnosed, 30 remissions and 30 refractory patients) and 40 healthy control to detect miR-448 expression by performing RT-qPCR analysis. Our results showed that the expression level of miR-448 was significantly higher in the newly diagnosed group and refractory group than that in healthy control group, respectively. Meanwhile, we found that miR-448 presented different expression manners in different stages of MM. Besides, we demonstrated that miR-448 expression was markedly higher in Durie–Salmon stage III patients than in stage I/II patients (P < 0.05), whereas showed no correlation to age, gender, ISS stage, type of heavy and light chain as well as renal damage. Consistent with this observation, Spearman correlation analysis revealed that miR-448 was positively correlated with the proportion of PC in the bone marrow and MP, whereas negatively correlated with Hb and showed no obvious significant association with β2-MG, Alb, as well as Ca2+.
Taken the above into consideration, we further conducted ROC analysis to verify the diagnostic value of miR-448 in plasma for MM patients. Results showed that the area under AUC is 0.888 (sensitivity = 76.70%, specificity = 87.50%, 95% CI 0.812–0963, P < 0.001), which further provided a theoretical basis that miR-448 may serve as a potential biomarker for the diagnosis of MM.
A number of studies have revealed that miR-448 was widely involved in the pathophysiology of tumors by regulating the corresponding carcinogenic pathway or tumor suppressor pathway. For example, a previous study showed that miR-448 was highly expressed in human colon cancer tissues as compared to noncancerous tissues. Over expression of miR-448 could enhance the CD8+ T cell response by inhibiting IDO1 expression, thereby inhibiting colon cancer progression [Citation13]. In another study, researchers found that miR-448 was downregulated in pancreatic cancer tissues compared to adjacent normal tissues, and overexpression of miR-448 significantly decreased cell proliferation and promoted apoptosis by targeting Rab2B [Citation14]. It is worth mentioning that miR-448 was confirmed to be low-expressed in Hodgkin’s lymphoma and could participate in a variety of pathological processes by targeting B-cell lymphoma-2 genes [Citation18,Citation24]. According to these reports, miR-448 may also regulate the pathogenesis of MM by targeting oncogenes or tumor suppressor genes, but the exact underlying mechanisms still need to be investigated.
Limitations in this study should be mentioned. First, we did not horizontally compare the expression levels of miR-448 in MM and other hematological diseases, which is very important to clarify the special role of miR-448 in blood system disease. Second, we did not follow up patients with newly diagnosed MM, implying that further analyzation between miR-448 and the prognosis of MM patients is unavailable. Finally, this study is a single center study with small sample, thereby further multicenter study with a large sample is urgently needed.
In conclusion, our results demonstrated that plasma miR-448 may serve as a biomarker for the diagnosis of MM and thereby provide certain evidence for the potential clinical applications of miR-448.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Li J, Zhang M, Wang C. Circulating miRNAs as diagnostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. J Clin Lab Anal. 2020;34(6):e23233.
- Rajkumar SV. Multiple myeloma: every year a new standard? Hematol Oncol. 2019;37(Suppl 1):62–65.
- Riccomi G, Fornaciari G, Giuffra V. Multiple myeloma in paleopathology: a critical review. Int J Paleopathol. 2019;24:201–212.
- Ahn JS, Okal R, Vos JA, et al. Plasmablastic lymphoma versus plasmablastic myeloma: an ongoing diagnostic dilemma. J Clin Pathol. 2017;70(9):775–780.
- Xiao LI, Lin F, Xiao R, et al. Multiple myeloma-associated skin light chain amyloidosis: a case of misdiagnosis. Oncol Lett. 2016;11(6):3617–3620.
- Chan HSH, Chen CI, Reece DE. Current review on high-risk multiple myeloma. Curr Hematol Malig Rep. 2017;12(2):96–108.
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972.
- Correia de Sousa M, Gjorgjieva M, Dolicka D, et al. Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci. 2019;20(24):6249.
- Huang W. MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol Biol. 2017;1617:57–67.
- Li P, Teng F, Gao F, et al. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol. 2015;35(3):433–447.
- Blondal T, Jensby Nielsen S, Baker A, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1–S6.
- Yuan X, Ma R, Yang S, et al. miR-520g and miR-520h overcome bortezomib resistance in multiple myeloma via suppressing APE1. Cell Cycle. 2019;18(14):1660–1669.
- Lou Q, Liu R, Yang X, et al. miR-448 targets IDO1 and regulates CD8(+) T cell response in human colon cancer. J Immunother Cancer. 2019;7(1):210.
- Jin J, Wu Y, Zhou D, et al. Mir448 targets Rab2B and is pivotal in the suppression of pancreatic cancer. Oncol Rep. 2018;40(3):1379–1389.
- Wu X, Tang H, Liu G, et al. miR-448 suppressed gastric cancer proliferation and invasion by regulating ADAM10. Tumour Biol. 2016;37(8):10545–10551.
- Li T, Yuan J, Lai Y, et al. The antitumor effect of miR-448 in epithelial ovarian cancer. Transl Cancer Res. 2020;9(8):4922–4930.
- Jiang X, Zhou Y, Sun AJ, et al. NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J Cell Physiol. 2018;233(11):8558–8566.
- Fan CB, Yan XH, Tian M, et al. Long non-coding RNA NEAT1 regulates Hodgkin's lymphoma cell proliferation and invasion via miR-448 mediated regulation of DCLK1. Eur Rev Med Pharmacol Sci. 2020;24(11):6219–6227.
- Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23.
- Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12.
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518.
- Brigle K, Rogers B. Pathobiology and diagnosis of multiple myeloma. Semin Oncol Nurs. 2017;33(3):225–236.
- Gerecke C, Fuhrmann S, Strifler S, et al. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113(27–28):470–476.
- Zhou P, Shi J, Wei L, et al. MicroRNA-448 suppresses the proliferation, migration, and invasion of glioma cell line U251 by targeting B-cell lymphoma-2. Chin Med J (Engl). 2020;133(1):114–116.