ABSTRACT
Objectives
The prognosis for adults with relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is poor. Blinatumomab is a CD3/CD19-directed BiTE® (bispecific T-cell engager) molecule approved globally for the treatment of BCP-ALL in adults and children. This multicenter open-label single-arm China registrational study evaluated the safety, efficacy, and pharmacokinetics of blinatumomab in Chinese adults with Philadelphia chromosome-negative (Ph−) R/R BCP-ALL (NCT03476239).
Methods
Patients aged ≥ 18 years were treated with up to 5 cycles of blinatumomab. The primary objective was to evaluate the hematological response rate (complete remission/complete remission with partial hematological recovery [CR/CRh]) within 2 cycles of blinatumomab.
Results
At the interim analysis (April 12, 2019), 90 patients (median age 31.5 years [range: 18-74]; 53.3% female; 77.8% with bone marrow blasts ≥ 50% at study entry) were enrolled at 23 study centers in China and had received blinatumomab. As of data cutoff, 43 patients (47.8%) continued the study. The CR/CRh rate within 2 cycles of blinatumomab was 45.6% (41/90 [CR, 37; CRh, 4]; 95% CI: 35.0–56.4). Median overall survival was 9.2 months (95% CI: 6.5–11.7); median relapse-free survival was 4.3 months (95% CI: 3.2–9.4). Mean serum concentration at steady-state and systemic clearance of blinatumomab in Chinese patients were within the range reported in adults from global clinical trials. No new safety risks were identified in Chinese patients.
Conclusions
The efficacy and safety of blinatumomab in these heavily pre-treated Chinese patients with Ph− R/R BCP-ALL is comparable to that for patients within global clinical trials.
Introduction
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy, with a global burden that results from impaired differentiation and overgrowth of malignant lymphoid progenitor cells in the bone marrow and/or extramedullary sites [Citation1]. In adult patients with ALL, more than 80% respond to induction chemotherapy with a complete response, and yet up to 50% relapse or are refractory to chemotherapy. Relapsed or refractory (R/R) B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is an aggressive malignant disease with a 5-year overall survival (OS) rate of approximately 10% [Citation2–6]. Allogeneic hematopoietic stem cell transplantation (alloHSCT) provides a curative treatment option for adults with R/R BCP-ALL. Only a small fraction of these adults receive alloHSCT, often due to failure to achieve complete remission (CR) with second-line therapies or complications from chemotherapy-related toxicities [Citation7,Citation8]. Therapies that enable adults with R/R BCP-ALL to achieve CR without complications from toxicities will allow patients to proceed to alloHSCT and improve survival.
The B-lineage surface antigen CD-19 is constitutively expressed on the surface of normal B cells and is highly conserved in B-cell malignancies. Blinatumomab is a BiTE® (bispecific T-cell engager) immune-oncology therapy that binds CD19 B cells and the CD3 receptor on T cells [Citation9–13]. Blinatumomab utilizes a patient’s own cytolytic T cells to attack CD19-positive cells, including those of BCP-ALL, and transiently connect malignant cells with T cells, thereby inducing T-cell-mediated killing of the bound malignant cell. The subsequent serial lysis of malignant cells by blinatumomab-activated T cells is highly effective and closely resembles the natural cytotoxic T-cell reaction.
Blinatumomab has demonstrated efficacy and safety in children, adolescents and young adults (AYAs), and adults with Philadelphia chromosome–negative (Ph−) R/R BCP-ALL [Citation14–17]. In TOWER, a phase 3 randomized controlled trial in adults, patients treated with blinatumomab had superior OS than patients treated with chemotherapy (7.7 vs 4.0 months) and a higher complete remission/complete remission with partial hematological recovery (CR/CRh) rate versus those treated with chemotherapy (42.4% vs 20.1%) [Citation14]. Blinatumomab also induced high rates of minimal/measurable residual disease (MRD)-negative response in adults, AYAs, and children with molecularly resistant B-ALL [Citation16–18]. Blinatumomab is the first single-agent immunotherapy and the only BiTE® molecule that has been approved globally in adults and children for the treatment of R/R BCP-ALL and BCP-ALL in first or second CR with MRD ≥ 0.1% (i.e. ≥ 10−3) [Citation9,Citation19,Citation20]. Clinical trials were conducted primarily in Caucasian patients [Citation14,Citation15], with a recent trial in Japanese adults [Citation21]. Blinatumomab was approved by the National Medical Products Administration in December 2020. This multicenter, open-label, single-arm China registrational study is the first study to evaluate the safety, efficacy, and pharmacokinetics (PK) of blinatumomab in Chinese patients with R/R BCP-ALL, and to evaluate any ethnic differences that may be present in the Chinese adult population. To our knowledge this study evaluates the largest number of East Asian adult patients with R/R BCP-ALL treated with blinatumomab.
Material and methods
Trial design and oversight
In this ongoing multicenter, open-label, single-arm China registrational study, investigators at 23 centers in China enrolled patients aged ≥ 18 years with Ph− R/R BCP-ALL in any of the following stages: primary refractory after induction therapy or relapsed within 12 months of first remission; or relapsed within 12 months of receiving alloHSCT; or relapsed or refractory after first salvage therapy or beyond. Patients were required to have > 5% blasts in the bone marrow at the time of enrollment. To ensure balance in terms of the number of prior salvage treatments and enroll a population representative of adult ALL patients, approximately 50% of patients received blinatumomab as a first salvage treatment. Patients with a history or presence of clinically relevant central nervous system (CNS) pathology or isolated extramedullary disease were excluded. Complete inclusion and exclusion criteria are provided in Supplemental Methods.
This study included a screening period, treatment period, and follow-up period (). Treatment includes up to 5 cycles of blinatumomab, with a single cycle consisting of a continuous intravenous (IV) infusion over 4 weeks followed by a treatment-free interval of 2 weeks. In the first induction cycle, the initial dose of blinatumomab was 9 μg/day for the first 7 days of treatment (to mitigate for potential cytokine release syndrome [CRS] and CNS events), which was increased (dose step) to 28 μg/day starting on day 8 (week 2) to the end of infusion (week 4). For all subsequent cycles, the dose of blinatumomab was 28 μg/day for all 4 weeks of continuous treatment. Premedication with dexamethasone IV was administered within 3 hours prior to the start of treatment in each cycle, before dose increase on day 8 and prior to restart after any interruption of more than 4 hours. Patients who achieved complete remission/complete remission with partial hematological recovery/complete remission with incomplete hematological recovery (CR/CRh/CRi) within 2 induction cycles of treatment could receive up to 3 additional consolidation cycles of blinatumomab. Patients were evaluated at a safety follow-up visit 30 days (± 3 days) after the end of the last dose of blinatumomab and were followed long-term for disease and survival status every 3 months (± 1 month) until death or a maximum of 2 years after the start of treatment, whichever occurred first. If patients were suitable for alloHSCT after 1 or additional cycles of blinatumomab, they could undergo alloHSCT instead of receiving further cycles of blinatumomab; at least 2 cycles of blinatumomab were recommended before alloHSCT.
Figure 1. Study Design. a9 µg/day in cycle 1 (days 1 to 7). bSafety follow-up visit at 30 days (± 3 days) after protocol-specific therapy; thereafter long-term follow-up was every 3 months (± 1 month). alloHSCT, allogeneic hematopoietic stem cell transplantation; CIVI, continuous intravenous infusion; CR, complete remission (CR was defined as ≤ 5% bone marrow blasts, no evidence of disease and full recovery of peripheral blood counts defined as platelets > 100,000/µL and absolute neutrophil count [ANC] > 1000/µL); CRh, complete remission with partial recovery of peripheral blood counts (≤ 5% bone marrow blasts, no evidence of disease, platelets > 50,000/ µL and ANC > 500/µL); CRi, complete remission with incomplete hematological recovery (≤ 5% bone marrow blasts, no evidence of disease, platelets > 100,000/µL or ANC > 1000/µL).
![Figure 1. Study Design. a9 µg/day in cycle 1 (days 1 to 7). bSafety follow-up visit at 30 days (± 3 days) after protocol-specific therapy; thereafter long-term follow-up was every 3 months (± 1 month). alloHSCT, allogeneic hematopoietic stem cell transplantation; CIVI, continuous intravenous infusion; CR, complete remission (CR was defined as ≤ 5% bone marrow blasts, no evidence of disease and full recovery of peripheral blood counts defined as platelets > 100,000/µL and absolute neutrophil count [ANC] > 1000/µL); CRh, complete remission with partial recovery of peripheral blood counts (≤ 5% bone marrow blasts, no evidence of disease, platelets > 50,000/ µL and ANC > 500/µL); CRi, complete remission with incomplete hematological recovery (≤ 5% bone marrow blasts, no evidence of disease, platelets > 100,000/µL or ANC > 1000/µL).](/cms/asset/ee5867e0-f0d4-46ec-9f4d-1572be1408dc/yhem_a_2111992_f0001_oc.jpg)
The trial protocol was approved by an Institutional Review Board or Independent Ethics Committee at each participating center. Patients or their legally acceptable representative provided written informed consent. This trial was conducted in accordance with the International Council for Harmonization Good Clinical Practices regulations and guidelines. (ClinicalTrials.gov number, NCT03476239). The study was conducted in accordance with the principles stated in the Declaration of Helsinki.
Disease assessment
Hematological response rates were assessed after the first 2 cycles of blinatumomab and during the entire treatment period. CR was defined as ≤ 5% bone marrow blasts, no evidence of disease and full recovery of peripheral blood counts defined as platelets > 100,000/μL, and absolute neutrophil count (ANC) > 1000/μL; CRh was defined as a CR with partial recovery of peripheral blood counts defined as platelets > 50,000/μL and ANC > 500/μL; and CRi was defined as a CR with incomplete recovery of peripheral blood counts defined as platelets > 100,000/μL or ANC > 1000/μL. Cytomorphological bone marrow assessments were conducted at Guangzhou KingMed Center for Clinical Lab. Co., Ltd. (Guangdong, China). MRD was assessed by the presence of at least 0.01% (i.e. ≥ 10−4) leukemic blasts in a bone marrow specimen using multicolor flow cytometry at Q2 Solutions (Beijing, China). MRD-negative response was defined as having < 10−4 detectable leukemic blasts in a bone marrow specimen. PK studies were conducted at WuXi AppTec (Shanghai, China).
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The Medical Dictionary for Regulatory Activities (MedDRA) was used to code AEs according to a system organ class and a preferred term. Treatment-emergent AEs included all AEs that began after the first dose of blinatumomab through 30 days after the end of treatment.
Statistical analyses
The primary endpoint was the CR/CRh rate within 2 cycles of treatment with blinatumomab. The response rate was calculated, and the exact binomial 95% confidence interval (CI) generated for the response rate. The secondary efficacy endpoints of CR, CR/CRh/CRi, alloHSCT, and MRD-negative response rates were calculated and the exact binomial 95% CI generated. The time-to-event endpoints including relapse-free survival (RFS) and OS were summarized with number of patients with events, number of patients censored, Kaplan-Meier curves, proportions at selected time points, and quartiles (when estimable). Continuous variables were summarized by the non-missing sample size, mean, standard deviation (SD), median, first and third quartiles, minimum, and maximum. Categorical variables were summarized by the percentage in each category.
Subgroup analyses of the CR/CRh rate were conducted to explore the consistency of the treatment effect including a logistic regression to estimate the odds ratio and its 95% CIs between subgroups. Subgroups included: sex (female vs male), age (< 35 years vs ≥ 35 to < 55 years vs ≥ 55 years), prior salvage therapy (yes vs no), prior alloHSCT (yes vs no), bone marrow blasts at baseline based on an analysis performed at a central lab (< 50% vs ≥ 50%), disease status (primary refractory vs first relapse vs second or later relapse), and refractory to last previous therapy (yes vs no).
The overall sample size of 120 patients, calculated using the exact method for a single proportion, was estimated to ensure 90% power to detect a significant difference in terms of CR/CRh rate between historical control with 30% CR/CRh rate and blinatumomab, assuming a 45% CR/CRh rate in the alternative hypothesis, at the 2.5% one-sided significance level. A Data Review Team oversaw a planned interim efficacy after 90 patients had a chance to complete 2 cycles of treatment and safety follow-up. The Data Review Team could recommend that the interim analysis become the primary analysis if the observed CR/CRh rate exceeded 42.2%, which was derived from an O’Brien-Fleming alpha spending function [Citation22]. Regardless, of the interim analysis results, the trial would continue to enroll to the planned 120 patients.
Pharmacokinetic analyses
The PK analysis set included a total of 491 blinatumomab samples from 103 patients who received ≥ 1 infusion of blinatumomab and had ≥ 1 PK sample collected as of the data cutoff date 14 June 2019. Of these, 116 samples (24%) were excluded from the PK parameter estimations and summary statistics. Reasons for exclusion included concentrations below the lower limit of quantification (LLOQ; n = 85) at post-dose timepoints and samples from patients who did not receive the specified doses (n = 5). All data for PK analyses were extracted from a secure folder and were mapped via the SAS_CDISC_v2 clinical connector into the Pharsight Knowledgebase Server (PKS) system version 04.0.3 (Certara™, Princeton, NJ). Blinatumomab PK analysis was performed using Phoenix WinNonlin version 6.4 software on Citrix (Certara™, Princeton, NJ) as part of the validated PKS system on individual serum blinatumomab concentrations to estimate the following PK parameters: Css (serum concentration at steady-state; collected during continuous IV infusion at each dose level and summarized as the average of the observed concentrations collected after 5 half-lives from start of infusion), systemic clearance (CL, calculated as CL = R0/Css; where R0 was the infusion rate [μg/hr] and Css was the average Css), terminal half-life (t½,z, calculated as t½,z = ln(2)/λz, where λz was the first-order rate constant estimated via linear regression of the terminal log-linear decay phase), and volume of distribution (Vz, calculated as Vz = CL/λz, where CL was averaged over multiple cycles and where λz was the first-order rate constant estimated via linear regression of the terminal log-linear decay phase as determined from the noncompartmental analysis). Actual doses administered and actual sampling times relative to the start of the continuous IV infusion were used in the noncompartmental analysis and nominal times were used for presenting data in tables. Blinatumomab concentrations below the LLOQ (50.0 pg/mL) were set to zero before data analysis. Mean graphs were prepared using SigmaPlot version 12.5 (Systat Software Inc, San Jose, CA), and individual concentration-time profiles were generated using SAS version 9.3 (SAS, Cary, NC). Individual and mean (SD) concentration-time profiles were summarized by cycle and plotted by time (in hours) post-dose.
Results
Patient disposition and baseline characteristics
In this ongoing multicenter, open-label, single-arm China registrational study, 123 patients were screened at 23 study centers in China. Overall, 106 patients were enrolled from October 18, 2017 to the interim analysis date of April 12, 2019. The first 90 patients who had a chance to complete 2 cycles of treatment and safety follow-up were analyzed in the interim analysis; patients had received blinatumomab for a mean (standard deviation [SD]) duration of 49.8 (38.5) days. Interim results met the criteria for this analysis to become the primary analysis based on the efficacious benefit assessment. As of the data cutoff, 43 patients (47.8%) continued the study and 47 patients (52.2%) had discontinued the study (39 [43.3%] died, 6 [6.7%] withdrew consent, 2 [2.2%] lost to follow-up). Two patients (2.2%) continued blinatumomab and 6 patients (6.7%) had completed blinatumomab at data cutoff; 82 patients (91.1%) had discontinued blinatumomab, with the most common reason for discontinuation being protocol-specified criteria (41 patients [45.6%]).
Of the 90 patients in this study, all patients were Chinese, 53.3% (48/90) were women, and the median age was 31.5 years (range: 18─74) (). Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (ECOG = 0: 32/90 [35.6%]; ECOG = 1: 40/90 [44.4%]), and 70 patients (77.8%) had bone marrow blasts ≥ 50% at study entry. Fifty-one of 90 patients (56.7%) had ≥ 1 prior salvage regimen (1 prior regimen: 32/90 [35.6%]; 2 prior regimens: 12/90 [13.3%]; ≥ 3 prior regimens: 7/90 [7.8%]). After the first induction therapy, 19 patients (21.1%) were MRD-negative (< 1 × 10−4), 27 patients (30.0%) were MRD positive (≥ 1 × 10−4), and the MRD status was unknown for 44 patients (48.9%). Seventeen of 90 patients (18.9%) had primary refractory disease after induction, 35 patients (38.9%) relapsed within 12 months of first remission, and 13 patients (14.4%) had prior alloHSCT ().
Table 1. Demographics and Baseline Characteristics.
Efficacy
Hematological response rate
The rate of CR/CRh within 2 cycles of blinatumomab treatment was 45.6% (41/90; 95% CI: 35.0–56.4); 37 patients (41.1%) achieved CR and 4 patients (4.4%) achieved CRh (). Of the 49 patients who did not achieve a CR/CRh response during the first 2 cycles, 2 (2.2%) had CRi, 2 (2.2%) had blast-free hypoplastic or aplastic bone marrow without CRh or CRi, 8 (8.9%) had progressive disease, 19 (21.1%) had not responded to treatment, 3 (3.3%) were not evaluable, and 15 (16.7%) had missing postbaseline response assessments. The CR/CRh/CRi rate within 2 cycles of blinatumomab treatment was 47.8% (43/90; 95% CI: 37.1–58.6).
Table 2. Best Response During First 2 Cycles of Blinatumomab.
Subgroup analyses were conducted to explore the consistency of the hematological response rate between subgroups (sex, age, prior salvage therapy, prior alloHSCT, baseline bone marrow blasts, disease status, and refractory status to last previous therapy). Except for the subgroup of baseline bone marrow blasts, no notable treatment-by-subgroup effects were observed for the other subgroups (). The CR/CRh rate within 2 cycles of blinatumomab treatment was better for patients who at baseline had bone marrow blasts < 50% versus ≥ 50% (70.0% [14/20] vs 38.6% [27/70]; odds ratio = 3.71; 95% CI: 1.27–10.84). Prior alloHSCT did not have a negative impact on the hematological response rate.
Figure 2. Forest Plot for CR/CRh During First 2 Cycles of Blinatumomab. The CR/CRh rate during the first 2 cycles of blinatumomab was compared within subgroups (sex [female vs male], age [< 35 vs ≥ 35 to < 55 vs ≥ 55 years], prior salvage therapy [yes vs no], prior alloHSCT [yes vs no], bone marrow blasts at baseline [< 50% vs ≥ 50%], disease status [primary refractory vs first relapse vs second or later relapse], and refractory to last previous therapy [yes vs no]). Logistic regression was used to estimate the odds ratio and its 95% CIs between subgroups. alloHSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.
![Figure 2. Forest Plot for CR/CRh During First 2 Cycles of Blinatumomab. The CR/CRh rate during the first 2 cycles of blinatumomab was compared within subgroups (sex [female vs male], age [< 35 vs ≥ 35 to < 55 vs ≥ 55 years], prior salvage therapy [yes vs no], prior alloHSCT [yes vs no], bone marrow blasts at baseline [< 50% vs ≥ 50%], disease status [primary refractory vs first relapse vs second or later relapse], and refractory to last previous therapy [yes vs no]). Logistic regression was used to estimate the odds ratio and its 95% CIs between subgroups. alloHSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.](/cms/asset/174724d7-6792-4c12-868e-f67d3feb9c16/yhem_a_2111992_f0002_oc.jpg)
Overall survival
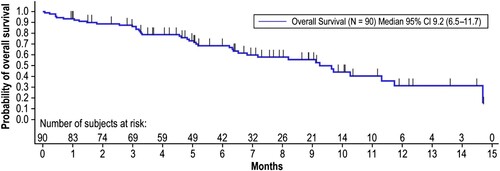
OS time was calculated from the time of first infusion of blinatumomab until death due to any cause. The median OS time was 9.2 months (95% CI: 6.5–11.7), with 40 deaths occurring in 90 patients (); 50 patients were censored (43 were alive at follow-up, 5 withdrew consent, and 2 lost to follow-up). The Kaplan–Meier estimate of survival was 86.2% at 3 months, 68.4% at 6 months, 55.5% at 9 months, 31.3% at 12 months, and not estimable at 18 and 24 months.
Figure 3. Overall Survival. Overall survival time was calculated from the time of first infusion of blinatumomab until death due to any cause. Patients still alive were censored at the date last known to be alive until the data cutoff date of April 12, 2019. Months are calculated as days from the first treatment to death/censor date, divided by 30.5. CI, confidence interval.
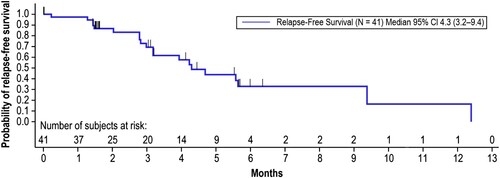
Relapse-free survival
Relapse-free survival (RFS) time was calculated from the first onset of CR/CRh within the 2 cycles of blinatumomab until documented hematological relapse, extramedullary disease, or death due to any cause, whichever occurred first. The median RFS time was 4.3 months (95% CI: 3.2–9.4) with 20 events occurring in 41 patients achieving CR/CRh during the first 2 cycles of treatment (). The Kaplan-Meier estimate of RFS was 69.4% at 3 months, 32.9% at 6 and 9 months, 16.4% at 12 months, and 0.0% at 18 and 24 months.
Figure 4. Relapse-Free Survival. Relapse-free survival time was calculated from the first onset of CR/CRh within the 2 cycles of blinatumomab until documented hematological relapse, extramedullary disease, or death due to any cause, whichever occurred first. Patients still alive and relapse-free were censored at the date of last disease assessment up until the data cutoff date of April 12, 2019. Months are calculated as days from the first onset of CR/CRh within the 2 cycles of blinatumomab until documented hematological relapse/extramedullary disease/death/censor date, divided by 30.5. CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.
Minimal residual disease-negative response
MRD-negative response is defined as < 10−4 leukemic cells detectable by flow cytometry within 2 cycles of blinatumomab treatment. Among the 41 patients who achieved CR/CRh within 2 cycles of treatment with blinatumomab and had evaluable MRD assessment, the MRD-negative response rate was 82.9% (34/41, 95% CI: 67.9, 92.8; ).
AlloHSCT
Of the 90 patients, 16 (17.8%) received alloHSCT during the long-term follow-up. Of the 41 patients who achieved CR/CRh during treatment, 9 patients (22.0%, 95% CI: 10.6–37.6) underwent alloHSCT while in remission (). Among these 9 patients, 1 patient died 264 days after receiving alloHSCT.
Table 3. Proportion of Patients Undergoing alloHSCT.
Pharmacokinetics
During continuous IV infusion of 9 and 28 μg/day blinatumomab to adult patients in cycle 1, mean (SD) concentration at steady-state (Css) was 112 (48.8) pg/mL and 488 (260) pg/mL, respectively (,). The estimated mean (SD) systemic clearance (CL), terminal half-life (t1/2,z), and volume of distribution (Vz) were 3.37 (2.17) L/hr, 2.41 (0.885) hours, and 9.53 (6.45) L, respectively. The PK parameter estimates in Chinese patients in this study were consistent with those reported in the Japanese phase 1b/2 clinical trial [Citation21] (NCT02412306) and global clinical trials (NCT01466179, NCT02000427, NCT02013167; ,).
Figure 5. Pharmacokinetics of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally. Css was determined after 5 half-lives from start of continuous IV infusion of 9 and 28 μg/day blinatumomab to adult patients in cycle 1 for the Chinese cohort, and compared with the Japanese [Citation21] and global cohorts (Amgen data on file, refer to Data Sharing Statement). Boxes display mean (dashed lines), median (solid lines), 25th (bottom) percentile, and 75th (top) percentile. Whiskers represent the 10th (bottom) and 90th (top) percentiles. ALL, acute lymphoblastic leukemia; Css, concentration at steady-state; IV, intravenous; R/R, relapsed/refractory.
![Figure 5. Pharmacokinetics of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally. Css was determined after 5 half-lives from start of continuous IV infusion of 9 and 28 μg/day blinatumomab to adult patients in cycle 1 for the Chinese cohort, and compared with the Japanese [Citation21] and global cohorts (Amgen data on file, refer to Data Sharing Statement). Boxes display mean (dashed lines), median (solid lines), 25th (bottom) percentile, and 75th (top) percentile. Whiskers represent the 10th (bottom) and 90th (top) percentiles. ALL, acute lymphoblastic leukemia; Css, concentration at steady-state; IV, intravenous; R/R, relapsed/refractory.](/cms/asset/6b2f66bc-f79d-4730-9ba5-0993558148cb/yhem_a_2111992_f0005_ob.jpg)
Table 4. Comparison of Demographics and Baseline Characteristics, Pharmacokinetics, and Treatment-Emergent Adverse Events of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally.
Safety
All 90 patients (100.0%) had treatment-emergent AEs (). Eighty-six patients (95.6%) had grade ≥ 3 AEs, and 29 patients (32.2%) had a serious AE. Eleven patients (12.2%) had AEs leading to discontinuation of blinatumomab. Ten patients (11.1%) had fatal AEs; 5 of these fatal AEs were reported as treatment-related by the investigator. Grade ≥ 3 events of interest include neutropenia and febrile neutropenia (53/90, 58.9%), infections (38/90, 42.2%), elevated liver enzymes (20/90, 22.2%), infusion reaction (9/90, 10.0%), CRS (6/90, 6.7%), and neurologic events (5/90, 5.6%) (). The safety profile observed for Chinese patients in this study is consistent with that observed in Japanese and global clinical trials evaluating blinatumomab in R/R BCP-ALL (). No new safety risks were identified based on these analyses of AEs in Chinese patients. The quality of life profile for the Chinese cohort was comparable to that for the global cohort. None of the patients tested positive for binding or neutralizing anti-blinatumomab antibodies.
Discussion
This multicenter, open-label, single-arm China registrational study was conducted to evaluate the efficacy and safety of blinatumomab in Chinese adults with Ph− R/R BCP-ALL. In these 90 heavily pre-treated Chinese patients, the efficacy and safety of blinatumomab was comparable to that for patients within global and Japanese clinical trials [Citation14,Citation15,Citation21]. To our knowledge this is the largest prospective study using blinatumomab in R/R BCP-ALL in Chinese adults conducted to date.
No chemotherapeutic regimen used in the treatment of adult R/R BCP-ALL is clearly superior [Citation14]. Historically, treatment options for adults with R/R ALL have been limited to conventional cytotoxic chemotherapies, which result in CR rates of about 30%–40% in first salvage and about 10%–20% in second salvage [Citation23]. There is no standard approach to care for this population. The MD Anderson Cancer Center group in the United States reported an overall CR rate of 31% in 314 adult patients in first relapse after a variety of salvage regimens. The median OS was 5 months and the OS at 5 years was 3% [Citation24]. Treatment with blinatumomab is now an option since its approval to treat adults with Ph− R/R ALL by the United States Food and Drug Administration (FDA) in 2014 and by the European Medicines Agency in 2015. In December 2020, blinatumomab was approved by the National Medical Products Administration. There is a high unmet need for this population of Chinese patients with R/R ALL. This study demonstrates similar response rates in Chinese patients compared with the global population.
In this study cohort, patient demographics, disease status at study entry, response rate, and AE profile were similar to the global population. The global phase 3 trial (TOWER) randomized patients to receive either blinatumomab (271 patients) or chemotherapy (134 patients). The median OS was significantly longer in the blinatumomab arm than in the chemotherapy arm (7.7 vs 4.0 months) [Citation14]. Remission rates within 12 weeks after treatment initiation were significantly higher in the blinatumomab arm than in the chemotherapy arm, both with respect to CR with full hematologic recovery (34% vs 16%, P < 0.001) and with respect to CR with full, partial, or incomplete hematologic recovery (44% vs 25%, P < 0.001) [Citation14]. Blinatumomab was generally well tolerated.
The results of this study closely align with the blinatumomab arm of the TOWER clinical trial. For this Chinese cohort, 56.7% of patients had ≥ 1 prior salvage regimen. The CR/CRh rate within 2 cycles of blinatumomab for these Chinese patients was 45.6% (41/90; 95% CI: 35.0–56.4), with 41.1% of patients achieving CR and 4.4% of patients achieving CRh. This hematological response rate is akin to the CR/CRh rate reported with blinatumomab in the TOWER clinical trial (42.4%, 115/271) [Citation14] and in the Japanese phase 1b/2 clinical trial (46.2%, 12/26) [Citation21], and is greater than the CR/CRh rate observed in Chinese patients with Ph− R/R BCP-ALL treated with conventional chemotherapy (31.1%, 84/270; first salvage without prior alloHSCT subgroup: CR/CRh = 34.8%; second or higher salvage without prior alloHSCT subgroup: CR/CRh = 17.6%) [Citation25]. Median OS in Chinese patients was comparable with the blinatumomab arm of the TOWER clinical trial (9.2 months [95% CI: 6.5–11.7] vs 7.7 months [95% CI: 5.6–9.6]) [Citation14].
MRD-negative response was a secondary endpoint in this study; however, the MRD-negative results were consistent with the blinatumomab arm of the TOWER clinical trial (82.9% for this trial vs 76.3% for the blinatumomab arm of the TOWER trial). This highlights the similarity in the depth and quality of remissions achieved with blinatumomab in Chinese versus global patients. Additionally, the mean Css and CL of blinatumomab in Chinese patients were within the range reported in adult patients with BCP-ALL from Japanese [Citation21] and global clinical trials (Amgen data on file, refer to Data Sharing Statement).
The AEs reported for this Chinese cohort were generally consistent with those reported in the global and Japanese clinical trials. Grade ≥ 3 neutropenia and febrile neutropenia occurred in a considerable proportion of patients across global [Citation14], Japanese [Citation21], and Chinese clinical trials. CRS and neurotoxicity are important AEs of interest in patients treated with targeted immunotherapy regimens such as blinatumomab. CRS and neurotoxicity events of grade ≥ 3 were reported in 6 (6.7%) and 5 (5.6%) Chinese patients, respectively, comparable to that reported in the Japanese [Citation21] and global [Citation14] clinical trials. In the blinatumomab arm of the TOWER clinical trial, the incidence of grade ≥ 3 CRS and neurotoxicity was 4.9% and 9.4%, respectively, with 1.0% and 4.0% of patients having their treatment discontinued due to CRS and neurotoxicity, respectively [Citation14]. No new or unexpected safety risks were identified in the Chinese patients in this trial.
Other targeted drugs such as CD19 chimeric antigen receptor (CAR) T cells, monoclonal antibody drug conjugates such as inotuzumab ozogamicin (IO, a CD22 monoclonal antibody conjugated to calicheamicin), may provide benefit in this population. Both CD19 CAR T cells and IO therapies have demonstrated higher rates of CR and higher rates of MRD negativity than the rates seen with chemotherapy alone [Citation26–29]. Neither IO nor CD19 CAR T are currently approved for use in China; but CD19 CAR T therapies are being investigated in over 150 registered clinical trials in China [Citation30]. However, there are important events of interest associated with such therapies. In comparison to blinatumomab therapy, there is a higher incidence of severe CRS associated with treatment with CD19 CAR T cells and a higher incidence of liver toxicity and myelosuppression associated with IO therapy. Severe CRS developed in 27% of patients receiving CD19 CAR T cells [Citation28], compared with 4.9% of patients in the blinatumomab arm of the TOWER clinical trial and 6.7% of patients in this study. Eleven percent of patients developed Sinusoidal Obstruction Syndrome (SOS)/Veno Occlusive Disease (VOD), after treatment with IO [Citation29]. No treatment-emergent events of SOS/VOD were reported in either the blinatumomab arm of TOWER [Citation14] or in this study. Additionally, blinatumomab is an off-the-shelf therapeutic whereas CAR T cells must be manufactured (a process requiring at least 2 weeks); patients may progress in disease during this manufacturing time frame and may need bridging therapy, which could be associated with a risk of infection and other toxicities.
Approximately 85% of patients in the TOWER trial were Caucasian. The distribution of geographic regions includes Europe (66.4%), United States or Canada (15.1%), and rest of the world including Latin America and Asia (18.5%). The similarity of our findings in efficacy and AEs between this Chinese cohort and the global cohorts suggests there are no ethnic genetic differences in drug mechanism of action or metabolism.
China is geographically vast, with the world’s largest population. There are differences in the level of economic development and social insurance systems between regions, with different clinical practices, resources, and cultural nuances among hospitals. Collection of data and maintenance of protocol integrity were therefore often challenging and may be a limitation of this trial. In addition, since the administration of blinatumomab requires continuous IV infusion, physicians and nurses needed to be well trained. Careful clinical and nursing management is required to extend real-world use of blinatumomab in China. Finally, MRD measurement could not be performed at any of the validated laboratories in the United States and Europe that performed MRD for our global trials as Chinese regulations do not allow patient samples to be exported outside of China. A laboratory in mainland China was therefore used, which could also be a limitation for this trial.
Conclusions
This multicenter, open-label, single-arm China registrational study demonstrated that the efficacy and safety of blinatumomab in 90 heavily pre-treated Chinese patients with Ph− R/R BCP-ALL was comparable to that for patients in global and Japanese clinical trials [Citation14,Citation15,Citation21]. Blinatumomab may be considered as a treatment option for Chinese adults with Ph− R/R BCP-ALL.
Disclosure of interest
Hongsheng Zhou, Qingsong Yin, Jie Jin, Zhen Cai, Bin Jiang, Yan Li, Yanjuan He, Liping Ma, and Jianxiang Wang report research funding from Amgen Inc. Yuqi Chen, Paul Gordon, and Dong Yu report Amgen employment and stockholdings. Gerhard Zugmaier reports Amgen employment, stockholdings, patents, royalties, and other intellectual property. The remaining authors have nothing to disclose.
Acknowledgments
Medical writing support was provided by Liz Leight, PhD, an employee of Amgen Inc.
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing
Additional information
Funding
References
- Yi M, Zhou L, Li A, et al. Global burden and trend of acute lymphoblastic leukemia from 1990 to 2017. Aging. 2020;12(22):22869–91.
- Barlev A, Lin VW, Katz A, et al. Estimating long-term survival of adults with Philadelphia chromosome-negative relapsed/refractory B-precursor acute lymphoblastic leukemia treated with blinatumomab using historical data. Adv Ther. 2017;34(1):148–55.
- Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–41.
- O'Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186–91.
- Wei G, Wang J, Huang H, et al. Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia. J Hematol Oncol. 2017;10(1):150.
- Oriol A, Vives S, Hernández-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA study group. Haematologica. 2010;95(4):589–96.
- El Fakih R, Ahmed S, Alfraih F, et al. Hematopoietic cell transplantation for acute lymphoblastic leukemia in adult patients. Hematol Oncol Stem Cell Ther. 2017;10(4):252–8.
- Marks DI, Alonso L, Radia R. Allogeneic hematopoietic cell transplantation in adult patients with acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2014;28(6):995–1009.
- Stein A, Franklin JL, Chia VM, et al. Benefit-risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Drug Saf. 2019;42(5):587–601.
- Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science (New York, NY). 2008;321(5891):974–7.
- Löffler A, Gruen M, Wuchter C, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17(5):900–9.
- Zhao J, Song Y, Liu D. Recent advances on blinatumomab for acute lymphoblastic leukemia. Exp Hematol Oncol. 2019;8:28.
- Li L, Wang Y. Recent updates for antibody therapy for acute lymphoblastic leukemia. Exp Hematol Oncol. 2020;9(1):33.
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
- Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66.
- Locatelli F, Zugmaier G, Rizzari C, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA. 2021;325(9):843–54.
- Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA. 2021;325(9):833–42.
- Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31.
- Brown PA, Wieduwilt M, Logan A, et al. Guidelines insights: acute lymphoblastic leukemia, version 1.2019. J Natl Compr Cancer Netw. 2019;17(5):414–23.
- Curran E, Stock W. Taking a “BiTE out of ALL”: blinatumomab approval for MRD-positive ALL. Blood. 2019;133(16):1715–9.
- Kiyoi H, Morris JD, Oh I, et al. Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia. Cancer Sci. 2020;111(4):1314–23.
- O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–56.
- Gökbuget N, Kelsh M, Chia V, et al. Blinatumomab vs historical standard therapy of adult relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J. 2016;6(9):e473.
- Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86(7):1216–30.
- Ma J, Liu T, Jin J, et al. An observational study of Chinese adults with relapsed/refractory Philadelphia-negative acute lymphoblastic leukemia. Int J Hematol Oncol. 2018;7(2):IJH06.
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87.
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
- Wei J, Guo Y, Wang Y, et al. Clinical development of CAR T cell therapy in China: 2020 update. Cell Mol Immunol. 2020; https://doi.org/10.1038/s41423-020-00555-x.
- Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–802.
