ABSTRACT
Objectives
Multiple myeloma (MM) is an incurable plasma cell malignancy associated with poor survival. Novel therapeutic drugs are urgently needed to improve MM therapy and patient outcomes. This study aimed to investigate the effect of formosanin C (FC), a Chinese medicine monomer, on MM in vitro and disclose the underlying molecular mechanism.
Methods
The effect of FC on the viability, proliferation, apoptosis, and autophagy of MM cell lines (NCI-H929 and ARP1) was studied through CCK-8, colony formation, flow cytometry, GFP-LC3, and western blotting assays, respectively. A pharmacological approach and network pharmacology technology were implemented to explore the potential mechanisms of the action of FC on MM cells.
Results
FC efficiently suppressed the viability and colony-forming capacity, but promoted the number of autophagic vacuoles with GFP-LC3 localization and the percentage of apoptotic cells in MM cells. Additionally, FC significantly increased the levels of the autophagy-related proteins LC3-Ⅱ and Beclin 1, as well as the apoptosis-related proteins Bax and cleaved caspase-3, but blocked the expression of the proapoptotic protein Bcl-2 in the cells; these effects were reversed by an inhibitor of autophagy, 3-methyladenine. What's more, we found that the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway was involved in the FC-mediated inhibition of MM. Pharmacological inhibition of this pathway dramatically relieved FC-triggered excessive expression of autophagy-related proteins and rescued MM cells from FC-induced apoptosis.
Conclusion
Our findings indicate that FC exhibits an anti-MM effect by activating cell autophagy through the PI3K/AKT/mTOR signaling pathway.
Introduction
Multiple myeloma (MM), displaying abnormal monoclonal plasma cell proliferation in the bone marrow, is the second most common hematological malignancy and considered incurable [Citation1]. MM incidence has annually increased [Citation2, Citation3]. In last 10 years, the survival of MM patients has improved owing to treatment agents, including proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, and apoptosis inducers [Citation3–5]. However, these drugs are relatively expensive and can cause adverse events, including fatigue, nausea, diarrhea, neuropathy, and loss of appetite, which restrict MM treatment and significantly decrease patients’ quality of life [Citation6]. Patients benefiting from the above mentioned drugs still face relapse risk and refractory disease [Citation6]. Thus, exploring reliable, economical, and effective novel drugs for treating MM is significant.
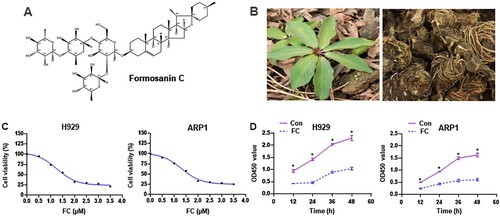
Natural products isolated from plants and herbs are candidate drugs for tumor treatment [Citation7]. Clinically, arsenic trioxide, etoposide, and homoharringtonine have shown notable anticancer activities against acute myeloid leukemia, acute promyelocytic leukemia, and Burkitt lymphoma via different mechanisms [Citation8–10]. For MM treatment, some phytochemicals, such as agaricus and curcumin, have been demonstrated to exert prominent antitumor effects by arresting the cell cycle, promoting anti-angiogenesis, regulating miRNAs, or inducing apoptosis [Citation6]. This suggests that some components extracted from plants could be used to treat MM and other hematologic tumors. Formosanin C (FC; A), also known as Paris saponin-II, is a monomer extracted from Rhizoma Paridis (B). FC has anti-inflammatory and antiproliferative activities [Citation11, Citation12] and exhibits anticancer effects against hepatocellular carcinoma [Citation13], lung cancer [Citation14], ovarian cancer [Citation15], and colon cancer [Citation16]. However, little is known about its effects on MM.
Figure 1. The chemical structure of FC, different parts of the Chinese herb Rhizoma Paridis, and the inhibitory effect of FC on MM cell viability. (A) The chemical structure of FC (C51H82O20, molecular weight, 1015.2). (B) Leaves and rhizomes of Rhizoma Paridis. (C and D) H929 and ARP1 cells were treated with indicated concentrations of FC for 24 h or with 2 μM FC for 12–48 h, cell viability and the OD values at 450 nm were assessed using the CCK-8 assay. The curves of cell viability (C) and the OD450 values (D) are shown (n = 5). *P < 0.05, difference with control group.
Autophagy is a lysosome-dependent protein degradation pathway that removes excess protein aggregates and damaged or dysfunctional organelles [Citation17]. Autophagy plays a double-edged role in tumors. It is linked to cell survival/proliferation and is beneficial for tumor maintenance; it can recycle damaged organelles, relieve metabolic stress, overcome nutrient deprivation, and regulate cellular homeostasis [Citation18, Citation19]. However, excessive autophagy may block tumor survival or result in cell death [Citation20]. In MM, chemotherapeutic drug-induced autophagy play a pro-survival and drug-resistance role by relieving cellular stress caused by toxic stimulation, or a subversive role by triggering autophagic cell death [Citation21]. Therefore, inhibiting MM cell survival/proliferation and promoting MM cell death by regulating autophagy may be novel therapeutic strategy [Citation22].
Herein, we aimed to evaluate the anti-proliferative and pro-apoptotic effects of FC on MM cells, and investigate the relationship between autophagy and the above mentioned effects of FC and the possible molecular mechanisms of the action of FC on MM cells.
Materials and methods
Chemicals and antibodies
FC was purchased from TargetMol (batch: 117278, purity: 98%; Boston, MA, USA). The autophagy inhibitor, 3-methyladenine (3-MA), was obtained from Selleck Chemicals (Houston, TX, USA). Insulin-like growth factor-1 (IGF-1) was acquired from Novoprotein (Shanghai, China). RPMI 1640 medium and fetal bovine serum (FBS) were obtained from Gibco (Rockville, MD, USA). Cell Counting Kit-8 (CCK-8) was purchased from Vazyme (Nanjing, JS, China). Adenovirus expressing GFP-LC3 fusion protein (Ad-GFP-LC3) was from Sciben Biotech Company (Nanjing, JS, China). The following antibodies were used: β-actin (Huaan Biotechnology, Hangzhou, ZJ, China), LC3 (Novus Biologicals, Centennial, CO, USA), Beclin1, Bcl-2, Bax, cleaved caspase-3, PI3K, p-PI3K, AKT, p-AKT, mTOR, and p-mTOR (Cell Signaling Technology, Beverly, MA, USA). Goat anti-rabbit IgG-horseradish peroxidase (HRP) and goat anti-mouse IgG-HRP (Pierce, Rockford, IL, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
Cell lines and cell culture
The human MM cell lines NCI-H929 (H929) and ARP1 were donated by Professor Ye Yang (Nanjing University of Chinese Medicine, China). Cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin (Hyclone, Logan, UT, USA) and cultured at 37 °C in a humidified incubator containing 5% CO2. All controls in this study were treated with DMSO equal to FC volume.
Cell viability, apoptosis and colony formation assays
H929 and ARP1 cells, seeded in 96-well or 6-well plates, were treated with FC (2 μM) for 24 h. Cell viability was evaluated using the CCK-8 assay (Vazyme Biotech Co., Ltd, Nanjing, China) according to manufacturer’s instructions. For cell apoptosis assay, the cells were stained with Annexin V-FITC/PI staining kit (Vazyme Biotech Co., Ltd, Nanjing, China) and analyzed using a fluorescence-activated cell sorter (FACS) Vantage SE flow cytometer (Beckman, CA, USA). Additionally, a colony formation assay was performed using Methocult medium (H4230; StemCell Technologies, Vancouver, Canada) according to manufacturer’s instructions. After incubation at 37°C and 5% CO2 in a humidified atmosphere for 10 days, the colonies were scored.
Network pharmacology analysis
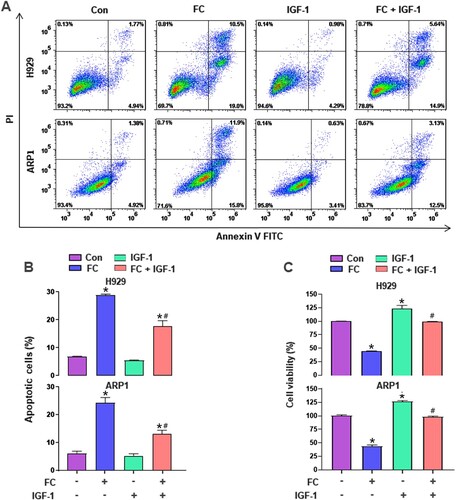
Relevant targets of the traditional Chinese medicine Rhizoma Paridis were obtained from Swiss Target Prediction (http://www.swisstargetprediction.ch/). All the protein names obtained by entering target protein names, restricting the species to ‘Homo sapiens’ and excluding non-human targets, were corrected to official names, based on the UniProKB search function in the UniProt database (https://www.uniprot.org/). The disease target dataset of myeloma was acquired from DrugBank (https://www.drugbank.ca/) and the DigSee database (http://210.107.182.61/geneSear). Venn analysis was performed on the drug and myeloma targets to identify common gene targets. The intersection targets were imported into STRING (http://www.string-db.org/), the protein–protein interaction (PPI) network was analyzed, and the hub gene–target network was ranked by degree values using Cytoscape 3.7.1. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed on the obtained intersection genes using the Metascape database (http://metascape.org/gp/index.htmL#/main/step1). KEGG pathway enrichment analysis was conducted with a cutoff level set at P-value <0.01 and enrichment >1.5.
Western blot analysis
H929 and ARP1 cells, treated with FC (2 μM) for 24 h, were washed with cold PBS, placed on ice, and lysed with RIPA (Beyotime, Shanghai, China). Lysates were sonicated for 10 s and centrifuged at 16 000 × g for 2 min at 4°C. The supernatants were collected and western blotting was performed as previously described [Citation23]. Briefly, lysates containing equivalent amounts of protein were separated on 10–12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated with PBS containing 0.05% Tween 20 and 5% non-fat dry milk to block nonspecific binding, followed by incubation with primary antibodies against LC3, Beclin 1, Bcl-2, Bax, cleaved caspase-3, PI3K, p-PI3K, Akt, p-Akt, mTOR, and p-mTOR overnight at 4 °C, and incubation with appropriate secondary antibodies, including horseradish peroxidase- conjugated goat anti-rabbit IgG or goat anti-mouse IgG, overnight at 4°C. Immunoreactive bands were visualized using enhanced chemiluminescence solution (Sciben Biotech Company, Nanjing, China). The optical density of each band was quantified using ImageJ software (NIH) and normalized with β-actin. Results of independent experiments were expressed as the fold change over the protein amount in the control group. Histograms of western blot quantification were generated using GraphPad Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA).
GFP-LC3 assay
H929 and ARP1 cells were infected with Ad-GFP-LC3 and seeded in a 6-well plate under standard culture conditions and kept overnight at 37 °C humidified incubator with 5% CO2. The next day, cells were treated with FC (2 μM) for 24 h. Subsequently, the cells were washed 3 times with PBS, followed by cell imaging under a fluorescence microscopy (Leica DMi8, Wetzlar, Germany) equipped with a digital camera. At least five independent fields per well were photographed. The number of LC3 puncta per cell was counted.
Statistical analysis
GraphPad Prism 8 software was used for data processing. All data are expressed as mean ± standard error of the mean (SEM). Student's t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using one-way analysis of variance (ANOVA) followed by the Bonferroni's post-test to compare replicate means. The criterion for statistical significance was p < 0.05.
Results
FC suppresses MM-cell viability and proliferation
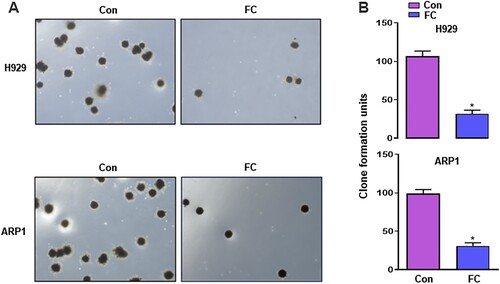
FC suppresses the progression of hepatocellular carcinoma [Citation13], lung cancer [Citation14], ovarian cancer [Citation15], and colon cancer [Citation16], however, its significance in MM treatment has not been reported. To confirm the effect of FC, we first treated H929 and ARP1 cells with FC (0.5, 1, 1.5, 2, 2.5, 3, 3.5 μM) for 24 h. Cell viability was assessed using the CCK-8 assay. FC suppressed the viability of H929 and ARP1 cells in a concentration-dependent manner, with IC50 values of 1.64 and 1.68 μM for H929 and ARP1 cells, respectively (C). Based on this result, we chose 2 μM as the drug concentration for subsequent experiments. H929 and ARP1 cells were treated with 2 μM FC for 12, 24, 36, and 48 h. A decrease was observed in culture OD values at 450 nm at all time points (D), confirming that FC suppressed MM cell viability. Subsequently, H929 and ARP1 cells were incubated in H4320 Methocult medium containing 2 μM FC to determine the inhibitory effect of FC on myeloma cell growth. 10 days later, the colony number reduced in cells exposed to FC (A and B). The results suggest that FC inhibits cell viability and proliferation in MM cell lines.
Figure 2. Inhibition of proliferation of MM cells by FC, demonstrated using colony formation assay. H929 and ARP1 cells were incubated in Methocult medium containing 2 μM FC for 10 days in 6-well plates in triplicate. Cell colonies were photographed under a microscope (A), and the numbers of colonies were calculated (B). 5 independent fields per well were included for the counting. All quantified data are expressed as mean ± SEM (n = 3). *P < 0.05, difference with control group.
FC induces apoptosis of H929 and ARP1 cells
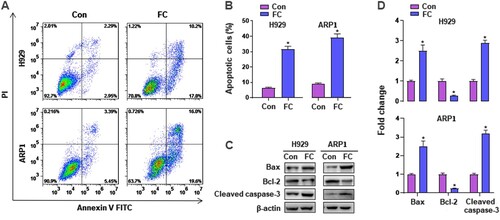
To investigate the effect of FC on apoptosis in MM cells, H929 and ARP1 cells were treated with or without 2 μM FC for 24 h, followed by Annexin V-FITC/PI staining. As shown in A and B, FC elicited a significant increase in the relative number of apoptotic H929 and ARP1 cells. Accordingly, western blotting revealed robust cleavage of caspase-3, overexpression of Bax, and downregulation of Bcl-2 evoked by FC in the cells (C and D). These findings clearly show that FC induces apoptosis in MM cells.
Figure 3. FC induces apoptotic death in MM cells. H929 and ARP1 cells were exposed to FC (2 μM) for 24 h. (A) The percentages of live (the third quadrant), early apoptotic (the fourth quadrant), late apoptotic (the first quadrant), and necrotic cells (the second quadrant) were determined by FACS using annexin-V-FITC/PI staining. Results from a representative experiment are shown. (B) Quantitative analysis of apoptotic cells using FACS. The rate of apoptotic cells corresponds to the sum of the rates of early and late apoptotic cells. (C) Total cell lysates were subjected to western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were observed in three independent experiments. (D) The blots for Bcl-2, Bax, and cleaved caspase-3 were semi-quantified. All quantified data are expressed as mean ± SEM (n = 3). *P < 0.05, difference with control group.
FC activates autophagy leading to apoptosis in H929 and ARP1 cells
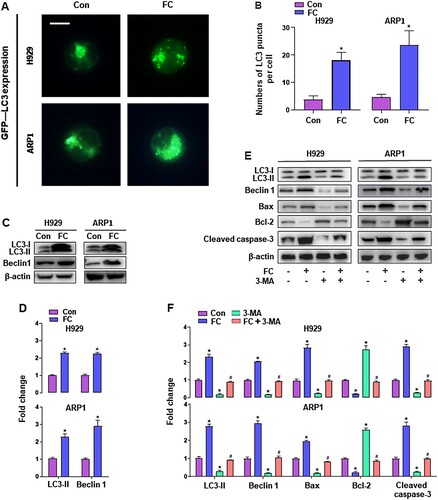
Since autophagy plays an important role in controlling cell survival and death [Citation24], we sought to determine the impact on autophagy in MM cells exposed to FC. To this end, we monitored autophagic vacuoles with GFP-LC3 localization in H929 and ARP1 cells using GFP-LC3 assay. Imaged and quantified results showed that when the cells, infected with Ad-GFP-LC3, were exposed to FC (2 μM) for 24 h, the number of the cells with LC3 puncta structures significantly increased compared with that of the vehicle-treated cells (A and B). What’s more, western blot analysis revealed that LC3-Ⅱ and Beclin 1 levels were remarkably upregulated by FC (C and D). These results clearly indicated that FC activated autophagy in MM cells. To study the role of activated autophagy in FC-induced apoptosis in MM cells, H929 and ARP1 cells were pre-treated with or without 3-MA (10 μM), an inhibitor of autophagy, for 2 h, followed by exposure to FC (2 μM) for 24 h. As shown in E and F, treatment with 3-MA substantially inhibited FC-activated autophagy in MM cells, as 3-MA strikingly attenuated FC-promoted LC3-Ⅱ and Beclin 1 expression. Notably, the effects of FC on Bax, cleaved caspase-3, and Bcl-2 were markedly reversed in MM cells by 3-MA administration. These results indicate that inhibition of autophagy by 3-MA rescues MM cells from FC-induced apoptosis, suggesting that excessive autophagy elicited by FC mediates apoptosis of MM cells.
Figure 4. FC triggers activation of autophagy contributing to MM cell death. H929 and ARP1 cells, or H929 and ARP1 cells infected with Ad-GFP-LC3 were treated with FC (2 μM) for 24 h or pre-treated with/without 3-MA (10 μM) for 2 h and then exposed to FC (2 μM) for 24 h. (A and B) Shown are representative GFP-LC3 fluorescence images (in green) (A) and quantified number (B) for GFP-LC3 puncta in the cells. Scale bar: 2 μm. (C and E) Total cell lysates were subjected to western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were observed in three independent experiments. (D and F) The blots for LC3-Ⅱ, Beclin 1, Bax, Bcl-2, and cleaved caspase-3 were semi-quantified. All quantified data are expressed as means ± SEM (n = 3-5). *P < 0.05, difference with control group; #P < 0.05, difference with FC group.
FC-inhibited PI3K/AKT/mTOR pathway contributes to activated autophagy-mediated apoptosis in MM cells
To further explore the mechanisms by which FC induced excessive autophagy resulting in apoptosis in MM cells, we first performed KEGG pathway enrichment analysis, which was based on Venn analysis and PPI network diagram. The results showed that there were 16 intersection genes between the drug targets of the traditional Chinese medicine Rhizoma Paridis and myeloma disease targets (A and B), and the PI3K/AKT signaling pathway is the most likely target of Rhizoma Paridis in MM (C). The results led us to wonder whether FC, one of the main components of Rhizoma Paridis, acts on the PI3K/AKT signaling pathway in MM cells. To test this, the activity of the PI3K/AKT pathway and its downstream target mTOR was detected through western blotting. As shown in A and B, the protein expression of p-PI3K, p-AKT, and p-mTOR was dramatically repressed by FC in H929 and ARP1 cells, compared with the control group. Additionally, to investigate the role of the PI3K/AKT/mTOR pathway in FC-triggered excessive autophagy-mediated apoptosis in MM cells, H929 and ARP1 cells were pre-treated with or without IGF-1 (100 ng/mL), an agonist of the PI3K/AKT/mTOR pathway, for 1 h, followed by treatment with FC (2 μM) for 24 h. As expected, pharmacological activation of the PI3K/AKT/mTOR pathway with IGF-1 significantly attenuated FC-promoted LC3-Ⅱ, Beclin 1, Bax, and cleaved caspase-3 and FC-suppressed Bcl-2 in the cells (C and D). What’s more, IGF-1 efficiently prevented MM cells from FC-triggered apoptosis and suppression of viability (A–C). The above results suggest that FC-mediated inhibition of the PI3K/AKT/mTOR pathway contributes to excessive autophagy-mediated apoptosis in MM cells.
Figure 5. The PI3K/AKT signaling pathway is a potential target of Rhizoma Paridis in myeloma, as revealed by network pharmacology analysis. (A) Venn diagram shows 16 potential therapeutic targets of Rhizoma Paridis in myeloma. (B) Hub targets ranked by degree value in PPI network. The size of the points depends on their value. (C) The top 12 significant terms of KEGG analysis.
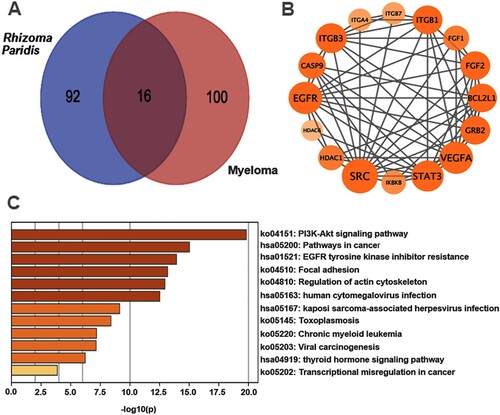
Figure 6. FC inhibits the PI3K/AKT/mTOR pathway, activating autophagy and leading to MM cell apoptosis. H929 and ARP1 cells were treated with FC (2 μM) for 24 h or pre-treated with/without IGF-1 (100 ng/mL) for 1 h and then exposed to FC (2 μM) for 24 h. (A and C) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-actin as a loading control. Similar results were observed in three independent experiments. (B and D) The blots for p-PI3K, p-AKT, p-mTOR, LC3-Ⅱ, Beclin 1, Bax, Bcl-2, and cleaved caspase-3 were semi-quantified. For (B) and (D), all data are expressed as means ± SEM (n = 3). *P < 0.05, difference with control group; #P < 0.05, difference with FC group.
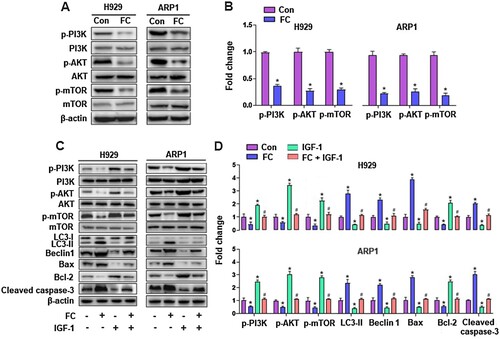
Figure 7. IGF-1 rescues MM cells from FC-triggered apoptosis and -suppressed viability. H929 and ARP1 cells were pre-treated with/without IGF-1 (100 ng/mL) for 1 h and then exposed to FC (2 μM) for 24 h. (A) The percentages of live (the third quadrant), early apoptotic (the fourth quadrant), late apoptotic (the first quadrant), and necrotic cells (the second quadrant) were determined by FACS using annexin-V-FITC/PI staining. Results from a representative experiment are shown. (B) Quantitative analysis of apoptotic cells using FACS. The rate of apoptotic cells corresponds to the sum of the rates of early and late apoptotic cells. (C) Cell viability were assessed using the CCK-8 assay. For (B) and (C), all data are expressed as means ± SEM (n = 3-5). *P < 0.05, difference with control group; #P < 0.05, difference with FC group.
Discussion
In the present study, we investigated the anti-MM effect of FC, the main active component of Rhizoma Paridis. To the best of our knowledge, this is the first report to show that FC exerts a notable inhibiting effect against MM cell lines, including H929 and ARP1, by inducing autophagy-mediated apoptosis via suppression of the PI3K/AKT/mTOR signaling pathway.
Several studies have indicated that FC is a promising antitumor drug in a variety of solid malignancies, including hepatocarcinoma [Citation13], lung cancer [Citation25], ovarian cancer [Citation15], and colon cancer [Citation16]. These findings prompted us to wonder whether FC exhibits an anti-MM effect. Using the CCK-8 assay, colony formation assay, and flow cytometry analysis, we demonstrated that FC dramatically inhibited the viability and proliferation of MM cells and induced notable apoptosis, suggesting that FC has remarkable efficiency against MM.
Emerging evidence has revealed that autophagy plays an anti-apoptotic role by raising the stress threshold required for the induction of apoptosis or by directly suppressing apoptosis [Citation21, Citation26, Citation27]. A specific level of autophagy is necessary for myeloma cells to sustain their life [Citation28, Citation29]. Hence, inhibiting autophagy is considered beneficial for MM [Citation30]. However, excessive autophagy leads to autophagic cell death in tumor cells by extreme degradation of the cytoplasm [Citation31]. In the present study, using western blot analysis, we found that FC-induced apoptosis was attributed to excessive autophagy in H929 and ARP1 cells. This result was supported by evidence that FC dramatically activated autophagy, as the levels of two crucial autophagy-related proteins, LC3-Ⅱ and Beclin 1, were remarkably promoted by FC in the cells. Suppression of excessive autophagy with 3-MA, a well-known inhibitor of autophagy, efficiently rescued cells from FC-induced apoptosis, as indicated by the results that 3-MA markedly reversed FC-upregulated Bcl-2, cleaved caspase-3, and FC-downregulated Bax expression. These results indicated that FC elicits apoptosis by activating autophagy, rather than inhibiting it, in MM cells. Having shown that FC activates autophagy in MM cells, we next examined the effect of FC on the activity of the proteasome in MM cells, which plays an essential role in the ubiquitin-proteasome degradation pathway, an alternative protein degradation pathway to autophagy. Chymotrypsin-like (CT-L) proteasome activity was measured in FC-treated H929 and ARP1 cells. Two proteasome inhibitors, MG-132 and bortezomib, were used as positive controls. We found that 2 μM FC did not influence the (CT-L) proteasome activity in MM cells, whereas both MG-132 and bortezomib, a proteasome inhibitor frequently used for MM treatment, extremely suppressed it at the same dose in the cells (Supplementary figure S1). The result suggests that FC inhibits MM cells may not by inhibiting proteasome activity.
Additionally, KEGG pathway enrichment analyses identified the PI3K/AKT signaling pathway, a key regulator of cell survival, growth, proliferation, and apoptosis, as a target of Rhizoma Paridis in MM cells. This hinted us that FC, a main component of Rhizoma Paridis, may acts on the PI3K/AKT signaling pathway. Using IGF-1, we revealed that the suppression of the PI3K/AKT/mTOR pathway contributes to FC-induced excessive autophagy, leading to apoptosis in MM cells. It’s known that internal reactive oxygen species is associated with the PI3K/AKT/mTOR pathway, autophagy and apoptosis in MM cells [Citation32, Citation33]. However, the role of internal reactive oxygen species in FC inhibition of the PI3K/AKT/mTOR pathway-induced autophagy-mediated apoptosis in MM cells is unclear, which deserves further study. In addition, one of the most significant side effects of bortezomib is peripheral neurotoxicity, which occurs in up to 50% of cases; bortezomib may severely reduce patients’ quality of life, and its clinical application is, therefore, significantly restricted [Citation34, Citation35]. Our recent study demonstrated that bortezomib results in peripheral neurotoxicity via activation of the mTOR pathway [Citation34]. Thus, we speculated that FC can be used to enhance the anti-MM efficacy of bortezomib and relieve bortezomib-induced peripheral neurotoxicity by targeting the mTOR pathway. Apparently, this speculation requires further investigation.
In conclusion, we identified that FC exhibits notable activity against MM, at least partly, by promoting autophagy, providing a proof-of-principle for the potential clinical application of FC as an autophagic activator in MM treatment. We further demonstrated that FC-induced autophagy-mediated inhibition of MM cells was attributed to the suppression of the PI3K/AKT/mTOR pathway. Our findings underscore that FC may be a novel drug acting as an autophagy activator to treat MM and provide an experimental basis for understanding the mechanism of the action of FC on MM cells.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplemental Material
Download MS Word (1.9 MB)Acknowledgments
We would like to express our great appreciation and gratitude to Professor Ye Yang, Nanjing University of Chinese Medicine, for providing MM cell lines.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Joshua DE, Bryant C, Dix C, et al. Biology and therapy of multiple myeloma. Med J Aust. 2019;210:375–380.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397:410–427.
- Bobin A, Liuu E, Moya N, et al. Multiple myeloma: An overview of the current and novel therapeutic approaches in 2020. Cancers (Basel). 2020;12:2885. https://doi.org/10.3390/cancers12102885
- Pinto V, Bergantim R, Caires HR, et al. Multiple myeloma: available therapies and causes of drug resistance. Cancers (Basel). 2020;12:407. https://doi.org/10.3390/cancers12020407
- Kang B, Park H, Kim B. Anticancer activity and underlying mechanism of phytochemicals against multiple myeloma. Int J Mol Sci. 2019;20:2302. https://doi.org/10.3390/ijms20092302
- Zhu F, Jiang D, Zhang M, et al. 2,4-Dihydroxy-3'-methoxy-4'-ethoxychalcone suppresses cell proliferation and induces apoptosis of multiple myeloma via the PI3K/akt/mTOR signaling pathway. Pharm Biol. 2019;57:641–648.
- Cicconi L, Platzbecker U, Avvisati G, et al. Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: update of the APL0406 Italian-German randomized trial. Leukemia. 2020;34:914–918.
- Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter study of risk-adapted therapy With dose-adjusted EPOCH-R in adults With untreated burkitt lymphoma. J Clin Oncol. 2020;38:2519–2529.
- Zhang Z, Jiang M, Borthakur G, et al. Acute myeloid leukemia with a novel CPSF6-RARG variant is sensitive to homoharringtonine and cytarabine chemotherapy. Am J Hematol. 2020;95:E48–E51.
- Yin L, Shi C, Zhang Z, et al. Formosanin C attenuates lipopolysaccharide-induced inflammation through nuclear factor-kappaB inhibition in macrophages. Korean J Physiol Pharmacol. 2021;25:395–401.
- Xiao X, Yang M, Xiao J, et al. Paris saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother Pharmacol. 2014;73:807–818.
- Li Y, Man S, Li J, et al. The antitumor effect of formosanin C on HepG2 cell as revealed by 1H-NMR based metabolic profiling. Chem Biol Interact. 2014;220:193–199.
- Man S, Zhang L, Cui J, et al. Curcumin enhances the anti-cancer effects of Paris saponin II in lung cancer cells. Cell Prolif. 2018;51:e12458.
- Yang M, Zou J, Zhu H, et al. Paris saponin II inhibits human ovarian cancer cell-induced angiogenesis by modulating NF-kappaB signaling. Oncol Rep. 2015;33:2190–2198.
- Chen M, Ye K, Zhang B, et al. Paris saponin II inhibits colorectal carcinogenesis by regulating mitochondrial fission and NF-kappaB pathway. Pharmacol Res. 2019;139:273–285.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662.
- White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410.
- Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120.
- Yun Z, Zhichao J, Hao Y, et al. Targeting autophagy in multiple myeloma. Leuk Res. 2017;59:97–104.
- Desantis V, Saltarella I, Lamanuzzi A, et al. Autophagy: A New mechanism of prosurvival and drug resistance in multiple myeloma. Transl Oncol. 2018;11:1350–1357.
- Zhang H, Dong X, Zhao R, et al. Cadmium results in accumulation of autophagosomes-dependent apoptosis through activating Akt-impaired autophagic flux in neuronal cells. Cell Signal. 2019;55:26–39.
- Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075.
- Zhang L, Man S, Wang Y, et al. Paris saponin II induced apoptosis via activation of autophagy in human lung cancer cells. Chem Biol Interact. 2016;253:125–133.
- Li A, Chen X, Jing Z, et al. Trifluoperazine induces cellular apoptosis by inhibiting autophagy and targeting NUPR1 in multiple myeloma. FEBS Open Bio. 2020;10:2097–2106.
- Wang G, Zhou P, Chen X, et al. The novel autophagy inhibitor elaiophylin exerts antitumor activity against multiple myeloma with mutant TP53 in part through endoplasmic reticulum stress-induced apoptosis. Cancer Biol Ther. 2017;18:584–595.
- Carroll RG, Martin SJ. Autophagy in multiple myeloma: what makes you stronger can also kill you. Cancer Cell. 2013;23:425–426.
- Pengo N, Scolari M, Oliva L, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14:298–305.
- Roy M, Liang L, Xiao X, et al. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6:2209–2224.
- Zheng Z, Wang L, Cheng S, et al. Autophagy and myeloma. Adv Exp Med Biol. 2020;1207:625–631.
- Zhao Y, Zhang E, Lv N, et al. Metformin and FTY720 synergistically induce apoptosis in multiple myeloma cells. Cell Physiol Biochem. 2018;48:785–800.
- Jaouadi O, Limam I, Abdelkarim M, et al. 5,6-Epoxycholesterol isomers induce oxiapoptophagy in myeloma cells. Cancers (Basel). 2021;13:3747. https://doi.org/10.3390/cancers13153747
- Dong X, Zuo Y, Zhou M, et al. Bortezomib activation of mTORC1 pathway mediated by NOX2-drived reactive oxygen species results in apoptosis in primary dorsal root ganglion neurons. Exp Cell Res. 2021;400:112494.
- Rampen AJ, Jongen JL, van Heuvel I, et al. Bortezomib-induced polyneuropathy. Neth J Med. 2013;71:128–133.
