ABSTRACT
Background:
The CYP2C19 gene is highly polymorphic, and CYP2C19 is involved in the broad interindividual variability of the clinical efficacy of certain clinical medications, such as clopidogrel. However, data on the CYP2C19 genotype in the Chinese population of the Foshan area of Guangdong Province are scarce. The purpose of this study was to determine CYP2C19 genetic polymorphisms in patients in the Foshan area and to compare the CYP2C19 genotype frequencies in different populations to determine the allele distribution pattern to identify the most appropriate prescription.
Methods:
The CYP2C19 gene was detected in 1231 patients on a gene chip platform, and the genotype frequencies of CYP2C19 in Foshan populations from different populations were compared.
Results:
The frequencies of CYP2C19*1, *2 and *3 in the Foshan population were 63.89%, 30.46% and 5.65%, respectively. For the three metabolic types, the frequency associated with the rapid metabolism type (*1/*1) was 41.51 [95% confidence interval (CI) 40.11 to 42.91%]; that for the intermediate metabolism type (*1/*2, *1/*3) was 44.76% (95% CI 43.34 to 46.18) and that for the slow metabolism type (*2/*2, *2/*3, *3/*3) was 13.73% (95% CI 12.75 to 14.71%). In the Foshan population, the frequencies of the CYP2C19 *2 and *3 alleles were similar to those previously reported for Chinese and other Asian populations.
Conclusion:
Our study is a report on the genetic basis of CYP2C19 polymorphism in the Foshan population. Our results will potentially contribute to the improvement of pharmacotherapy effectiveness by providing personalized medicine for the Foshan population.
Introduction
Coronary heart disease is one of the main causes of disability and death worldwide [Citation1]. Clopidogrel, which blocks the platelet adenosine diphosphate (ADP) receptor pathway to inhibit platelet aggregation, is an antiplatelet drug that is usually used in the treatment of thromboembolic diseases such as coronary artery disease (CAD), ischaemic stroke or peripheral atherosclerotic diseases [Citation2]. However, the pharmacodynamic efficacy of clopidogrel is often reduced because of interindividual variability. According to report, ∼4–30% of patients appear nonresponsive or show low responsiveness to clopidogrel (clopidogrel resistance), which could increase the risk of major adverse cardiovascular events (MACE) [Citation3].
Cytochrome P450 (CYP450) is an enzyme that is essential for the degradation and biosynthesis of medications, toxins, and endogenous substances [Citation4]. The CYPP450 enzymes are superfamily subsets of hemeproteins that are thought to have a fundamental action in the metabolic phase of different medications and steroid compounds [Citation5,Citation6]. CYP2C19 participates in the metabolism of approximately 10% of commonly prescribed medications, such as antidepressants, antipsychotics, clopidogrel, proton pump inhibitors, and antiepileptics [Citation7,Citation8]. Genetic variations in CYP2C19, which is highly polymorphic, may affect the ability to metabolize the related drugs. Interindividual differences in enzyme activity divide the population into the extensive metabolizer (EM), intermediate metabolizer (IM) and poor metabolizer (PM) phenotypes [Citation9,Citation10]. CYP2C19 exhibits 35 different types of gene alleles of gene polymorphism. CYP2C19*3 and CYP2C19*2 are the most frequent loss-of-function (LOF) alleles and are responsible for the reduced efficacy of clopidogrel and increased rate of cardiovascular events [Citation11–13]. In March 2010, the US Food and Drug Administration (FDA) proposed a boxed warning about clopidogrel, which suggests that physicians might consider the use of replacement treatment strategies in individuals with PM genotypes [Citation14]. The polymorphism of CYP2C19 varies greatly among regions and ethnic groups [Citation15]. Multiple studies on the differences in the genotype frequencies of CYP2C19 among different populations have been performed to date.
There are three populations of Han inhabitants lived in Guang dong Province,namely Chaoshan population, Cantonese and Hakka. Guandong is the most populous province of China with over 126.8 million inhabitants, among which Foshan is a typical city where majority of citizens are Cantonese-speaking groups. Unlike the Hakka-speaking groups in Guangdong, the CYP2C19 polymorphism in the Cantonese-speaking groups of this city remains incompletely understood.
To the best of our knowledge, no data is available on CYP2C19 polymorphism in the Cantonese-speaking groups in Guangdong Province. Only one study on CYP2C19 genetic polymorphisms has been conducted among Hakka populations of Guangdong Province. Our study aims to determine the CYP2C19 genetic polymorphisms among patients in Foshan City of Guangdong Province of China. We also compared the genotype frequencies of CYP2C19 in different populations.
Materials and methods
Subjects
Our study was retrospective. We selected 1231 patients (ages 19–92 years) who visited The Sixth Affiliated Hospital, South China University of Technology between January 2016 and May 2018 for inclusion in this study. All subjects were Han Chinese individuals residing in the Foshan area of Guangdong Province with more than three generations of paternal ancestry of their ethnicity. Subjects with liver diseases or severe heart failure or kidney disease was excluded. All of the chosen subjects were non-consanguineous. This study protocol was performed in accordance with the principles of the Declaration of Helsinki. The study was approved by the ethics committee of The Sixth Affiliated Hospital, South China University of Technology, and written informed consent was obtained from all participants (number: 20160106).
DNA extraction
Venous blood samples were taken from each subject in 5 ml tubes containing ethylene diaminetetraacetic acid (EDTA). Genomic DNA was isolated using the MagPure Fast Blood DNA KF Kit (Magen, Chuangzhou, China) according to the manufacturer’s instructions.
DNA genotyping
Detection of the CYP2C9*1, CYP2C9*2 and CYP2C19*3 alleles was performed by using a commercially available kit (BaiO Technology Co, Ltd, Shanghai, China). In brief, 5 μl of sample DNA was mixed with 19 μl of CYP2C19 amplification solution 1 or 2 and 1 μl of reaction solution A provided in the kit. Each polymerase chain reaction (PCR) assay included positive and negative controls. Amplification was carried out with an ABI VeritiDx PCR system (Applied Biosystems, USA). The cycling conditions were as follows: 50°C for 5 min, an initial denaturation step of 5 min at 94°C, followed by 35 cycles of 94°C for 25 s for denaturation, 48°C for 40 s and 72°C for 30 s, with a final extension step at 72°C for 5 min. Then, the PCR products were transferred to a hybridization reaction chamber for hybridization reactions. Finally, we put the gene chip into the gene chip reading instrument (BaiO Technology Co, Ltd, Shanghai, China) and utilized BaioRBE-2.0 software (BaiO Technology Co, Ltd, Shanghai, China) to detect the CYP2C19 alleles. Positive and negative controls were included in each experiment. showed the different genotype distributions on the chips.
Statistical analysis
Statistical analyses were performed with Excel and SPSS 19.0.
The gene counting method was used to calculate allele frequencies and genotype frequencies. The chi-square test Chi-square and Fisher’s exact tests were used to analyse the differences in the allele frequencies between our population and other populations. P values < 0.05 were considered significant. Hardy-Weinberg equilibrium was calculated for each allele using the chi-square test.
Results
CYP2C19 genotype and allele frequencies of all subjects.
A total of 1231 individuals (range, 19–92 years old) were included in the study.
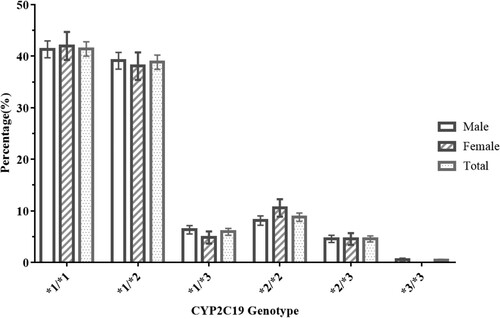
The frequencies of the CYP2C19*1, CYP2C19*2 and CYP2C19*3 alleles were 63.89%, 30.46% and 5.65%, respectively. The allele frequency of CYP2C19*3 was lower than that of CYP2C19*2 by nearly one-fifth. Among the 1231 subjects, 511 (41.51%, 95% CI 40.11 to 42.91%) were wild-type homozygous; 478 (38.83%, 95% CI 37.44 to 40.22%) were heterozygous; and 108 (8.77%, 95% CI 7.96 to 9.58%) were mutant homozygous for CYP2C19*2 (). There were 73 (5.93%, 95% CI 5.26 to 6.60%) heterozygotes and 5 (0.41%, 95% CI 0.23 to 0.59%) mutant homozygotes for CYP2C19*3. There were 56 (4.55%, 95% CI 3.96 to 5.14%) mutant heterozygous for CYP2C19*2 and CYP2C19*3.
The total 1231 subjects were divided into three phenotypes on the basis of genetic polymorphism. As shown in , there were 511 (41.51%, 95% CI 40.11 to 42.91%) patients with EM (CYP2C19*1/*1); 551 (44.76%, 95% CI 43.34 to 46.18%) patients with IM (CYP2C19*1/*2 or CYP2C19*1/*3); and 169 patients (13.73%, 95% CI 12.75 to 14.71%) with PM (CYP2C19*2/*2, CYP2C19*3/*3, or CYP2C19*2/*3). The CYP2C19 allele and genotype frequencies in all subjects were in Hardy-Weinberg equilibrium (χ2 = 8.00, P > 0.05).
CYP2C19 allele frequency in our study compared with previous reports.
We further compared the CYP2C19 allele frequencies between our data and previously published studies from different countries and ethnic groups (). Our results showed that the CYP2C19*1 allele frequency in our population was lower than those in other Caucasian and Asian populations. In addition, the CYP2C19*2 and CYP2C19*3 allele frequencies in the subjects of our study were higher than those in studies of Caucasians.
Table 1. CYP2C19 allele frequencies in our study and in previous studies.
Discussion
CYP2C19 participates in the metabolization of approximately 10% of commonly prescribed medications, such as antidepressants, antipsychotics, clopidogrel, proton pump inhibitors, and antiepileptics [Citation7,Citation8]. The genetic polymorphism of CYP2C19 plays an important role in related drug metabolism and may lead to inter-individual and inter-ethnic variation in patient responsiveness and adverse drug reactions [Citation16]. Currently, at least 34 alleles of CYP2C19 have been identified. Among these alleles, the CYP2C19*2 and CYP2C19*3 alleles are responsible for the reduced activation of metabolized drugs and an increased rate of serious adverse effects that undermine clinical therapeutics [Citation17,Citation18]. Several studies have analysed genetic polymorphisms of the CYP2C19 gene in Chinese populations, but no study has focused on Han populations from Guangdong Province. Therefore, our results provide new data on the CYP2C19 allele frequency that could help to establish a new database for functional research and guide medicine for the Han populations of Guangdong. The CYP2C19 allele and genotype frequencies in all subjects were in Hardy-Weinberg equilibrium (χ2 = 8.00, P > 0.05).
In our current study, a total of three different alleles and six genotypes were detected. The CYP2C19*1 allele (wild-type) frequency in the Foshan population was lower than that in Caucasian populations and Asian populations [Citation19–26]. According to previous studies, the highest CYP2C9*2 allele frequency was found in Asians (21.0–49.39%) [Citation18,Citation27–32]; however, the lowest frequency was found in Caucasians (2.9–14%) [Citation19–23]. The CYP2C19*2 allele frequency in the subjects of our study was higher than that reported for Caucasians. Interestingly, the CYP2C19*2 allele frequency in our group (30.46%) was closer to those of Hakka (31.06%) and Chinese-Dai (30%) populations [Citation27,Citation28]. As shown in , the CYP2C19*2 allele frequency in our group was closer to that reported for other Asian populations.
The CYP2C9*3 allele frequency shows an increasing order in different groups of subjects as follows: Caucasians (0.0–0.3%) [Citation19–23], Africans (0.0–2.0%) [Citation23–25], Asians (1.0–11.1%) [Citation18,Citation27–32]. The CYP2C19*3 allele frequency was reported to be 5.65% in the Foshan population, which is consistent with those in other Chinese populations [Citation28–30]. However, the frequency of the CYP2C19*3 variant in our population was lower than has been reported in the Japanese, Korean, Vietnamese, and Thai populations [Citation31–33]. In addition, the CYP2C19 *2 and CYP2C19 *3 alleles cause diverse responses to medications such as clopidogrel; therefore, clinicians should be cautious when using clopidogrel in populations with a high loss-of-function allele frequency, especially in China.
Recent reports have provided evidence that the polymorphisms of CYP2C19 might affect its activity in the metabolization of related drugs. Interindividual differences in enzyme activity divide the population into EM, IM and PM phenotypes [Citation9,Citation10]. In our current study, the proportion of the IM phenotype (44.76%) was slightly higher than that of the EM phenotype (41.51%). The proportion of the PM phenotype was 13.73%, which is closer to that recorded in other Asian populations. Similar to findings in the Hakka population, the CYP2C19*2 allele was the most important variation in our group and was chiefly responsible for the PM phenotype, accounting for 80.48% of the study population.
In summary, our study provides the CYP2C19 genetic polymorphisms present in the Foshan population. The CYP2C19 allele frequencies and genotype frequencies were compared with those of other populations. This study demonstrates that the CYP2C19 allele frequency in the Foshan population is closest to that of Hakka populations and is distinct from that of African and Caucasian populations. In conclusion, our study provides important information about CYP2C19 polymorphisms in the Han population of Guangdong.
Abbreviations
CI: confidence interval; EM: extensive metabolizer; IM: intermediate metabolizer; PM: poor metabolizer; PCR: POLYMERASE chain reaction.
Author contributions
GXW, ZXW and SYY coordinated and performed all sample analyses. XWY, and ZYG drafted and revised the manuscript. All the authors read and approved the final manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to protecting the participants’ anonymity but are available from the corresponding author on reasonable request.
Acknowledgements
We give special thanks to the staff for their help in carrying out this study. We also thank the staff of the American Journal Experts language service for their academic editing services.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305.
- Sabatini MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(12):1179–1189.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49(14):1505–1516.
- Scott SA, Sangkuhl K, Gardner EE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90(2):328–332.
- Zheng X, Fang P, Bao SS, et al. Function of 38 variants CYP2C9 polymorphism on ketamine metabolism in vitro. J Pharmacol Sci. 2017;135:8–13.
- Chhonker YS, Chandasana H, Bala V, et al. In-vitro metabolism, CYP profiling and metabolite identification of E- and Z- guggulsterone, a potent hypolipidmic agent. J Pharm Biomed Anal. 2018;160:202–211.
- Scott SA, Sangkuhl K, Shuldiner AR, et al. PharmGKB summary: Very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 2012;22(2):159–165.
- Eltalal S, Ayouty ME, El-Said A, et al. CYP2C9 (*2&*3) and CYP2C19 (*2&*3) polymorphisms among children. Acta Neurol Belg. 2021;121(6):1623–1631.
- Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–2247.
- Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI. JAMA. 2010;304(16):1821–1830.
- PharmaVar. Pharmacogene Variation Consortium; 2021. Available from: https://www.pharmvar.org/gene/CYP2C19.
- Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration. J Am Coll Cardiol. 2010;56(2):134–143.
- Sorich MJ, Vitry A, Ward MB, et al. Prasugrel vs. clopidogrel for cyto-chrome P450 2C19-genotyped subgroups: Integration of the TRITON-TIMI38 trial data. J Thromb Haemost. 2010;8(8):1678–1684.
- Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; Writing Committee Members, et al. ACCF/AHA Clopidogrel clinical alert: Approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122(5):537–557.
- Shuldiner DAR, O’Connell DJR, Bliden MKP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857.
- Kobori L, Kohalmy K, Porrogi P, et al. Drug-induced liver graft toxicity caused by cytochrome P450 poor metabolism. Br J Clin Pharmacol. 2008;65(3):428–436.
- Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15(12):1929–1937.
- Shimatani T, Inoue M, Kuroiwa T, et al. Effect of omeprazole 10 mg on intragastric pH in three different CYP2C19 genotypes, compared with omeprazole 20 mg and lafutidine 20 mg, a new H2-receptor antagonist. Aliment Pharmacol Ther. 2003;18(11–12):1149–1157.
- Yamada H, Dahl ML, Lannfelt L, et al. CYP2D6 and CYP2C19 genotypes in an elderly Swedish population. Eur J Clin Pharmacol. 1998;54(6):479–481.
- Gaikovitch EA, Cascorbi I, Mrozikiewicz PM, et al. Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol. 2003;59(4):303–312.
- Scordo MG, Caputi AP, D’Arrigo C, et al. Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res. 2004;50(2):195–200.
- Bravo-Villalta HV, Yamamoto K, Nakamura K, et al. Genetic polymorphism of CYP2C9 and CYP2C19 in a bolivian population: an investigative and comparative study. Eur J Clin Pharmacol. 2005;61(3):179–184.
- Halling J, Petersen MS, Damkier P, et al. Polymorphism of CYP2D6, CYP2C19, CYP2C9 and CYP2C8 in the Faroese population. Eur J Clin Pharmacol. 2005;61(7):491–497.
- Herrlin K, Massele AY, Jande M, et al. Bantu Tanzanians have a decreased capacity to metabolize omeprazole and mephenytoin in relation to their CYP2C19 genotype*. Clin Pharmacol Ther. 1998;64(4):391–401.
- Persson I, Aklillu E, Rodrigues F, et al. S-mephenytoin hydroxylation phenotype and CYP2C19 genotype among ethiopians. Pharmacogenetics. 1996;6(6):521–526.
- Masimirembwa C, Bertilsson L, Johansson I, et al. Phenotyping and genotyping of S-mephenytoin hydroxylase (cytochrome P450 2C19) in a shona population of Zimbabwe*. Clin Pharmacol Ther. 1995;57(6):656–661.
- Zhong Z, Hou J, Li B, et al. Analysis of CYP2C19 genetic polymorphism in a large Ethnic Hakka population in Southern China. Med Sci Monit. 2017;23:6186–6192.
- He N, Yan FX, Huang SL, et al. CYP2C19 genotype and S-mephenytoin 4′-hydroxylation phenotype in a Chinese Dai population. Eur J Clin Pharmacol. 2002;58(1):15–18.
- Ding Y, Xu D, Zhang X, et al. Genetic polymorphisms and phenotypic analysis of drug-metabolizing enzyme CYP2C19 in a Li Chinese population. Int J Clin Exp Pathol. 2015;8(10):13201–13208.
- Zuo LJ, Guo T, Xia DY, et al. Allele and genotype frequencies of CYP3A4, CYP2C19, and CYP2D6 in Han, Uighur, Hui, and Mongolian Chinese populations. Genet Test Mol Biomarkers. 2012;16(2):102–108.
- Kimura M, Ieiri I, Mamiya K, et al. Genetic polymorphism of cytochrome P450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monit. 1998;20(3):243–247.
- Yamada S, Onda M, Kato S, et al. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol. 2001;36(10):669–672.
- Sukasem C, Tunthong R, Chamnanphon M, et al. CYP2C19 polymorphisms in the Thai population and the clinical response to clopidogrel in patients with atherothrombotic-risk factors. Pharmgenomics Pers Med. 2013;6:85–91.