Abstract
Interactions between three genotypes (Ljsym 71-1, Ljsym 71-2 and Ljsym 72) of Lotus japoicus and one isolate from each of four species of arbuscular mycorrhizal fungi (Glomus sp. R-10, Glomus intraradices, Glomus etunicatum, and Gigaspora margarita) were investigated and compared with the wild-type ‘Gifu’ B-129. All the three genotypes showed no or defective internal colonization after inoculation with these AM fungi. In Ljsym72 mutant, the AM fungi produced deformed appressoria on the root surface, but failed to form any internal structures (internal hyphae, arbuscules and vesicles) except only in Glomus intraradices. The Ljsym71-1 and Ljsym71-2 mutants had more deformed appressoria and occasionally formed internal hyphae, arbuscules and vesicles, depending on AM fungi used. Wild-type ‘Gifu’ (nod+myc+) plants had typical colonization. The colonization of mutants by several fungi varied and provides a basis for studying recognition and compatibility between plants and mycorrhizal fungal species. These mutants also will be useful in studies of the genetics of the symbiosis between plant species and AM fungi.
Introduction
Arbuscular mycorrhizal (AM) fungi are obligate biotrophic organisms that are estimated to live symbiotically with the roots of about 80% terrestrial plant species (Brundrett & Abbott Citation2002). The establishment of functional symbioses between AM fungi and host plants involves sequences of recognition events and have several development phases: Spore germination, hyphal growth, appressorium formation, intraradical penetration and intraradical growth up to the formation of arbuscules (Smith & Read Citation1997). The growth of the fungi are triggered by host signals which induce changes in gene expression and a process leading to unequivocal recognition between the two partners of the symbiosis (Giovannetti & Sbrana Citation1998).
In this symbiosis genetic control exercised by each symbiont is poorly understood. The use of plant mutants in mycorrhizal research has potential to increase contribution to this area of research (Bradbury et al. Citation1991, Duc et al. Citation1989, Wegel et al. Citation1998, Wyss et al. Citation1990). Duc et al. (Citation1989) isolated mycorrhizal (myc−) mutants of pea (Pisum sativum L.) and faba bean (Vicia faba L.) that were defective in colonization. These mutants were also unable to form functional root nodules (nod−). Subsequently, Gianinazzi-Pearson et al. (Citation1991) reported that the myc− mutants have aborted infections. In contrast, Wyss et al. (Citation1990) reported that nod− mutants of soybean were colonized by Glomus mossae to the same extent as wild-type (nod+) soybean plants. Marsh and Schlutze (Citation2001) summarized most of the mycorrhizal mutants screened primarily from a collection of nodulation mutants in several host plants including a non-nodulating tomato mutant rmc (Barker et al. Citation1998). Gao et al. (Citation2001) showed that some of AM fungi can colonize the tomato mutant rmc but some others did not.
The myc− mutants should be screened against different populations of AM fungi in a range of soils to see whether resistance is horizontal or if some fungal strains can overcome resistance as in the case of certain nod− plants in the presence of different Rhizobium populations (Lie & Timmermans Citation1983). This would provide a new tool for exploring the genetic variability in AM fungi and would open the possibility of controlling plant-fungus specificity in the presence of mixed fungal populations in field soils.
The fully sequenced model plant Arabidopsis thaliana is unable to establish either mycorrhizal and rhizobial symbioses. Recently two model legumes Medicago truncatula and Lotus japonicus were used by plant scientists (Cook Citation1999, Kawaguchi et al. Citation2002, Schauser et al. Citation1998, Senoo et al. Citation2000, Solaiman et al. Citation2000, Stougaard Citation2001, Parniske Citation2004). M. truncatula and L. japonicus are diploid and carry significant advantages over alternative legumes for studying plant-mycorrhizal interactions (Handberg & Stougaard Citation1992, Harrison Citation1999, Jiang & Gresshoff Citation1997), because these are diploid having relatively smaller genomes and efficient transformation system. In other legumes, genetic analysis remains difficult due to features such as polyploidy, large genomes, few mapped genes and/or the lack of efficient methods for transformation. In L. japonicus, nodulation mutants were obtained by ethyl-methane sulfonate (EMS)-mutagenesis followed by backcrossing with parent ‘Gifu’ B-129 plants (Kawaguchi et al. Citation2002) and these mutants were screened for mycorrhizal phenotypes (Senoo et al. Citation2000, Solaiman et al. Citation2000). We screened three symbiotic mutants characterized and categorized (Senoo et al. Citation2000). But this screening was carried out using only one AM fungal strain, Glomus sp. R-10. Therefore, the present research was designed to study these defective mutants including wild-type parent inoculating with four different AM fungi (Glomus sp. R-10, Glomus etunicatum, Glomus intraradices, and Gigaspora margarita).
Materials and methods
Plant material
Screening for phenotypes defective in stages of mycorrhizal colonization was carried out using a population of Lotus japonicus B-129 ‘Gifu’ mutants showing non-nodulating, ineffectively nodulating and hypernodulating phenotypes, mutagenized by EMS-treatment (Imaizumi-Anraku et al. Citation1997, Senoo et al. Citation2000). These mutant lines are extensively characterized (Kawaguchi et al. Citation2002). The Ljsym72, Ljsym71-1 and Ljsym71-2 mutants are non-nodulating (nod−) mutants in which the initial stage of nodulation prior to nodule primordia formation is blocked. All of the mutated loci were confirmed to be monogenic and recessive. Mycorrhizal characteristics and phenotypes of these mutants were reported earlier (Senoo et al. Citation2000) and it was confirmed that these are myc− mycorrhizal mutants when inoculated with a Glomus sp. R-10 (it is an unknown species of Glomus named as a Glomus sp. R-10 for commercial use by Idemitsu Kosan Co. Ltd., Japan).
Potting medium and inoculum
Washed sand was mixed with Akadama soil (subsoil of volcanic ash soil, Kodaira Engei Shizai Co., Ltd., Japan) in equal volumes, sterilized by autoclaving at 121°C for 1 h and amended with NH4NO3, KH2PO4 and KCl at the rate of 0.53 g, 0.027 g, and 0.107 g l−1 mix, respectively. Arbuscular mycorrhizal fungal inoculum containing spores of Glomus sp. R-10 was mixed well with the potting mixture (1:10, v/v). Glomus etunicatum was obtained from Dr Tatsuhiro Ezawa of Nagoya University, Japan. Glomus intraradices and Gigaspora margarita were obtained from Dr Masanori Saito of National Grassland Research Institute, Japan. These inocula were also mixed well with potting mixture (1:10, v/v).
Screening procedure
Seeds from wild-type L. japonicus ‘Gifu’ and symbiotic (nod−myc−) mutants (Ljsym72, Ljsym71-2, and Ljsym71-1) were scratched with sand paper, surface sterilized by 2% sodium hypochlorite containing 0.02% Tween 20 for 10 min, rinsed in sterile distilled water and then germinated on sterilized moist filter paper in Petri dishes at 25°C under dark conditions. After germination, the seedlings were transplanted individually in nursery trays containing a sand-soil-inoculum mixture. Seedlings of ‘Gifu’ were grown without inoculum to check the contamination of pathogens in the sterilized mix, if any. Plants were grown for three months in a growth chamber (day: 20 h, 25°C, photosynthetic photon flux density of 45 µmol m−2 s−1; night: 4 h, 22°C). After 30 days, the light intensity of the growth chamber was changed to 70 µmol m−2 s−1. Sampling was done at 90 days after transplanting of seedlings for assessment of mycorrhizal colonization.
Evaluation of mycorrhizal colonization
Root samples were cleared in 10% KOH and stained with trypan blue using a modification of the method of Phillips and Hayman (Citation1970), in which lactoglycerol was used instead of lactophenol. Colonization in the inoculated control ‘Gifu’ B-129 and mutant plant was determined as the percentage of root length colonized, under a dissecting microscope following the grid-line intersect method (Tennant, Citation1975). The detailed assessment of colonization in the mutants was carried out by the method of McGonigle et al. (Citation1990). Briefly, cleared and stained whole root segments were mounted on slides. Intersects between the roots and an ocular crosshair were scored for the presence of different mycorrhizal structures at ×100 magnification. Five to 10 fields were counted for each slide. The evaluation of mycorrhizal colonization was carried out in three replicated samples (each replicate contained a mixture of six root systems). Results were expressed as the percent of intersects having external hyphae, appressoria, internal hyphae, arbuscules, and vesicular colonization.
Statistical analysis
All the data were the mean of 3 replications and two-way analysis of variance was carried out to determine whether the effects of the mutants, fungal species and interactions between them were significant in Lotus japonicus. The percentages of colonization data were arcsine transformed before analysis of ANOVA. Least significant difference (LSD) test was used to separate the treatment means.
Results
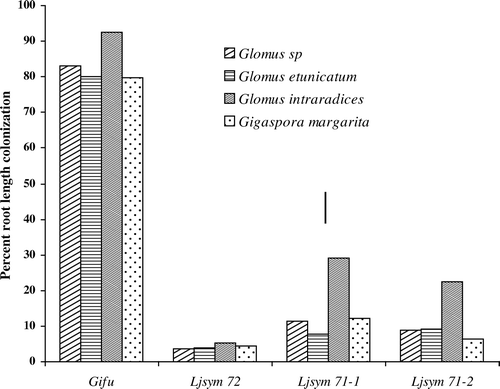
Root length colonized by four different AM fungi
Light microscopic observation following staining of the roots with trypan blue indicated that mycorrhizal colonization of wild-type ‘Gifu’ by Glomus sp., Glomus etunicatum, Glomus intraradices, and Gigaspora margarita was typical and was significantly higher than colonization in mutants (). The colonization in mutant was only by extraradical and intraradical hyphae. Mycorrhizal colonization processes were observed step by step including appressoria formation, hyphal penetration and development of internal hyphae and arbuscules. Glomus and Gigaspora produced vesicles or auxiliary cells, respectively. The percentage of the root length colonized by the fungi was 83% (Glomus sp.), 79% (Glomus etunicatum), 93% (Glomus intraradices), and 76% (Gigaspora margarita). The significantly higher colonization by Glomus intraradices was observed in the wild-type compared to the other three fungi. All three mutants (Ljsym71-1, Ljsym71-2 and Ljsym72) showed highly reduced colonization compared to wild-type ‘Gifu’ after inoculation with four different fungi. G. intraradices also produced higher colonization compared to other three fungi, in Ljsym71-2 and Ljsym71-1 mutants.
Detailed quantitative analysis of colonized roots
The quantitative measurement of structural components of all four AM fungi in roots were carried out and analysis of variance data is presented in . All the structural components of colonization were significantly influenced by different mutants except external hyphae. In Ljsym72, there are no arbuscules and vesicle formation observed. Some arbuscules and vesicles were observed in Ljsym71-1 and Ljsym71-2 mutants. Ljsym72 had no internal hyphae except with G. intraradices whereas some were present in Ljsym71-1 and Ljsym71-2. All mutants including wild-type had similar development of external hyphae. Appressoria formation was lower in Ljsym72 compared to that in Ljsym71-1 and Ljsym71-2.
Table I Percentage root length colonized by external hyphae, appressoria, internal hyphae, arbuscules and vesicles of four different AM fungi.
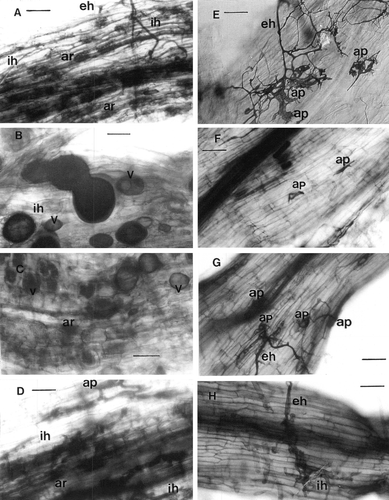
Phenotypic observation of mycorrhizal mutants
Typical colonization by four different AM fungi in wild-type ‘Gifu’ root was observed (A–2D). The hyphal colonization in the root of Ljsym72 was greatly reduced (E–2H), and most hyphae grew on the root surface in the form of runner hyphae with distinctive branching. Appressoria were visible on the root surface but they were extremely abnormal in shape, showing extraordinary branching and swelling (E–2F). Following appressoria formation, hyphae attempted to enter the root exodermis (the cortical cell layer adjacent to epidermis), but they were halted at root epidermis where abnormal branching and swelling developed. No arbuscules or vesicles were present in any root of Ljsym72. There are some intraradical hyphae produced by Glomus intrardices (H).
Figure 2. Light microscopy of cleared and trypan blue stained roots of wild-type ‘Gifu’ (Figures A–D) and mutant Ljsym 72 (Figures E–H) of Lotus japonicus, colonized by Glomus sp. R-10 (A, E), Glomus etunicatum (B, F), Glomus intraradices (C, G) and Gigaspora margarita (D, H). eh, external hypahe, ap, appressoria, ih, internal hyphae, ar, arbuscules, v, vesicles. Bar represents 50 µm.
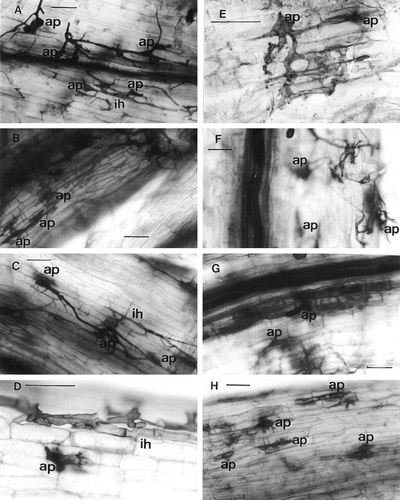
In Ljsym71-1 and Ljsym71-2 mutants, hyphal colonization of the root was significantly reduced compared with the wild-type (). The development of deformed appressoria followed by aborted internal hyphae were often observed. Several types of irregularly shaped appressoria were observed on the root surface or along the adjacent walls of epidermal cells, showing complex branching, enlarged, and swollen appearance (A–3D). Also, the number of appressoria formed on the root surface was significantly higher than those on the wild-type root. Elongation of internal hyphae from the appressoria was observed, but they were aborted soon after penetration within one or two cell layers, sometimes showing a swollen appearance (C). Most often, the mycorrhizal colonization was arrested at this stage. Colonization by internal hyphae at the root cortex was observed at a low frequency, but rather complex branching or swelling of hyphae were visible (B). Only a limited number of hyphae was developed to form arbuscules, and the morphology of the arbuscules occasionally appeared to be degenerated (G). The amount of internal hyphae seemed to be greater in Ljsym71-1 than in Ljsym71-2 (A–3H) but it showed no significant differences when measured quantitatively ().
Figure 3. Light microscopy of cleared and trypan blue stained roots of mutant Ljsym 71-1 (Figures A–D) and mutant Ljsym 71-2 (Figures E–H) of Lotus japonicus, colonized by Glomus sp. R-10 (A, E), Glomus etunicatum (B, F), Glomus intraradices (C, G) and Gigaspora margarita (D, H). ap, appressoria, ih, internal hyphae. Bar represents 50 µm.
Discussion
The results of this study clearly indicated that mycorrhizal mutants of Lotus japonicus obtained by EMS-mutagenesis are stable and showed almost similar defective colonization patterns when inoculated with several AM fungi. The Ljsym72 mutant is very resistant and showed no colonization in roots when inoculated with different fungi except with G. intraradices. The Ljsym71-2 and Ljsym71-1 mutants showed slight variation in percent root colonization by internal hyphae. Duc et al. (Citation1989) reported that all the ineffectively nodulating pea mutants (nod+fix−) could form some internal mycorrhizal structures. In our experiment, we did not find any internal structures in the Ljsym72 mutant except with G. intraradices but Ljsym71 had some defective internal colonization.
We further report the identification of three mutants affecting arbuscular mycorrhizal colonization in a model legume plant Lotus japonicus. They are apparently recessive and markedly reduce the mycorrhizal colonization when inoculated with four fungal species representing the two genera of arbuscular mycorrhizal fungi (Glomus and Gigaspora). The identification of mutations affecting mycorrhizal establishment indicated that some of the genes involved are neither genetically redundant nor essential for plant function, reflecting the fact that the symbiosis is not genetically obligate for these plants and this has also been reported in tomato (Barker et al. Citation1998).
The mutation of Ljsym72 blocked colonization at the root surface, in a similar way to the reduced mycorrhizal colonization (rmc) in tomato (Barker et al. Citation1998) and myc−1 mutants in pea (Pisum sativum L.) (Duc et al. Citation1989), in Phaseolus vulgaris L. (Shirtliffe & Vessey Citation1996) and in the model legumes Medicago truncatula (Gaertn.) (Sagan et al. Citation1995). Colonization in Ljsym72 is normally blocked at the surface of the root so that it is apr+pen− in the developmental framework proposed by Smith (Citation1995) and it has been reported that a mutant Ljsym15 showed responsible for surface opening and arbuscules formation (Demchenko et al. Citation2004). At this stage, we do not know they are alleles to Ljsym72 or not. But it is necessary to carry out an allelic test among the Lotus japonicus mutants already available in several countries.
An important feature of Ljsym72 was that no formation of internal hyphae, arbuscules or vesicles occurred with three fungal strains tested and has some internal hyphae with G. intrardices. The Ljsym72 mutant can be classified as mcbep (mycorrhizal colonization blocked at epidermis) irrespective of fungal strain tested. In the case of Ljsym71-1 and 71-2, an important feature was that colonization by internal hyphae, arbuscules and vesicles were greatly reduced. These mutants showed arrested hyphal penetration at root exodermis (the cortical cell layer adjacent to epidermis), produced low amount of internal structures and these can be categorized that the two mutants are truly mcbex (mycorrhizal colonization blocked at exodermis), irrespective of the fungal strains tested. These features are similar to those of the Ljsym4-1 and Ljsym4-2 mutants in which fungal infection attempts generally aborted within the epidermis (Bonfante et al. Citation2000).
In conclusion, the Ljsym72, 71-1, and 71-2 can be categorized as mcbep and mcbex, respectively even when tested with different fungal strains. We confirmed that these phenotypes were true because we adopted a rather long period of plant growth (3 months) in our screening procedure. Other research groups observed mycorrhizal phenotype within shorter periods of plant growth, and the defined phenotype was sometimes altered at the later stage, as shown by Wegel et al. (Citation1998). The mcbep phenotype is the earliest blockage against AM colonization among the mutants that have been reported. Cloning of the responsible gene is essential. Isolation and cloning of the gene will allow us to understand its function, and probe a range of hosts to determine its distribution and expression. The complexity of this symbiosis makes genetic approaches essential, and the identification of more interesting plant mutants, particularly those that are specific for the mycorrhizal symbiosis, should be emphasized in the future (Harrison Citation1999). Mycorrhizal mutants will play a pivotal role in improving our understanding of the plant controls at various stages of development of the mycorrhizal symbiosis (Parniske Citation2004).
The authors thank Dr Motoshi Suzuki and Mr Akihiko Narutaki of Idemitsu Kosan Co, Ltd., Tokyo, Japan for supplying inoculum of Glomus sp. R-10. We also thank Dr Masayoshi Kawaguchi of Tokyo University for supplying mutant seeds. ZS is grateful to the Japan Society for the Promotion of Science (JSPS) for providing a postdoctoral fellowship and research grant. This study was also partly supported by grant-in-aid for Scientific Research on Priority Areas (Grant no. 10182102). The authors are grateful to Prof Lyn Abbott and Dr Susan Barker at the University of Western Australia for critical reading of the manuscript.
References
- Barker , SJ , Stummer , B , Gao , L , Dispain , I , O'Conor , PJ and Smith , SE . 1998 . A mutant in Lycopersicon esculentum Mill. with highly reduced VA mycorrhizal colonisation: Isolation and preliminary characterization . Plant J , 15 : 791 – 797 .
- Bonfante , P , Genre , A , Faccio , A , Martin , I , Schauser , L , Stougaard , J , Web , J and Parniske , M . 2000 . The Lotus japonicus Ljsym4 gene is required for the successful symbiotic infection of root epidermis cells . Mol Plant Microbe Interaction , 13 : 1109 – 1120 .
- Bradbury , SM , Peterson , RL and Bowley , SR . 1991 . Interactions between three alfalfa nodulation genotypes and two Glomus species . New Phytol , 119 : 115 – 120 .
- Brundrett , MC and Abbott , LK . 2002 . “ Arbuscular mycorrhizas in plant communities ” . In Microorganisms in plant conservation and biodiversity , Edited by: Sivasithamparam , K , Dixon , KW and Barrett , RL . 151 – 193 . Dordrecht : Kluwer Academic Publishers .
- Cook , DR . 1999 . Medicago truncatula: A model in the making! . Curr Opin Plant Biol , 2 : 301 – 304 .
- Demchenko , K , Winzer , T , Stougaard , J , Parniske , M and Pawlowski , K . 2004 . Distinct roles of Lotus japonicus SYMRK and SYM15 in root colonization and arbuscule formation . New Phytol , 163 : 381 – 392 .
- Duc , G , Trouvelot , A , Gianinazzi-Pearson , V and Gianinazzi , S . 1989 . First report of non-mycorrhizal plant mutants (myc−) obtained in pea (Pisum sativum L.) and faba bean (Vicia faba L.) . Plant Sci , 60 : 215 – 222 .
- Gao , LL , Delp , G and Smith , SE . 2001 . Colonisation patterns in a mycorrhiza-defective mutant tomato vary with different arbuscular-mycorrhizal fungi . New Phytol , 151 : 477 – 491 .
- Gianinazzi-Pearson , V , Gianinazzi , S , Guillemin , JP , Trouvelot , A and Duc , G. 1991 . “ Genetic and cellular analysis of resistance of vesicular-arbuscular mycorrhizal fungi in pea mutants ” . In Advances in molecular genetics of plant-microbe interactions , Edited by: Hennecke , H and Varma , DPS . 336 – 342 . Dordrecht : Kluwer Academic Publishers .
- Giovannetti , M and Sbrana , C . 1998 . Meeting a non-host: The behaviour of AM fungi . Mycorrhiza , 8 : 123 – 130 .
- Handberg , K and Stougaard , J . 1992 . Lotus japnicus, an autogamous, diploid legume species for classical and molecular genetics . Plant J , 2 : 487 – 496 .
- Harrison , MJ . 1999 . Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis . Ann Rev Plant Physiol Plant Mol Biol , 50 : 361 – 389 .
- Imaizumi-Anraku , H , Kawaguchi , M , Koiwa , H , Akao , S and Syono , K . 1997 . Two ineffective-nodulating mutants of Lotus japonicus – different phenotypes caused by the blockage of endocytotic bacterial release and nodule maturation . Plant Cell Physiol , 38 : 871 – 881 .
- Jiang , Q and Gresshoff , PM . 1997 . Classical and molecular genetics of the model legume Lotus japonicus . Mol Plant-Microbe Interact , 10 : 59 – 68 .
- Kawaguchi , M , Imaizumi-Anraku , H , Koiwa , H , Niwa , S , Ikuta , A , Syono , K and Akao , S . 2002 . Root, root hair, and symbiotic mutants of the model legume Lotus japonicus . Mol Plant-Microbe Interact , 15 : 17 – 26 .
- Lie , TA and Timmermans , PCJM . 1983 . Host-genetic control of nitrogen fixation in the legume-Rhizobium symbiosis: Complication in the genetic analysis due to material effects . Plant Soil , 75 : 449 – 453 .
- Marsh , JF and Schultze . 2001 . Analysis of arbuscular mycorrhizas using sysmbiosis-defective plant mutants . New Phytol , 150 : 525 – 532 .
- McGonigle , T , Miller , MH , Evans , DG , Fairchild , GL and Swan , JA . 1990 . A new method which gives an objective measure of colonisation of roots by vesicular-arbuscular mycorrhizal fungi . New Phytol , 115 : 495 – 501 .
- Parniske , M . 2004 . Molecular genetics of the arbuscular mycorrhizal symbiosis . Curr Opinion Plant Biol , 7 : 414 – 421 .
- Phillips , JM and Hayman , DS . 1970 . Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection . Trans Br Mycol Soc , 55 : 158 – 160 .
- Sagan , M , Morandi , D , Tarenghi , E and Duc , G . 1995 . Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis . Plant Sci , 111 : 63 – 71 .
- Senoo , K , Solaiman , MZ , Kawaguchi , M , Imaizumi-Anraku , H , Akao , S , Tanaka , A and Obata , H . 2000 . Isolation of two different phenotypes of mycorrhizal mutants in the model legume plant Lotus japonicus after EMS-treatment . Plant Cell Physiol , 41 : 726 – 732 .
- Schauser , L , Handberg , K , Sandal , N , Stiller , J , Thykjaer , T , Pajuelo , E , Nielsen , A and Stougaard , J . 1998 . Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus . Mol Gen Genet , 259 : 414 – 423 .
- Shirtliffe , SJ and Vessey , JK . 1996 . A nodulation (nod + fix− mutant of Phaseolus vulgaris L. has nodule like structures lacking peripheral vascular bundles (Pvb−) and is resistant to mycorrhizal infection (myc−) . Plant Sci , 118 : 209 – 220 .
- Smith , SE . 1995 . “ Discoveries, discussions and directions in research on mycorrhizae ” . In Mycorrhiza, structure, function, molecular biology and biotechnology , Edited by: Varma , A and Hock , B . 3 – 24 . Berlin : Springer Verlag .
- Smith SE , Read DJ . 1997 . Mycorrhizal symbiosis . 2nd ed. London : Academic Press .
- Stougaard , J . 2001 . Genetics and genomics of root symbiosis . Curr Opin Plant Biol , 4 : 328 – 335 .
- Solaiman , MZ , Senoo , K , Kawaguchi , M , Imaizumi-Anraku , H , Akao , S , Tanaka , A and Obata , H . 2000 . Characterization of mycorrhizas formed by Glomus sp. on roots of hypernodulating mutants of Lotus japonicus . J Plant Res , 113 : 443 – 448 .
- Tennant , D . 1975 . A test of a modified line intersect method of estimating root length . J Ecol , 63 : 995 – 1001 .
- Wegel , E , Schauser , L , Sandal , N , Stougaard , J and Parniske , M . 1998 . Mycorrhiza mutants of Lotus japonicus define genetically independent steps during symbiotic infection . Mol Plant-Microbe Interact , 11 : 933 – 936 .
- Wyss , P , Mellor , RB and Wiemken , A . 1990 . Vesicular-arbuscular mycorrhizas of wild-type soybean and non-nodulating mutants with Glomus mossae contain symbiosis-specific polypeptides (mycorrhizins), immunologically cross-reactive with nodulins . Planta , 182 : 22 – 26 .