Abstract
Coffee senna (Senna occidentalis L.) plants were subjected to five phosphorus levels: 0, 25, 50, 75 and 100 mg P per kg soil (P0, P1, P2, P3 and P4, respectively). A pot culture experiment was conducted in a net house, AMU, Aligarh, India, under phosphorus-deficient soil. The present data indicates that soil-applied phosphorus significantly ameliorates most of the attributes studied. Out of five phosphorus levels, 75 mg P per kg soil (P3) proved best and enhanced fresh and dry weights, total chlorophyll and carotenoid content, nitrate reductase activity and leaf-NPK and Ca content, analyzed at 120, 270 and 300 days after sowing (DAS). The number of pods, seed-yield per plant and seed-protein content (330 DAS) were significantly enhanced by the P3 level, except the number of seeds per pod, 100-seed weight and total anthraquinone glycosides content, respectively. Transpiration rate, stomatal conductance and net photosynthetic rate were also enhanced by this treatment.
Introduction
Coffee senna (Senna occidentalis L.) commonly known as ‘Badi Kasondi’, belongs to the family Fabaceae. It is an erect, foetid annual herb or under shrub, 60–150 cm in height. Leaves, 15–20 cm long, leaflets are ovate to ovate-lanceolate. The pods are brown, flat, slightly curved and 5–12 cm long. It contains 40 or more brown to dark-olive green, hard, shining, ovoid seeds about 4 mm long (The Wealth of India Citation1992). It is grown throughout tropical and subtropical countries of the world including India, for its roots, flowers and seeds which have medicinal properties. It is a decongestant and used for the treatment of cough, whooping cough, convulsions, and heart disease (The Wealth of India Citation1992).
Anthraquinones () are an important group of natural products occurring in higher plants as glycosides. They are found in a large number of plant families including family Fabaceae. The leaves and seeds of coffee senna contain anthraquinone glycosides which are analgesic, antibacterial, anti-hepatotoxic, antifungal, anti-inflammatory, antiseptic, laxative and purgative. The plant parts (leaves and seeds) contain anticancer properties. It is also used for skin fungal diseases (Thomson Citation1987, Citation1996; Gritsanapan et al. Citation2005). Furthermore, the seeds have been used as a substitute for coffee (The Wealth of India Citation1992; Morris Citation1999). Medicinal herbs are used to combat illness and support the body's own defense to regain good health. However, the supply of these medicines lags behind their demand in the market. One of the best solutions to this problem is the cultivation of these plants on scientific lines. This would augment the yield and quality of these herbs ensuring their steady supply in the market. In fact, mineral nutrients applied basally or through foliar application, enhance the plant productivity and could be used as tools to ameliorate the quality of the herbal-medicines.
Most of the agricultural soils in India are phosphorus-deficient (Ghosh and Hassan Citation1977). The application of phosphorus fertilizers is used to overcome soil-phosphorus deficiency. However, plant biological yield appears to be comparatively low in Aligarh soils which are also phosphorus-deficient (Khan and Mohammad Citation2006). Our hypothesis was that the poor phosphorus level of the soil in this region may be one of the prime causes of the low productivity of coffee senna. Therefore, the present study was undertaken to test this hypothesis.
Materials and methods
Soil analysis
Prior to seed sowing, a 5.0 kg homogenous mixture of soil and farmyard manure (5:1) was collected. The physico-chemical characteristics of the soil tests were: texture-sandy loam, pH (1:2) −8.0, E.C. (1:2) −0.50 m mhos/cm, available N, P and K – 96.87, 7.68 and 149.8 mg per kg soil, respectively, and calcium carbonate 0.10%. These soil samples were tested at the Government Soil Testing Laboratory, Quarsi Farm, Aligarh.
Plant material
Healthy seeds of coffee senna were received from the USDA-ARS, Plant Genetic Conservation Resources Unit, Griffin, GA, USA. Healthy seeds of uniform size were selected, surface sterilized with 95% ethanol, washed in distilled water and sown at a depth of 2 cm in earthen pots containing sandy-loam soil.
Experimental design
A pot experiment was conducted in the net house at the Botany Department, A.M.U., Aligarh (27° 52′ N latitude, 78° 51′ E longitude, and 187.45 m altitude). The experiment was randomized complete block design using five levels of phosphorus, namely, 0, 25, 50, 75 and 100 mg P per kg soil (P0, P1, P2, P3 and P4, respectively) applied as potassium dihydrogen orthophosphate (KH2PO4). Each treatment was replicated three times and each replicate had two plants. The plants were irrigated as required. All the plants were arranged according to the experiment and grown in a greenhouse under sunlight.
Growth analysis
A simple pot experiment was designed in terms of the growth, physiological and biochemical, yield and quality attributes to analyze the complete life cycle of coffee senna. The plants were sampled at vegetative stage, 120 days after sowing (DAS), flowering stage (270 DAS) and fruiting stage (300 DAS), respectively. At all the three growth stages (120, 270 and 300 DAS), three plants from each treatment were harvested with the roots and washed with tap water to remove adhering foreign particles. Water adhering to the roots was removed with blotting paper and fresh weights were recorded. The plants were dried for 24 h in a hot air oven at 80°C. Dry weights were then recorded.
Yield analysis
For yield components (number of pods, number of seeds per pod, 100-seed weight and seed-yield), six plants from each treatment were collected at harvest (330 DAS). Pods were threshed and cleaned. The number of pods (per plant) and number of seeds per pod were recorded. The seeds were sun-dried to maintain a constant moisture content for recording the 100-seed weight. Seed-yield was calculated accordingly.
Physiological and biochemical analysis
Fresh leaves were used for the analysis of various physiological and biochemical attributes, namely, transpiration, stomatal conductance, net photosynthetic rates, total chlorophyll and carotenoid content, and nitrate reductase activity, respectively. However, NPK and Ca content were analyzed in dried leaves. All the physiological and biochemical attributes were measured at 120, 270 and 300 DAS, whereas photosynthesis was determined only at 270 DAS.
Determination of transpiration rate, stomatal conductance and net photosynthetic rate
Transpiration, stomatal conductance and net photosynthetic rates were measured on clear days at 11.00 a.m. on fully expanded leaves of coffee senna using a IRGA (Infra Red Gas Analyzer, LICOR 6200 Portable Photosynthesis System, Nebraska, USA). The IRGA was calibrated and zero was adjusted approximately every 30 min during the measurement period. Pre leaflet was enclosed in 1-l gas exchange chamber for 60 sec. These measurements were recorded three times in each treatment. Photosynthesis was measured only at 270 DAS.
Estimation of total chlorophyll and carotenoid content
Total chlorophyll and carotenoid content from fresh leaves were estimated using the method of Mac Kinney (Citation1941) and MaClachlan and Zalik (Citation1963), respectively. Fresh tissue from interveinal leaf areas was ground in a mortar and pestle in 80% acetone. The optical density (OD) of the solution was read at 645 and 663 nm for chlorophyll content and at 480 and 510 nm for caroteniod content estimation using a spectrophotometer (Spectronic 20D, Milton Roy, USA). Contents were expressed as mg g−1 FW.
Determination of nitrate reductase (NR) activity
The enzyme activity was estimated by the intact tissue method developed by Jaworski (Citation1971), which is based on the reduction of nitrate to nitrite as per the following biochemical reaction:
Nutrient analysis
Leaf samples of each treatment were digested (Lindner Citation1944) for the estimation of leaf-NPK and Ca contents. The leaves were dried in a hot air oven at 80°C for 24 h. Dried leaves were powdered and the powder was passed through a 72 mesh. The sieved powder was used for NPK and Ca content. One hundred mg of oven-dried leaf powder was carefully transferred to a digestion tube and 2 ml of AR Grade concentrated sulphuric acid was added to it. It was heated on a temperature controlled assembly for about 2 h. After heating, the contents of the tube turned black. It was cooled for about 15 min at room temperature and then 0.5 ml 30% hydrogen peroxide (H2O2) was added drop by drop. The addition of H2O2 followed by heating was repeated until the contents of the tube turned colorless. The prepared aliquot (peroxide-digested-material) was used to estimate NPK and Ca content.
Estimation of nitrogen content
Leaf-nitrogen content was estimated (Lindner Citation1944). The leaf-powder was digested in H2SO4 using a digestion tube. A 10 ml aliquot (peroxide-digested-material) was taken in a 50 ml volumetric flask. To this, 2 ml of 2.5 N sodium hydroxide and 1 ml of 10% sodium silicate solutions were added to neutralize the excess of acid and to prevent turbidity, respectively. In a 10 ml graduated test tube, 5 ml aliquot of this solution was taken and 0.5 ml Nessler’s reagent was added. The contents of the test tubes were allowed to stand for 5 min for maximum color development. The OD of the solution was read at 525 nm, using a spectrophotometer. The reading of each sample was compared with the standard calibration curve of ammonium sulphate to estimate the percentage nitrogen content.
Estimation of phosphorus content
The method of Fiske and Subba Row (Citation1925) was used to estimate the leaf-phosphorus content in the digested material. The same aliquot was used to determine the leaf-P content. A 5 ml aliquot was taken in a 10 ml graduated test tube. To it, 1 ml molybdic acid (2.5%) was added carefully, followed by addition of 0.4 ml 1-amino-2-naphthol-4-sulphonic acid. When the color turned blue, the volume was made up to 10 ml with the addition of double distilled water. The solution was shaken for 5 min. The OD of the solution was read at 620 nm using a spectrophotometer.
Estimation of potassium and calcium content
Potassium and calcium contents were analyzed with flame-photometrics. In the flame-photometer, the solution (peroxide-digested material) is discharged through an atomizer in the form of a fine mist into a chamber, where it is drawn into a flame. Combustion of the elements produces light of a particular wavelength (λ max for K = 767 nm (violet). The light produced was conducted through the appropriate filters to impinge upon a photoelectric cell that activates a galvanometer. Potassium and calcium contents were estimated in the same aliquot with the help of emission spectra using specific filters in a flame-photometer. Leaf-NPK and Ca contents were expressed in terms of percent dry weight.
Estimation of seed-protein content
Seed-protein was estimated using the method by Lowry et al. (Citation1951). The seed (50 mg) was ground into a powder using a mortar and pestle with 5% cold trichloroacetic acid (TCA). To it, 0.5 ml of Folin phenol reagent was added rapidly with immediate mixing. The content started turning blue. It was left for 30 min for maximum color development. Extracted protein was measured at 660 nm using a spectrophotometer. The reading was compared with a calibration curve obtained using a known dilution of standard egg albumin solution and percent seed-protein content was calculated on a dry weight basis.
Estimation of total anthraquinone glycosides content
Total anthraquinone glycosides content in seeds were analyzed spectrophotometrically according to the method given in Standard of ASEAN Herbal Medicine (ASEAN Countries Citation1993). The OD of the pink color developed was recorded at 515 nm and expressed as percentage dry weight.
Statistical analysis
Analysis of variance (ANOVA) was carried out and F-values were calculated. Least significant difference (LSD) was calculated at 5% according to Gomez and Gomez (Citation1984) and Duncan's Multiple Range Test (DMRT) was employed to separate means.
Results
Depending upon the growth stage of plants, various growth, physiological and biochemical, yield and quality attributes were recorded at 120, 270 and 300 DAS. The values for the optimum treatment (P3) studied at three growth stage were analyzed. We observed that at each stage (120, 270 and 300 DAS), values were highest for most of the attributes at 270 DAS.
Growth attributes
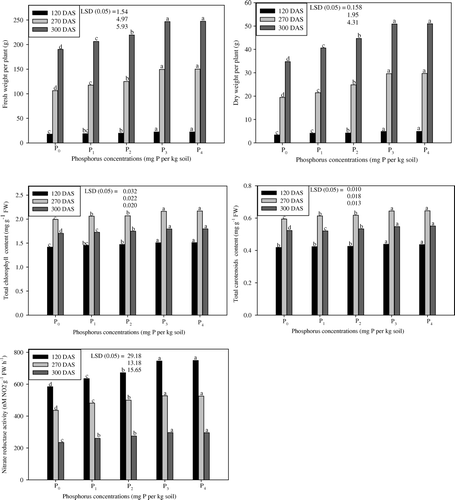
depicts the changes in fresh and dry weights of coffee senna at different phosphorus levels over their respective controls at 120, 270 and 300 DAS. Out of five levels of phosphorus, P3 proved optimum and found effective in increasing the fresh and dry weights by 40.5% and 52.2% at 270 DAS, respectively, when compared to the Control (p≤0.05). However, the effect of the P4 treatment was found equal with that of P3 at all three stages ().
Figure 2. Effect of five phosphorus levels (P0, P1, P2, P3 and P4) on fresh and dry weights per plant, total chlorophyll and carotenoid contents and nitrate reductase activity of coffee senna (Senna occidentalis L.) studied at 120, 270 and 300 DAS (means of three replicates). Duncan's Multiple Range Test (DMRT) was employed to separate the means with letters in the column (p≤0.05).
Yield attributes
The plants supplemented with phosphorus exhibited a significant response in yield attributes including number of pods and seed-yield, respectively. The pronounced effect of phosphorus was more prominent for the number of pods and seed-yield per plant as compared to the non-treated plants (see ). Treatments P3 and P4 produced the maximum number of pods and seed-yield per plant over P0. However, the number of seeds per pod and 100-seed weight were not influenced by the phosphorus treatments at 5% level (). The response of plants treated with phosphorus at P3 and P4 levels gave maximum increases of 41.2 and 35.5% for the number of pods and seed-yield per plant, respectively (). Moreover, the positive correlation of leaf-N (r=0.968**, 0.995**, 0.980**), leaf-P (r=0.987**, 0.993**, 0.985**), leaf-K (r=0.982**, 0.990**, 0.993**), leaf-Ca content (r=0.986**, 0.961**) as well as of dry weight (r=0.992**, 0.985**, 0.995**) at 120, 270 and 300 DAS and number of pods per plant (r = 0.976**) with seed-yield depict their contribution to seed-yield.
Table 1 Effect of five phosphorus levels (P0, P1, P2, P3 and P4) on yield attributes and seed protein and anthraquinone glycosides contents of coffee senna (Senna occidentalis L.) studied at 330 DAS (means of three replicates).
Physiological and biochemical attributes
Physiological and biochemical attributes namely, transpiration rate, stomatal conductance and net photosynthetic rate, total chlorophyll content, total carotenoid content, nitrate reductase activity, and leaf-NPK and Ca content were studied at 120, 270 and 300 DAS, respectively, and were found to be significantly increased by phosphorus application.
Transpiration rate, stomatal conductance and net photosynthetic rate
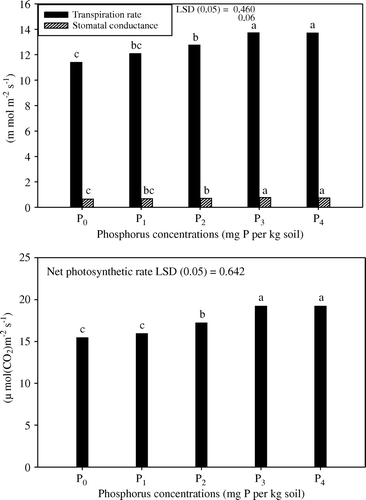
Data mentioned in indicate that the application of phosphorus significantly enhanced the transpiration rate, stomatal conductance and rate of photosynthesis at 270 DAS. Of the five levels, P3 proved optimum and accelerated the transpiration rate, stomatal conductance and rate of photosynthesis by 20.4, 18.6 and 24.5% only at 270 DAS in comparison with no phosphorus-supplied plants ().
Figure 3. Changes in transpiration rate, stomatal conductance and net photosynthetic rate in 270-days old plants of coffee senna (Senna occidentalis L.) subjected to five levels of phosphorus (P0, P1, P2, P3 and P4, respectively). Duncan's Multiple Range Test (DMRT) was employed to separate the means with letters in the column (p ≤ 0.05).
Chlorophyll and carotenoid content
The application of phosphorus to coffee senna plants favorably influenced the concentrations of the photosynthetic pigments (chlorophyll and carotenoid) at 120, 270 and 300 DAS (). The concentrations of chlorophyll and carotenoid content were higher as the phosphorus level increased. The total chlorophyll content was at a maximum in phosphorus-treated plants over the Control at all stages. P3 increased total chlorophyll content by 6.4, 8.5 and 5.4% at 120, 270 and 300 DAS, respectively. Like total chlorophyll content, total carotonoid content was also increased by phosphorus levels. P3 proved superior and gave higher value for total carotenoid content by 4.8, 8.4 and 4.6% at 120, 270 and 300 DAS, respectively, over P0. However, the value of P4 was equaled by P3 at all three stages ().
Nitrate reductase activity
Significant differences were found in the NR activity between the treatments (). The data revealed that phosphorus enhanced NR activity significantly over the Control plants at 120, 270 and 300 DAS. P3 generated maximum activity of 27.6, 20.8 and 26.1% at 120, 270 and 300 DAS, whereas, P0 gave the lowest activity. P4, however, gave equal value with that of treatment P3 ().
Nutrient elements
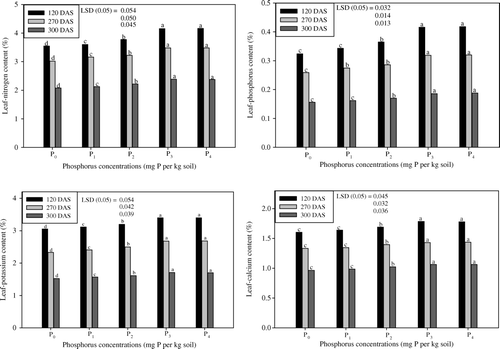
Phosphorus treatment caused a high significant increase in leaf-NPK and Ca content at all three stages (). The highest concentration of nitrogen content in the leaves was recorded for P3 and P4, while the lowest was for P0 (p≤0.05). Nitrogen content was higher using the application of P3 over P0. P3 increased the nitrogen content by 17.1, 15.3 and 14.8% at 120, 270 and 300 DAS, respectively, over the respective Control. Phosphorus at the level of P3 increased the phosphorus content in leaves at all three stages. The P3 treatment increased the phosphorus content at all stages over the Control by 28.4, 23.2 and 19.2% at 120, 270 and 300 DAS, respectively (). Where the potassium content was maximally affected by the P application, P3 gave the higher value (11.2, 15.1 and 12.3%) for potassium content at all three growth stages (). The calcium content increased as P rates were added. P3 produced leaf-calcium content of 11.4, 7.2 and 10.1% higher than P0 at 120, 270 and 300 DAS, respectively (). However, the effect of P4 showed parity with that of P3 at all three stages ().
Figure 4. Effect of five phosphorus levels (P0, P1, P2, P3 and P4) on leaf-NPK and Ca content of coffee senna (Senna occidentalis L.) studied at 120, 270 and 300 DAS (means of three replicates). Duncan's Multiple Range Test (DMRT) was employed to separate the means with letters in the column (p≤0.05).
Seed protein and total anthraquinone glycosides content
Data in showed that protein content (12.8%) was accumulated in seeds, maximally affected by P3 treatment in comparison to the control plants. On the other hand, the influence of different levels of phosphorus on total anthraquinone glycosides content in seeds was found non-significant (). The significant positive correlation of seed protein content with leaf-N (r=0.987**, 0.997**, 0.995**), leaf-P (r=0.998**, 0.999**, 0.997**,) leaf-K (r=0.995**, 0.999**, 0.999**) and leaf-Ca content (r=0.998**, 0.979**, 0.992**) at three stages of growth further confirms the involvement of these nutrients in protein synthesis.
Discussion
The data () revealed that growth attributes increased with increasing concentrations of phosphorus. Among the growth attributes, dry weight is considered the most meaningful as all physio-chemical activities culminate in the production of dry matter. The plant dry weight is being mainly dependent upon the photosynthetic activity. Among the many inorganic nutrients required by plants, phosphorus is one of the most important elements that significantly affect plant growth and metabolism (Raghothama Citation1999; Marschner Citation2002). It is well documented that phosphorus is required for several physiological processes including cell division, cell elongation and bud growth (Marschner Citation2002). Thus, on the basis of the metabolic roles played by phosphorus, such an increase in plant fresh and dry weights could be expected as a result of phosphorus application. The pronounced effect of phosphorus on growth attributes has been recorded in many medicinal plants (Khan et al. Citation2000; Samiullah and Khan Citation2003; Naeem and Khan Citation2005; Khan and Mohammad Citation2006).
The yield attributes were increased after various levels of P application was pointed out in Table 2. Seed yield is a cumulative performance of pod numbers per plant, seed numbers per pod and 100-seed weight, respectively. The enhanced values of yield attributes with phosphorus fertilization is not surprising as phosphorus is important for root development, energy translocation and other metabolic processes of plants (Marschner Citation2002). The optimum supply of phosphorus in the early stage of plant growth is expected to cause rapid cell division and cell elongation in the meristematic regions, leading to the full development of seeds and increased seed yield (Spencer and Chan Citation1991; Turk et al. Citation2003).
Most of the physiological and biochemical attributes were significantly affected as a result of soil-applied phosphorus at three growth stages of the plant. The P3 treatment proved optimum and gave maximum values for these attributes studied at 120, 270 and 300 DAS (Figures ). A higher transpiration rate noted in phosphorus treated plants could be expected as transpiration is known to depend on stomatal conductance (Johnson et al. Citation1987; McMurtrie Citation1993). Since the stomatal conductance having shown considerable improvement in the phosphorus-treated plants, a significant increase in the rate of transpiration is obviously expected. Phosphorus promotes ribulose-1,5-bisphosphate regeneration (Rao and Terry Citation1989; Fredeen et al. Citation1990), ribulose-1,5-bisphosphate carboxylase and adenosine triphosphate synthesis (Dietz and Foyer Citation1986) and carbon dioxide assimilation (Longstreth and Nobel Citation1980). Thus, phosphorus is directly or indirectly helpful in enhancing the photosynthesis process observed in the present study. Moreover, another contributing factor responsible for the increased rate of photosynthesis is the prompt and adequate supply of carbon dioxide to the mesophyll cells of the plant and is controlled to a large extent, by stomatal conductance.
It is observed that concentrations of photosynthesis pigments were affected by different levels of exogenous applications of phosphorus; the leaves of phosphorus-treated plants facilitating to trap more sunlight to increase the rate of photosynthesis as compared to the Control plants. Let us consider that total chlorophyll and carotenoid content were found to be at a maximum at 270 DAS and at a minimum at 300 DAS in phosphorus-treated plants (). In general, breakdown of the chlorophyll and caroteniod content began with leaf-senescence as a result of the aging effect; however, the highest concentrations of these pigments occurred when the leaf blade was fully mature and remained at a maximum with slight fluctuations for photosynthesis (Lopez-Cantarero et al. Citation1994).
Nitrate reductase activity in plants is influenced by different growth conditions including not only the environmental factors such as light and temperature (Lillo Citation1994; Campbell Citation1999), but also the application of phosphorus fertilizers (Oaks Citation1985). It is noteworthy that we supplied high phosphorus to the plants and an increase in the concentration of nitrogen led to an increased capacity of the plant for nitrate assimilation. The presence of phosphorus in the nutrient solution has been reported to induce higher assimilation of NO3 in corn (De Magalhaes et al. Citation1998) and bean (Gniazdowaska et al. Citation1999). The ameliorative effect of phosphorus application on NR activity has been confirmed in our earlier study on Cassia tora (Naeem and Khan Citation2005). It is pointed out that nitrate reductase activity was found to be at a maximum at 120 DAS as shown in . Furthermore, it decreased with the increasing age of the plants, comparatively more slowly from vegetative (120 DAS) to the flowering stage (270 DAS) and more rapidly from flowering to the fruiting stage (300 DAS) of Senna occidentalis L.
The effect of phosphorus application was found significant for increasing leaf-NPK and Ca content of coffee senna at 120, 270 and 300 DAS (). It was observed that the maximum enhancement in leaf-NPK and Ca contents due to applied-phosphorus in the soil appears to roots which allowed the plants to absorb more nutrients. The increased uptake of other nutrients with increasing levels of phosphorus could presumably be attributed to the beneficial effect of phosphorus on overall growth of the plant owing to the balanced plant nutrition that accordingly resulted into a better crop yield. Enhancement in leaf-NPK and Ca contents by phosphorus application has been reported by several researchers (Akhtar et al. Citation1987; Khan et al. Citation2000; Naeem and Khan Citation2005; Siddiqui Citation2005). Considering the stages of the leaf-NPK and Ca, a decrease in the concentration of all these nutrients was noted, with the increase in the age of the crop (). Such a decrease in NPK and Ca contents of leaf may be due to continuous utilization of these nutrients by the developing pods (sink) and their translocation from vegetative parts (source). The present study on leaf-NPK content confirms the findings of Lundegardh (Citation1951) of a close relationship between the growth of a plant with its leaf N, P and K status.
The increase in protein content of seeds is mainly due to increased nitrogen content in leaves indicating high amino acid synthesis and thereby improved seed protein content. Furthermore, phosphorus proved effective due to its assured availability and continuous utilization in carbon skeleton and amino acid synthesis as well as in the synthesis of energy-rich molecules such as (ATP). This enhancement was perhaps responsible for the enhanced synthesis of protein during seed development. The significant response of phosphorus on seed protein content is confirmed by the findings of Akhtar et al. (Citation1987), Menna et al. (Citation2001) and Naeem and Khan (Citation2005).
indicates that the effect of phosphorus application on anthraquinone glycosides content in the seeds of coffee senna was found non-significant. Anthraquinones compound in plants, occurring as glycosides with one or more sugar molecules (). They possess astringent, purgative, anti-inflammatory, antitumor and bactericide effects. Anthraquinone glycosides act as stimulant cathartics and increase the tone of the smooth muscle in the wall of large intestine. They also participate in the processes of metabolism, respiration, division of cells, oxidative phosphorylation, complexation with DNA and RNA, and perhaps, in other physiological processes of vital importance. In higher plants, anthraquinones are present in oxidized, reduced, glycosides and condensed forms. Furthermore, anthraquinones are used as dyes, pigments, analytical reagents and chemical means for plant protection (Thomson Citation1987, Citation1996; Muzychkina Citation1998).
Conclusions
The present work confirms that a deficiency of phosphorus in the soil in this region (Aligarh) may be one of the prime causes of low productivity for the coffee senna plant. Thus, it may be concluded that a plant basal dose of phosphorus P3, i.e., 75 mg P per kg soil proved optimum. It may form the basis for undertaking field trials and select economic amounts of phosphorus to augment the productivity and quality of coffee senna, used as a drug in the modern as well as traditional systems of medicine.
Acknowledgements
The authors are grateful to Dr Brad Morris, USDA, USA, for a generous supply of healthy seeds of coffee senna. We are also thankful to Seshadri Kannan (ret'd), Plant Physiologist, BARC, Mumbai for critical examination of the paper.
References
- Akhtar M , Khan FA Samiullah , Afridi MMRK . 1987 . Leaf NPK content as indicator of yield of protein content of lentil (Lens culinaris L. Medic) cv.T-36 . Geobios 14 : 162 – 167 .
- ASEAN Countries . 1993 . Standard of ASEAN Herbal Medicine. Jakarta , Vol. 1. Aksara Buana Printing .
- Campbell , WH. 1999 . Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology . Ann Rev Plant Physiol Plant Mol Biol. , 50 : 277 – 303 .
- De Magalhaes , JV , Alves , VMC , De Novais , RF , Mosquim , PR , Magalhaes , JR , Bahia Filho , AFC and Huber , DM. 1998 . Nitrate uptake by corn under increasing periods of phosphorus starvation . J Plant Nutr. , 21 : 1753 – 1763 .
- Dietz , KJ and Foyer , C. 1986 . The relationship between phosphate status and photosynthesis in leaves – reversibility of the effects of phosphate deficiency on photosynthesis . Planta , 167 : 376 – 381 .
- Fiske , CH and Subba Row , Y. 1925 . The Colorimetric determination of phosphorus . J Biol Chem. , 66 : 375 – 400 .
- Fredeen , AL , Raab , TK , Rao , IM and Terry , N. 1990 . Effects of phosphorus nutrition on photosynthesis in Glycine max (L.) Merr . Planta , 181 : 399 – 405 .
- Ghosh , AB and Hassan , R. 1977 . Phosphorus in soils, crops and fertilizers . Bull Indian Soc Soil Sci. , 12 : 1 – 8 .
- Gniazdowaska , A , Krawczak , A , Mikulska , M and Rychter , AM. 1999 . Low phosphate nutrition alters bean plants ability to assimilate and translocate nitrate . J Plant Nutr. , 22 : 551 – 563 .
- Gomez , KA and Gomez , AA. 1984 . Statistical procedure for agricultural research , 2nd ed , New York : John Wiley and Sons .
- Gritsanapan W , Phadumgrakwitya , Nualkaew S . 2005 . Investigation of alternative anthraquinone sources from Cassia spp. growing in Thailand . Proceedings of International Conference on Promotion and Development with International Coordination: Exploring Quality, Safety, Efficacy and Regulations . 25 – 26 February; Kolkata, India .
- Jaworski , EJ. 1971 . Nitate reductase assay in intact plant tissues . Biochem Biophys Res Commun. , 43 : 1247 – 1279 .
- Johnson , RC , Mornhinweg , DW , Ferris , DM and Heitholt , JJ. 1987 . Leaf photosynthesis and conductance of selected Triticum species at different water potentials . Plant Physiol. , 83 : 1014 – 1017 .
- Khan , MMA and Mohammad , F. 2006 . “ Mineral nutrition of medicinal plants – a review ” . In Medicinal plants: an ethnobotanical approach , Edited by: Trivadi , PC . 347 – 358 . Jodhpur : Agrobios Publishers .
- Khan , MMA , Samiullah , Afaq SH and Afridi , RM.. 2000 . Response of black nightshade (Solanum nigrum L.) to phosphorus application . J Agron Crop Sci. , 184 : 157 – 163 .
- Lillo , C. 1994 . Light regulation of nitrate reductase in green leaves of higher plants . Plant Physiol Biochem. , 18 : 10 – 13 .
- Lindner , RC. 1944 . Rapid analytical methods for some of the more common inorganic constituents of the plant tissues . Plant Physiol. , 19 : 76 – 79 .
- Longstreth , DJ and Nobel , PS. 1980 . Nutrient influences on leaf photosynthesis . Plant Physiol. , 65 : 541 – 543 .
- Lopez-Cantarero Lorente , FA. and Romero , L. 1994 . Are chlorophylls good indicators of nitrogen and phosphorus levels? . J Plant Nutr. , 17 : 979 – 990 .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall , RJ. 1951 . Protein measurement with folin phenol reagent . J Biol Chem. , 193 : 265 – 275 .
- Lundegardh , H. 1951 . Leaf analyses , London : Hilger & Watts .
- Mac Kinney , G. 1941 . Absorption of light by chlorophyll solutions . J Biol Chem. , 140 : 315 – 322 .
- MaClachlan , S and Zalik , S. 1963 . Plastid structure, chlorophyll concentration, free amino acid composition of chlorophyll mutant of barley . Canadian J Bot. , 41 : 1053 – 1062 .
- Marschner , H. 2002 . Mineral nutrition of higher plants , 2nd ed , London : Academic Press .
- McMurtrie , RE. 1993 . “ Modeling of canopy carbon and water balance ” . In Photosynthesis and production in a changing environment , Edited by: Hall , DO , Scurlock , JMO , Bolhar-Norden Kamp , HR , Leegood , PC and Long , SP . 220 – 231 . London : Chapman and Hall .
- Menna , KN , Pareek , RG and Jat , RS. 2001 . Effect of phosphorus and biofertilizers on yield and quality of chickpea (Cicer arietinum L.) . Ann Agric Res New Series. , 22 : 388 – 390 .
- Morris , JB. 1999 . “ Legume genetic resources with novel “value added” industrial and pharmaceutical use ” . In Perspectives on new crops and new uses , Edited by: Janick , J . 196 – 200 . Alexandria, VA : ASHS Press .
- Muzychkina , RA. 1998 . Natural anthraquinones. Biological and physicochemical properties , Moscow : PHASIS Publishing House .
- Naeem , M and Khan , MMA. 2005 . Growth, physiology and seed yield of Cassia tora (syn. Cassia obtusifolia) as affected by phosphorus fertilization . J Med Arom Plant Sci. , 27 : 4 – 6 .
- Oaks , A. 1985 . Nitrogen metabolism in roots . Ann Rev Plant Physiol Plant Mol Biol. , 36 : 407 – 414 .
- Raghothama , KG. 1999 . Phosphate acquisition . Ann Rev Plant Physiol Plant Mol Biol. , 50 : 665 – 693 .
- Rao , IM and Terry , N. 1989 . Leaf phosphate status, photosynthesis and carbon partitioning in sugarbeet. I. Changes in growth, gas exchange, and Calvin cycle enzymes . Plant Physiol. , 90 : 814 – 819 .
- Samiullah , Khan NA. 2003 . Physiological investigation on interactive effect of P and K on growth and yield of chickpea . Indian J Plant Physiol. , 81 : 165 – 167 .
- Siddiqui MH. 2005 . Study on the effect of N, P and S application on the performance of rapeseed-mustard. [PhD thesis] . Aligarh : Aligarh Muslim University .
- Spencer , K and Chan , CK. 1991 . Critical phosphorus levels in sunflower plants . Austral J Exp Agric Anim Husb. , 21 : 91 – 97 .
- The Wealth of India . 1992 . Raw materials (revised edition) . Ambusta CS . Vol. 2 . New Delhi, Publication and Information Directorate, CSIR .
- Thomson , RH. 1987 . Naturally occurring quinones. III. Recent advances , London : Chapman and Hall .
- Thomson , RH. 1996 . Naturally occurring quinones. IV , London : Chapman and Hall .
- Turk , MA , Tawaha , AM and Samara , N. 2003 . Effects of seeding rate and date and phosphorus application on growth and yield of narbon vetch (Vicia narbonensis) . Agronomie , 23 : 355 – 358 .