Abstract
Aluminum (Al) toxicity has been considered an important factor in limiting the growth and nutrient acquisition of sensitive tree species in acidic soils. Mycorrhizal fungi may offset the negative impacts of Al in the root zone. Here, we report our studies on the effect of Al on the growth and mineral nutrition of Populus deltoides in the presence of the ectomycorrhizal fungus Paxillus involutus. Mycorrhizal and non-mycorrhizal plants were exposed to Al levels of 0, 50, 100 and 200 mg/l for 10 weeks. The biomass of mycorrhizal plants increased significantly than non-mycorrhizal plants. The mycorrhizal plants showed higher levels of mineral nutrients such as phosphorus, calcium and magnesium compared to nonmycorrhizal plants in different concentrations of Al. Al content significantly decreased in shoots of mycorrhizal plants compared to non-mycorrhizal plants. The oxalic acid concentration was significantly increased in mycorrhizal plants over non-mycorrhizal plants. These results suggest that ectomycorrhizal colonization confer Al tolerance to P. deltoides plants and Al induced enhancement of organic acids by P. involutus is very likely to be associated with Al tolerance.
Introduction
Aluminum (Al) toxicity is the primary factor limiting crop production on acid soils that predominate under tropical climate. At soil pH values at or below 5, toxic forms of Al are solubilized into the soil solution, inhibiting root growth and function resulting in poor plant growth and yield (Kochian Citation1995). Al interferes with the uptake, transport and utilization of essential nutrients including Ca, Mg, P and K (Schöll et al. Citation2005). It is assumed that Al3+ ions may bind to the phospholipid heads of the plasma membrane, alter the lipid-protein interaction, and modify the activity of the nutrient transporters (Suhayda and Haug Citation1986 ). Alternatively, Al3+ may reduce the negative charge associated with the plasma membrane phospholipids and proteins by binding to these charged groups or shielding the surface potential (Kinraide et al. Citation1992). Schroeder (Citation1988) reported that Al3+ was bound directly to the transport proteins, thereby impairing their function.
Mycorrhizal symbiosis plays a significant role in the establishment and survival of plants in contaminated sites (Hartley et al. Citation1997). Some ectomycorrhizal fungi have been shown to not only increase the nutrient status of their host plant but also improve the ability of the plant to tolerate toxic elements. The effects of Al on tree growth and uptake of Ca and Mg have been investigated mostly with non-mycorrhizal tree seedlings. The role of mycorrhizal relationships in altering plant response to Al exposure is poorly understood, particularly in response to tree species (Koslowsky and Boerner Citation1989). Fungal mechanisms, including the maintenance of ion uptake, the binding of Al to fungal hyphae, or extracellular detoxification of Al in the rhizosphere, may be important for overcoming the stress associated with Al exposure (Gadd Citation1993; Meharg and Cairney Citation2000). It has been postulated that ectomycorrhizal fungi mediate the uptake of Ca and Mg in tree roots and thereby mitigate the negative effect of Al on the uptake of Ca and Mg and tree growth. In non-mycorrhizal roots, the highest uptake rates of Ca and Mg occur at the root apex or at sites where lateral roots pass through the Casparian band (Häussling et al. Citation1988). As these are the sites of ectomycorrhiza formation, and ectomycorrhizal hyphae have been found to transport Ca (Melin and Nilsson Citation1955) and Mg (Jentschke et al. Citation2000) to the tree roots, it seems likely that uptake of Ca and Mg in ectomycorrhizal roots is mediated by the ectomycorrhizal fungi (Bücking et al. Citation2002). Binding of Al into fungal cell walls or sequestration of Al into fungal cell vacuoles (Schier and McQuattie Citation1996) may reduce the flux of Al into the root cortex. Furthermore, Al can be detoxified by complexation with low molecular weight organic anions (Ma et al. Citation2001; Ryan et al. Citation2001). Ectomycorrhizal tree seedlings have been found to excrete more oxalate than non-mycorrhizal tree seedlings in response to Al in axenic conditions (Ahonen-Jonnarth et al. Citation2000). Although the role of ectomycorrhizal fungi to Al toxicity is reported, many of these studies are confined to coniferous plants (Cumming and Weinstein Citation1990; Hentschel et al. Citation1993; Schier and McQuattie Citation1996; Ahonen-Jonnarth et al. Citation2003; Schöll et al. Citation2005) and the concentrations of Ca and Mg in ectomycorrhizal tree seedlings are higher, lower or equal compared to non-mycorrhizal seedlings under Al toxicity.
In this paper, we assess the role that mycorrhizal fungi play in modulating the effects of Al on biomass and nutrient acquisition of Populus deltoides plants. An attempt was also made to understand the mechanism of Al tolerance by mycorrhizal plants compared to non-mycorrhizal plants. The ectomycorrhizal fungus P. involutus was used in this study as it readily forms mycorrhizal association with P. deltoides and also has high tolerance to Al. Populus deltoides was chosen due to its association with P. involutus, it accumulates relatively high levels of certain metals, is fast growing, has a wide spread root system (Lingua et al. Citation2008) and the added advantage of producing biomass that can be used for energy production. We hypothesized that P. involutus would confer Al tolerance to P. deltoides plants by reducing the availability of relative Al and associated effects of Al on nutrient acquisition.
Materials and methods
The ectomycorrhizal fungus Paxillus involutus (PI-MAR) was obtained from INRA, France, isolated from Populus euramericana (Populus x euramericana). The culture was maintained on Melins medium (Melin Citation1921) with Heller's micronutrients (Heller Citation1953). Micropropagated plants of Populus deltoides were used in this study.
The culture of P. involutus was tested for its ability to tolerate different concentrations of Al. Fifteen-day-old mycelial discs (2×7 mm) cut from the actively growing mycelium were transferred to 250 ml Erlenmeyer flasks containing 50 ml of Melin's liquid medium. Aluminum was added in the form of Al2 (SO4)3. 16H2O to give final concentrations of Al: 0, 50, 100 and 200 mg/l. After three weeks of incubation at 25°C, the mycelium was harvested and dried at 70°C for 48 h to determine the dry weight.
To study the effect of Al on the growth and mineral nutrition of P. deltoides, the fungus was inoculated (4×7 mm diam. mycelial discs) to 500 ml glass jars containing 120 ml of soil rite and perlite (1:1v/v) (pH.5.0) moistened with 30 ml of Melin's medium. The jars were incubated at 25°C for one week. After one week of incubation, micropropagated plants of P. deltoides (about 2 cm height) were transferred to these jars and 30 ml of Melin's liquid medium containing different concentrations of Al was added to reach final concentrations of Al: 0, 50, 100 and 200 mg/l. The plants were placed in a growth room at 25°C under 16 h light and 8 h dark for 10 weeks. Six replicates were maintained for each Al level and were completely randomized in the experimental system. The plants were harvested after 10 weeks and assessed for growth and ectomycorrhiza formation. Percentage mycorrhizal colonization was calculated as total number of short roots colonized by fungus/total number of short roots×100 (Reddy and Satyanarayana (Citation1998). For mineral element analysis (Al, P, Ca and Mg), the dried shoots of each plant were ground and digested with conc. HNO3 and perchloric acid (3:1) according to Page et al. (Citation1982). The contents of Ca, Mg and Al were measured using inductively coupled plasma atomic emission spectrophotometry (ICP-AES) and total P was estimated by the method of Kitson and Mellon (Citation1944).
Oxalic acid released in the medium by both mycorrhizal and non-mycorrhizal plants was assayed using HPLC method. The plants of P. deltoides were grown for 2 weeks at 25°C under 16 h light and 8 h dark. They were inoculated with mycelial discs (4×7 mm) of P. involutus and grown for four weeks (Reddy and Satyanarayana Citation1998). Then the plants (mycorrhizal and non-mycorrhizal) were transferred to test tubes containing the glass beads with MMN medium supplemented with different concentrations of Al (0, 50, 100 and 200 mg/l). The root portion was covered with black paper to prevent light and grown for two weeks. Then, the culture filtrates were collected from both mycorrhizal and non-mycorrhizal treatments and passed through 0.22 µm filters and subjected to HPLC with the polypore H column (Perkin Elmer, USA). The mobile phase consisted of 0.008N H2SO4 with a flow rate of 0.3 ml/min. Detection was performed by a UV/VIS detector at 210 nm. HPLC profile of the culture filtrates were analyzed for oxalate by comparison with the elution profiles of pure oxalic acid (Bio-Rad, USA).
Treatments were set up in a factorial design with six replicate plants per treatment. Treatment factors were mycorrhizal inoculation (two levels) and Al (four levels). Treatment effects on plant height, biomass and elemental ion concentrations were evaluated using two-way analysis of variance. Means of mycorrhizal and non-mycorrhizal treatments were examined using the Tukey-Kramer honestly significant difference test. The relationship between nutrients and biomass were analyzed with correlation coefficient. All analyses were conducted using the statistical package GraphPad Prism version 4.03.
Results
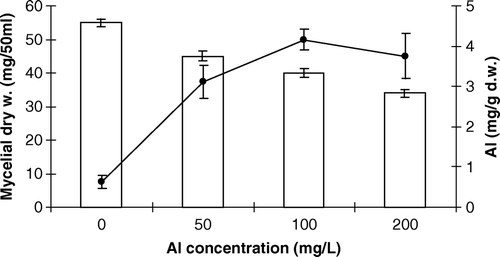
The mycelial growth of P. involutus decreased with an increase of Al concentration in the nutrient solution. The growth of P. involutus was almost inhibited by 50% (EC50) at 200 mg/l of Al. Maximum accumulation of Al in the mycelium was found at 100 mg/l ().
Figure 1. Effect of aluminum on the growth (bar) and Al content (line) in the mycelium of Paxillus involutus. The values are the means±standard error of means (n=3).
Mycorrhizal colonization of plants by P. involutus was highly successful with root colonization ranging between 35 and 61% in inoculated plants. The mycorrhizal colonization of P. involutus for different treatments of Al (0, 50,100 and 200 mg/l) were 61, 45, 44 and 35%, respectively. The mycorrhizal colonization was significantly decreased with increasing concentration of Al in the growth medium.
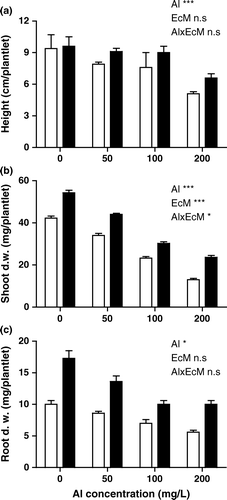
The biomass of mycorrhizal plants was greater than that of non-mycorrhizal plants across all Al treatments. Shoot and root biomass were 1.5- to 2-fold greater in mycorrhizal than in non-mycorrhizal plants (p<0.01) (). A significant difference in shoot and root biomass was observed in relation to Al treatments. The shoot biomass was significantly increased by P. involutus compared to non-mycorrhizal plants. Shoot height was higher in mycorrhizal plants compared to non-mycorrhizal plants ().
Figure 2. Shoot height, shoot and root biomass of mycorrhizal and non-mycorrhizal P. deltoides plants grown under different aluminum concentrations. Mean of six replicates±SEM. (Al: significant between Al concentration, EcM: significant between mycorrhizal and non-mycorrhizal plants; Al×EcM: interaction, *p<0.05, **p<0.01, ***p<0.001; n.s, not significant. Empty bars: non-mycorrhizal; filled bars: mycorrhizal plants).
The accumulation of Al in shoot tissues was dependent on mycorrhizal colonization (p<0.001 for mycorrhizae×Al interaction). Mycorrhizal plants accumulated a lesser amount of Al compared to non-mycorrhizal plants. The accumulation of Al increased significantly with an increase in Al concentration in the nutrient medium in non-mycorrhizal plants. Non-mycorrhizal plants accumulated 2 to 3 times more Al than mycorrhizal plants ().
Figure 3. Concentrations of Al, Ca, Mg and P in shoots of mycorrhizal and non-mycorrhizal P. deltoides plants grown under different aluminum concentrations. Mean of six replicates±SEM. (Al: significant between Al concentration, EcM: significant between mycorrhizal and non-mycorrhizal plants; Al×EcM: interaction, *p<0.05, **p<0.01, ***p<0.001; n.s, not significant. Empty bars: non-mycorrhizal; filled bars: mycorrhizal plants).
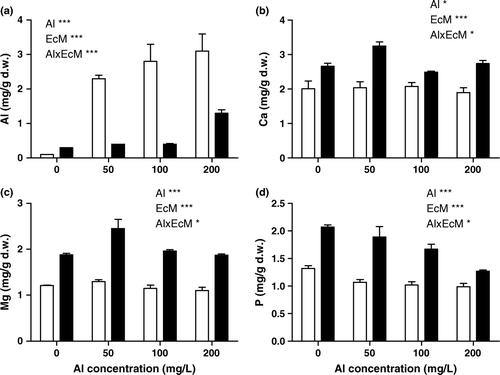
Both mycorrhizal colonization and Al in the medium altered the concentrations of macronutrients in tissues of P. deltoides (). Mycorrhizal plants had higher Ca concentrations than non-mycorrhizal plants. Ca concentrations were reduced with increasing Al concentration in the presence of ectomycorrhizal treatment and were more or less constant in the absence of mycorrhizal treatment. The maximum concentration of Ca was found at 50 mg/l of Al concentration (59% increases) in mycorrhizal plants. The Ca/Al ratio was also higher in mycorrhizal plants compared to non-mycorrhizal plants in different Al treatments. Mg concentrations were also higher in mycorrhizal plants than in non-mycorrhizal plants. Al in the medium reduced foliar Mg concentrations in non-mycorrhizal plants only (). Mycorrhizal plants showed higher (50–80%) Mg compared to non-mycorrhizal plants in the presence of Al. The Mg concentrations were influenced by mycorrhizal colonization (p<0.01 for mycorrhizae×Al interaction) (). The concentration of P was significantly higher in mycorrhizal plants than in non-mycorrhizal plants, and the response of P concentrations to Al treatment depended on mycorrhizal status () (p<0.01 for mycorrhizae×Al interaction). The P concentration increased 0.5- to 1-fold in mycorrhizal plants in different Al treatments (). The correlation between the biomass and different elements were not significant both in mycorrhizal and non-mycorrhizal plants except for P in mycorrhizal plants where it showed a positive correlation (r2=0.974).
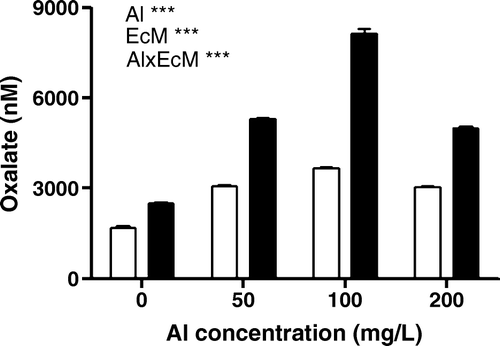
Oxalic acid exudation seemed to increase in response to Al addition in both mycorrhizal and non-mycorrhizal plants. The oxalate concentration was significantly increased in mycorrhizal plants than in non-mycorrhizal plants. The maximum amount of oxalate was observed at 100 mg/l of Al concentration in mycorrhizal plants. Significant variation was observed between the Al concentration and mycorrhizal treatment () (p<0. 01 for mycorrhizae and Al interaction).
Figure 4. Oxalic acid exudation in non-mycorrhizal (empty bars) and mycorrhizal (filled bars) plants in response to Al. Mean of six replicates±SEM. (Al: significant between Al concentration, EcM: significant between mycorrhizal and non-mycorrhizal plants; Al×EcM: interaction, *p<0.05, **p<0.01, ***p<0.001; n.s, not significant).
Discussion
Responses of ectomycorrhizal fungi to toxic metals are of importance in view of interest in the reclamation of polluted sites and influence on plant growth and productivity. It has been suggested that tolerance of the mycobiont may be an important factor in conferring plant tolerance (Colpaert and van Assche Citation1987). Higher concentrations of Al were chosen in this study keeping in view the reclamation of bauxite mined soils where the Al levels range between 150 and 200 mg/kg and soil pH 5.0–5.5 (Khosla and Reddy Citation2008). In order to select a fungus for high soluble Al conditions, the fungus must show rapid growth and root colonization so that the toxic effects brought about by the Al may be ameliorated quickly, thereby allowing the root to retain its normal growth. Populus involutus was selected in this study due to its high tolerance to Al and was able to accumulate high Al content in the mycelium ().
Though the mechanisms of nutrient acquisition in mycorrhizas are well described, their contributions to metal tolerance are not. The colonization of P. deltoides by P. involutus negated the impacts of Al on plant biomass and increased nutrient acquisition. Our results support the hypothesis that mycorrhizal fungi mediate the uptake of nutrients under Al toxicity as suggested by Finlay (Citation1995), Løkke et al. (Citation1996) and Breemen et al. (Citation2000). Mycorrhizal associations greatly enhance the uptake of nutrients through multiple mechanisms, including the activation of high affinity transport systems in fungi, increased physical exploitation of the root zone by hyphae and chemical alteration of P in the soil by fungal exudates (Bolan Citation1991). In the present study, the concentration of P of non-mycorrhizal plants not exposed to Al (1.3 mg/g) were well below the normal range (1.8–2.0 mg/g, Schier Citation1990), whereas P concentrations in mycorrhizal plants were well within the range. The P levels decreased with increase of Al concentrations in the medium in mycorrhizal plants but not in non-mycorrhizal plants. A positive correlation was observed between the mycorrhizal colonization and P levels. This result suggests that Al affects P transport occurring through the hyphae but not through root uptake. Phosphorus is a critical macronutrient involved in many structural and functional plant processes and low P acquisition rates would limit plant growth. The extensive hyphal network associated with the roots of mycorrhizal seedlings might have functioned to absorb P by accessing a greater substrate volume. In addition, mycorrhizal fungi may increase nutrient absorption as a result of high affinity fungal transport systems operating in mycorrhizal plants (Burleigh and Harrison Citation1999). The physiological effects of Al on plants are numerous, with Al inhibiting cell division, root growth and altering ion transport processes (Kochian Citation1995). In the present study, significant reductions in biomass were noted in non-mycorrhizal plants at Al concentration of 50 mg/l. These results were consistent with the findings of Schöll et al. (Citation2005) and Lux and Cumming (Citation2001) and suggest that Al toxicity limits the growth of tree species in regions receiving elevated additions of acidic deposition.
As reported in many studies on other tree species (Godbold et al. Citation1988; Kruger and Sucoff Citation1989; Lux and Cumming Citation2001), reductions in Ca and Mg concentrations were also observed with increasing Al concentrations in P. deltoides (). These reductions in Ca and Mg accumulation may be due to competition between Al and Ca or Mg in the Donnan free space, leading to reduced transfer of these cations to the stele (Godbold et al. Citation1988). The levels of Ca and Mg did not differ significantly in non-mycorrhizal plants in Al treatments. This might be due to the tolerance of poplar to Al (Bojarczuk Citation2004) where the levels of Ca and Mg are mediated by poplar plants under non-mycorrhizal conditions.
There is an extensive literature on the role of Al/Ca ratios on woody plant growth (Kelly et al. Citation1990; Ryan et al. 1990 ), these studies differ in their findings. Indeed, all demonstrate the variability between the plant and experimental systems used. Studies on the physiology of Al toxicity in non-woody plants have further highlighted the lack of a direct impact of Al on Ca relations (Ryan et al. Citation1994). Thus, while Al may displace Ca from the cell walls and the cell surface, this displacement may not be the primary factor influencing plant growth and nutrient relations (Kinraide Citation1998).
The significantly low Al concentrations measured for mycorrhizal seedlings in this study suggest that P. involutus is either adsorbing and retaining Al in hyphae or is producing diffusible ligands, such as low molecular weight organic acids, which may be chelating and detoxifying Al in the root zone. The oxalic acid exuded by mycorrhizal plants over non-mycorrhizal plants in this study clearly confirms this hypothesis. Eldhuset et al. (Citation2007) also reported that mycorrhizal roots of Piea abies/Laccaria bicolor exuded significantly more oxalate than non-mycorrhizal roots providing Al tolerance. Furthermore, comparing oxalate exudation, both mycorrhizal and non-mycorrhizal plants increased oxalate levels with increase of Al concentration in the medium, though the levels are higher in mycorrhizal plants compared to non-mycorrhizal plants. These results suggest that the tolerance response in P. deltoides might be operated by roots and triggered by P. involutus in this study.
Response of mycorrhizal seedlings to metal exposure varies widely, and the species of fungi and perhaps its fungal ecotype appear to be highly significant in plant response (Meharg and Cairney Citation2000). The results of the present study clearly indicate that P. involutus has the ability to regulate Al absorption by P. deltoides and is therefore a suitable tool to make possible the introduction of poplar on Al contaminated soil. Poplars also form both ecto- and AM fungal association and are suitable for phytoremediation purposes (Bradshaw et al. Citation2000); introduction of this plant along with P. involutus helps establishment in Al-contaminated soil.
Al tolerance is a complex phenomenon involving multiple genes and probably multiple physiological mechanisms (Maron et al. Citation2008). A number of genes have been shown to be differentially regulated by Al stress in different plant species (Mao et al. Citation2004). Further studies are needed to identify the genes involved in Al tolerance in ectomycorrhizal fungi to understand the mechanism details of Al tolerance.
Acknowledgements
The authors are thankful to Department of Science & Technology, Govt. of India for financial support (SP/SO/A-36/2001) and TIFAC-CORE for facilities.
References
- Ahonen-jonnarth , U , Goransson , A and Finlay , RD . 2003 . Growth and nutrient uptake of ectomycorrhizal Pinus sylvestris seedlings in a natural substrate treated with elevated Al concentrations . Tree Physiol. , 23 : 157 – 167 .
- Ahonen-jonnarth , U , van Hees , PAW , Lundstrom , US and Finlay , RD . 2000 . Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminum and heavy metal concentrations . New Phytol. , 146 : 557 – 567 .
- Bojarczuk , K . 2004 . Effect of toxic metals on the development of poplar (Populus tremula L. x P. alba L.) cultured in vitro . Polish J Env Stud. , 13 : 115 – 120 .
- Bolan , NS . 1991 . A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants . Plant Soil. , 134 : 189 – 207 .
- Bradshaw , H , Ceulemans , R , Davis , J and Stettler , R . 2000 . Emerging model systems in plant biology: Poplar (Populus) as a model forest tree . J Plant Growth Regul. , 19 : 306 – 313 .
- Breemen , NV , Finlay , RD , Lundstrom , US , Jongmans , AG , Giesler , R and Melkerud , PA . 2000 . Mycorrhizal weathering: A true case of mineral plant nutrition? . Biogeochemistry , 49 : 53 – 67 .
- Bücking , H , Kuhn , AJ , Schroder , WH and Heyser , W . 2002 . The fungal sheath of ectomycorrhizal pine roots: an apoplastic barrier for the entry of calcium, magnesium, and potassium into the root cortex? . J Exp Bot. , 53 : 1659 – 1669 .
- Burleigh , SH and Harrison , MJ . 1999 . “ Expression of phosphate transporters and the phosphate starvation-inducible gene Mt4 in mycorrhizal roots ” . In Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes , Edited by: Lynch , JP and Lindsey , DL . 261 – 270 . Rockville, MD : American Society of Plant Physiologists .
- Colpaert , J and van Assche , J . 1987 . Heavy metal tolerance in some ectomycorrhizal fungi . Funct Ecol. , 1 : 415 – 421 .
- Cumming , J and Weinstein , L . 1990 . Aluminum-mycorrhizal interactions in the physiology of pitch pine seedlings . Plant Soil. , 125 : 7 – 18 .
- Eldhuset , TD , Swensen , B , Wickstrøm , T and Wøllebæk , G . 2007 . Organic acids in root exudates from Picea abies seedlings influenced by mycorrhiza and aluminum . J Plant Nutr Soil Sci. , 170 : 645 – 648 .
- Finlay , RD . 1995 . Interactions between soil acidification, plant growth and nutrient uptake in ectomycorrhizal associations of forest trees . Ecol Bull. , 44 : 197 – 214 .
- Gadd , GM . 1993 . Interactions of fungi with toxic metals . New Phytol. , 124 : 25 – 60 .
- Godbold , DL , Dictus , K and Huttermann , A . 1988 . Influence of aluminum and nitrate on root growth and mineral nutrition of Norway spruce (Picea abies) seedlings . Can J For Res. , 18 : 1167 – 1171 .
- Hartley , J , Cairney , JWG and Mehard , AA . 1997 . Do ectomycorrhizal fungi exhibit adaptive tolerance to potentially toxic metals in the environment? . Plant Soil. , 189 : 303 – 319 .
- Häussling , M , Jorns , CA , Lehmbecker , G , Hecht-Buchholz , C and Marschner , H . 1988 . Ion and water uptake in relation to root development in Norway spruce (Picea abies [L.] Karst . J Plant Physiol. , 133 : 486 – 491 .
- Heller , R . 1953 . Recherches sur la nutrition minerale des tissues vegetaux cultives in vitro . Ann Sci Nat Bot Biol Veg. , 14 : 1 – 21 .
- Hentschel , E , Godbold , D , Marschner , P , Schlegel , H and Jentschke , G . 1993 . The effect of Paxillus involutus Fr. on aluminum sensitivity of Norway spruce seedlings . Tree Physiol. , 12 : 379 – 390 .
- Jentschke , G , Brandes , B , Kuhn , AJ , Schroder , WH , Becker , JS and Godbold , DL . 2000 . The mycorrhizal fungus Paxillus involutus transports magnesium to Norway spruce seedlings. Evidence from stable isotope labeling . Plant Soil. , 220 : 243 – 246 .
- Kelly , JM , Schadle , M , Thornton , FC and Joslin , JD . 1990 . Sensitivity of tree seedlings to aluminum. II. Red oak, sugar maple, and European beech . J Environ Qual. , 19 : 172 – 179 .
- Khosla , B and Reddy , MS. 2008 . Response of ectomycorrhizal fungi on the growth and mineral nutrition of Eucalyptus seedlings in bauxite mined soil . Am-Eurasian J Agric Environ Sci. , 3 : 123 – 126 .
- Kinraide , TB . 1998 . Three mechanisms for the calcium alleviation of mineral toxicities . Plant Physiol. , 118 : 513 – 520 .
- Kinraide , TB , Ryan , PR and Kochian , LV . 1992 . Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential . Plant Physiol. , 99 : 1461 – 1468 .
- Kitson , RE and Mellon , MG . 1944 . Colorometric determination of phosphorus as molybdo-vandophosphoric acid . Ind Eng Chem Ann Ed. , 16 : 379
- Kochian , LV . 1995 . Cellular mechanisms of aluminum toxicity and resistance in plants . Ann Rev Plant Physiol Plant Mol Biol. , 46 : 237 – 260 .
- Koslowsky , SD and Boerner , REJ . 1989 . Interactive effects of aluminum, phosphorus and mycorrhizae on growth and nutrient uptake of Panicum virgatum L. (Poaceae) . Environ Pollut. , 61 : 107 – 125 .
- Kruger , E and Sucoff , E . 1989 . Growth and nutrient status of Quercus rubra L. in response to Al and Ca . J Exp Bot. , 215 : 653 – 658 .
- Lingua , G , Franchin , C , Todeschini , V , Castiglione , S , Biondi , S , Burlando , B , Parravicini , V , Torrigiani , P and Berta , G . 2008 . Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones . Environ Poll. , 153 : 137 – 147 .
- Løkke , H , Bak , J , Falkengren Grerup , U , Finlay , RD , Ilvesniemi , H , Nygaard , PH and Starr , M . 1996 . Critical loads of acidic deposition for forest soils: Is the current approach adequate? . Ambio. , 25 : 510 – 516 .
- Lux , HB and Cumming , JR . 2001 . Mycorrhizae confer aluminum resistance to tulip-poplar seedlings . Can J For Res. , 31 : 694 – 702 .
- Ma , JF , Ryan , PR and Delhaize , E . 2001 . Aluminum tolerance in plants and complexing role of organic acids . Trends Plant Sci. , 6 : 273 – 278 .
- Mao , C , Yi , K , Yang , L , Zheng , B , Wu , Y , Liu , F and Wu , P . 2004 . Identification of aluminum regulated genes by cDNA-AFLP in rice (Oryza sativa L.): aluminum-regulated genes for the metabolism of cell wall components . J Exp Bot. , 55 : 137 – 143 .
- Maron , LG , Kirst , M , Mao , C , Milner , J , Menossi , M and Kochian , LV . 2008 . Transcriptional profiling of aluminum toxicity and tolerance response in maize roots . New Phytol. , 179 : 116 – 128 .
- Meharg , AA and Cairney , JWG . 2000 . Co-evolution of mycorrhizal symbionts and their hosts to metal-contaminated environments . Adv Ecol Res. , 30 : 69 – 112 .
- Melin , E . 1921 . Uber die mykorrhizenpilze von Pinus silvestris (L). und. Picea abies (L). karst . Svensk Bot Tidskr. , 15 : 192 – 203 .
- Melin , E and Nilsson , H . 1955 . Ca45 used as indicator of transport of cations to pine seedlings by means of mycorrhizal mycelium . Svensk Bot Tidskr. , 49 : 119 – 122 .
- Page AL , Miller RH , Keeney DR . 1982 . Methods of soil analysis – Part 2 (Ed. No. 9), Agronomy series . Madison, WI : ASA-SSSA Publishers .
- Reddy , MS and Satyanarayana , T . 1998 . Ectomycorrhizal formation in micropropagated plantlets of Populus deltoides . Symbiosis , 25 : 343 – 348 .
- Ryan , PR , Delhaize , E and Jones , DL . 2001 . Function and mechanism of organic anion exudation from plant roots . Annu Rev Plant Physiol Plant Mol Biol. , 52 : 527 – 560 .
- Ryan , PR , Kinraide , TB and Kochian , LV . 1994 . Al3+ – Ca2+ interactions in aluminum rhizotoxicity. I. Inhibition of root growth is not caused by reduction of calcium uptake . Planta. , 192 : 98 – 103 .
- Schier , GA . 1990 . Response of yellow-poplar (Liriodendron tulipifera L.) seedlings to simulated acid rain and ozone. 2. Effect on throughfall chemistry and nutrients in leaves . Environ Exp Bot. , 30 : 325 – 331 .
- Schier , GA and McQuattie , CJ . 1996 . Response of ectomycorrhizal and nonmycorrhizal pitch pine (Pinus rigida) seedlings to nutrient supply and aluminum: growth and mineral nutrition . Can J For Res. , 26 : 2145 – 2152 .
- Schöll , LV , Keltjens , WG , Hoffland , E and Van Breemen , N . 2005 . Effect of ectomycorrhizal colonization on the uptake of Ca, Mg and Al by Pinus sylvestris under aluminum toxicity . For Ecol Manage. , 215 : 352 – 360 .
- Schroeder , JI . 1988 . K+ transport properties of K+ channels in the plasma membrane of Vivcia faba guard cells . J Gen Physiol. , 92 : 667 – 683 .
- Suhayda , CG and Haug , A. 1986 . Organic acids reduce aluminum toxicity in maize root membranes . Physiol Plant. , 68 : 189 – 195 .