Abstract
The effects of drought on water relations, gas exchanges, solutes accumulation, and catalase (CAT), ascorbato peroxidase (APX), and guaiacol peroxidase (GPX) activities were studied in five Arachis genotypes, grown under control or withholding water conditions. Drought stress reduced plant growth of all genotypes; the genotypes A. duranensis 7988 and A. stenosperma SV2411 being characterized as the most drought-sensitive and A. ipaensis as the most drought-tolerant. Data of transpiration and stomatal conductance confirmed the findings that A. ipaensis was more tolerant to drought conditions. Water deficit increased organic solutes content and reduced leaf water potential in all genotypes. The data suggest that solutes accumulation in roots may, at least in part, explain the greater tolerance of A. ipaensis to drought stress. CAT activity showed a significant increase in stressed leaves of sensitive genotypes. APX and GPX activities either increased or were not affected by drought in leaves of all genotypes.
Introduction
Among the various abiotic stresses, drought is the major factor that limits crop productivity worldwide (Valliyodan and Nguyen Citation2006). In water-limiting environments, the decrease on growth and productivity results of osmotic effect, and different plant species appears to activate various physiological and biochemical mechanisms to endure the stress (Munns Citation2002). Osmotic adjustment by organic solutes accumulation, reduction of photosynthetic activity (Valliyodan and Nguyen Citation2006), and changes on antioxidative metabolism (Lima et al. Citation2002), are typical physiological and biochemical responses to water stress.
The osmotic adjustment in both roots and leaves reduces the cellular osmotic potential in drought-stressed plants and contribute to the maintenance of water uptake and cell turgor, allowing physiological processes, such as stomatal opening, photosynthesis, and cell expansion (Serraj and Sinclair Citation2002). Organic solutes include soluble carbohydrates, amino acids, proline, and betaines (Hasegawa et al. Citation2000). In addition to their role on cell water relations, the organic solutes accumulation may help with the maintenance of ionic homeostasis and C/N relation, and stabilization of macromolecules and organelles, such as proteins, protein complexes and membranes (Bohnert and Shen Citation1999; Bray et al. Citation2000). These solutes may also help in the control of pH in the citosol, detoxification of excess (Gilbert et al. Citation1998), and removal of free radicals (Smirnoff and Cumbes Citation1989).
The reduction in photosynthetic activity by water deficit is due to several coordinated events, such as stomatal closure and the reduced activity of photosynthetic enzymes. Upon moderate water deficit conditions, photosynthesis decreases due mainly to stomatal closure. As the stress progresses, biochemical constraints may limit the photosynthetic CO2 fixation more directly (Lawlor Citation1995, Munns and Tester Citation2008). Whatever the case, as the limitation of CO2 assimilation precedes inactivation of electron transfer reactions, an excess of reducing power is frequently generated in water-stressed plants. Thus, over-reduction of a photosynthetic electron chain may result in the formation of reactive oxygen species (ROS) such as superoxide (), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) (Alscher et al. Citation1997; Mittler Citation2002). These ROS are highly reactive and can alter normal cellular metabolism through oxidative damage to lipids, proteins, and nucleic acids (Alscher et al. Citation1997; Imlay Citation2003; Azevedo Neto et al. Citation2008).
To mitigate the oxidative damage initiated by ROS, plants have developed a complex defence antioxidative system, including low-molecular mass antioxidants as well as antioxidative enzymes such as catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) (Noctor and Foyer Citation1998; Azevedo Neto et al. 2008). CAT, which is found in peroxisomes, citosol, and mitochondria, dismutates H2O2 into H2O and O2 (McKersie and Leshem Citation1994). Peroxidases (APX and GPX) are distributed throughout the cell and catalyze the reduction of H2O2 to H2O. APX uses ascorbate as electron donor in the first step of the ascorbate-glutathione cycle and is considered the most important plant peroxidase in H2O2 detoxification (Noctor and Foyer Citation1998). GPX, which is less specific to electron donor substrate, decomposes H2O2 by oxidation of co-substrates such as phenolic compounds and/or ascorbate.
The main Brazilian Northeast areas of peanut cultivation are characterized by frequent long periods without rains. Although peanut is one of the most adapted oleaginous crops cultivated under high temperature and water shortage (Santos Citation2005), drought is seen as a problem for peanut cropping in this region. Therefore, genetic improvement for drought tolerance in the Brazilian Northeast is prevalent to current agricultural research due to social importance. The use of wild Arachis species in breeding programs is an excellent perspective to generate variability and to widen the genetic base of the populations. However, the knowledge of the physiological and biochemical traits is essential for further planning of their use in programs of direct transference of genes.
Therefore, the aim of this study was to evaluate the effects of water stress on water relations, gas exchanges, organic solutes accumulation and activities of antioxidative enzymes of different peanut genotypes, in order to better understand the physiological and biochemical mechanisms of drought tolerance, and indicate promising materials for further use in a peanut breeding program.
Materials and methods
Plant material, growth and treatment conditions
Seeds of five Arachis genotypes (four wild: A. duranensis V14167, A. ipaensis, A. duranensis 7988, and A. stenosperma SV2411 and one cultivated: A. hypogaea 55437), were sown in trays containing sand soil, watered daily and kept in a greenhouse. Ten days after seedling emergence they were transferred to plastic containers containing 8 L of soil/manure mixture appropriated to peanut crop, according recommendation described in Santos (Citation1998) . To prevent the losses of evaporation water, the soil surface was covered with polyethylene discs. The irrigation (100% field capacity) was daily until two days after transplantation, when water treatments started: control (100% field capacity) or water stress (withholding water). Field capacity was determined by gravimetric method after 72 h of draining. The containers of both treatments were weighed daily and, in the control treatment, the water lost by transpiration was replaced. In the water stress treatment, soil moisture was measured every five days with a WP4 dew point meter (Decagon Devices, Inc. Pullman, WA, USA), and showed that the average water lost by transpiration was nearly 40 ml day−1 for all genotypes. The mean values of temperature, air relative humidity and photosynthetic active radiation (at noon) were 27°C, 65% and 1200 µmolm−2 s−1, respectively. Plants were harvested 20 days after the start of water stress treatment (where stomatal closure took place). Samples (nearly 1g) of leaves and roots were cut, frozen in liquid nitrogen, and utilized for biochemical analyses. Shoot dry mass (SDM) was determined after drying in an oven at 65°C for 72 h.
Transpiration, stomatal conductance, and leaf water potential
Measurements of stomatal conductance (gs) and transpiration (E) were performed on abaxial and adaxial surfaces of youngest fully expanded leaf, at 3, 5, 9, 12, 16, and 20 days after the start of the water treatments, at 10–11 a.m. A steady-state porometer, model LI-1600 (LI-COR Inc., Lincoln, NE, USA) was used for the measurements. Temperature, air relative humidity, and photosynthetically active radiation during the measurement ranged from 30–32.5°C, 38–42%, and 550–900 µmol m−2 s−1, respectively. At the end of experimental period, the same leaves used for gas exchange determinations were utilized for leaf water potential (Ψw) measurements, using a model 3035 pressure chamber (Soil Moisture Equipment Corp, Santa Barbara, CA, USA).
Extract preparation
The extract used for all determinations was prepared by grinding 1 g of leaf and root fresh tissue with 4 ml of ice-cold extraction buffer (100 mM potassium phosphate buffer, pH 7.0, 0.1 mM EDTA). The homogenate was filtered through muslin cloth and centrifuged at 16,000 g for 15 min. The supernatant fraction was used as crude extract for soluble carbohydrates, free amino acids, free proline and soluble protein determinations, and enzyme activity assays. All operations were carried out at 4°C.
Organic solutes content
The soluble carbohydrates were determined at 490 nm, by sulphuric acid-phenol method (Dubois et al. Citation1956), using D(+)-glucose as standard. Free amino acids were determined at 570 nm, by ninhydrin method (Yemm and Cocking Citation1955), using L-glycine as standard. Proline was determined at 520 nm, by acid-ninhydrin method (Bates et al. Citation1973), using L-proline as standard. Soluble proteins were determined at 595 nm by protein-dye binding method (Bradford Citation1976), using bovine albumin as standard.
Enzyme assays
Total CAT (EC 1.11.1.6) activity was measured according the method of Beers and Sizer (Citation1952), with minor modifications. The reaction mixture (2.0 ml) consisted of 100 mM phosphate buffer (pH 7.0), 0.1 µM EDTA, 25 mM H2O2, and 100 µl enzyme extract. The reaction was started by addition of the extract. The decrease of H2O2 was monitored at 240 nm and quantified by its molar extinction coefficient (36 M−1 cm−1), and the results expressed as µmol H2O2 min−1 g−1 FW.
Total APX (EC 1.11.1.1) activity was assayed according to Nakano and Asada (Citation1981). The reaction mixture (1.95 ml) contained 50 mM phosphate buffer (pH 6.0), 0.1 µM EDTA, 0.5 mM ascorbate, 1.0 mM H2O2, and 65 µl enzyme extract. The reaction was started by addition of H2O2 and ascorbate oxidation measured at 290 nm for 1 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM−1 cm−1), and the results expressed in µmol ascorbate min−1 g−1 FW, taking into consideration that two mol ascorbate are required for reduction of one mol H2O2 (McKersie and Leshem Citation1994).
Total GPX (EC 1.11.1.7) activity was determined as described by Urbanek et al. (Citation1991) in a reaction mixture (2.0 ml) containing 100 mM phosphate buffer (pH 7.0), 0.1 µM EDTA, 5.0 mM guaiacol, 15.0 mM H2O2, and 10 µl enzyme extract. The addition of enzyme extract started the reaction and the increase in absorbance was recorded at 470 nm for 1 min. Enzyme activity was quantified by the amount of tetraguaiacol formed using its molar extinction coefficient (26.6 mM−1 cm−1). The results were expressed as µmol tetraguaiacol min−1 g−1 FW, taking into consideration that four mol H2O2 are reduced to produce one mol tetraguaiacol (Plewa et al. Citation1991).
Statistical analysis
The experimental design was a completely randomized factorial five (genotypes) × two (water treatments) with five replicates per treatment. Data were subjected to analysis of variance, and the means were compared by Tukey's test at 5% probability level.
Results
Growth, transpiration, stomatal conductance, and leaf water potential
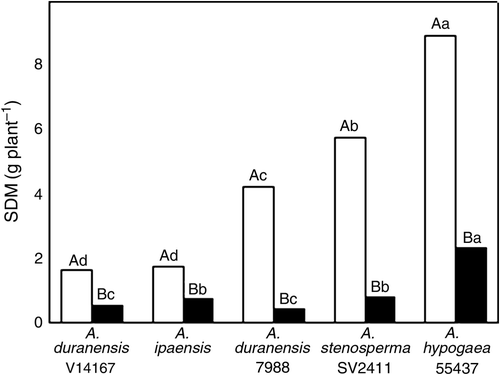
Data of shoot dry mass in both, control and withholding water conditions are given in . It may be observed that water deficit reduced SDM of all peanut genotypes. A. duranensis 7988 and A. stenosperma SV2411 showed the largest SDM reductions, 90.1 and 86.3%, respectively, while the smallest reduction was observed in A. ipaensis (57.6%).
Figure 1. Shoot dry mass (SDM) of five peanut genotypes after 20 days grown under control (open bars) or withholding water (closed bars) conditions. Values are means of five replicates. Means followed by the same capital letters (for water treatments) and lower-case letters (for genotypes) are not statistically different by Tukey's test at 5% probability level (n=5).
Stomatal behavior in the abaxial and adaxial leaf surfaces changed throughout the experimental period, mainly in control plants (). After nine days of water deficit, it was observed that stomatal closure in the abaxial surface of leaves from all genotypes resulted in a strong decrease in transpiration rates (89–94%). In adaxial surface, stomatal closure happened at 20 days of water stress in A. duranensis 7988, A. hypogaea 55437, and A. stenosperma SV2411, while A. duranensis V14167 and A. ipaensis presented transpiration rates near to 1.00 mmol m−2 s−1 (). At the same time, A. ipaensis presented the highest value of gs in adaxial surface, in contrast with A. duranensis 7988 and A. stenosperma SV2411 that presented the lowest values (). Water stress significantly reduced leaf water potential in all genotypes (); however, this decrease was more conspicuous in A. hypogaea 55437 genotype (5.7-fold), than in the other ones (nearly 3.4-fold).
Figure 2. Transpiration (E) and stomatal conductance (gs) in abaxial and adaxial leaf surfaces of five peanut genotypes grown under control or withholding water conditions.
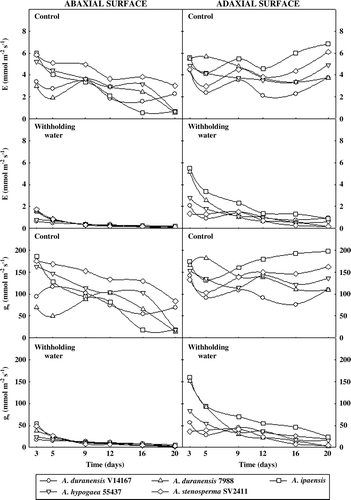
Table 1. Means and standard errors of transpiration (E) and stomatal conductance (gs) of five peanut genotypes after 20 days grown under control or withholding water conditions.
Table 2. Means and standard errors of leaf water potential (MPa) of five peanut genotypes after 20 days grown under control or withholding water conditions.
Organic solutes
Water stress increased the contents of soluble carbohydrates in leaf (a) and roots (b) of all genotypes studied. In leaves, the highest increases were observed in A. duranensis 7988 (171%) and A. stenosperma SV2411 (123%) and the lowest in A. hypogaea 55437 (52%), A. ipaensis (47%), and A. duranensis V14167 (28%). The stressed roots of A. duranensis V14167, A. ipaensis, A. duranensis 7988, and A. stenosperma SV2411 genotypes presented a medium increase of 108% in the carbohydrates content, while in A. hypogaea 55437 this increase was 52%. However, it is important to observe that carbohydrates in stressed roots of A. ipaensis genotype were significantly higher than in the other ones.
Figure 3. Content of organic solutes in five peanut genotypes after 20 days grown under control (open bars) or withholding water (closed bars) conditions. (a) Soluble carbohydrates in leaves and (b) in roots; (c) free amino acids in leaves and (d) in roots; (e) free proline in leaves and (f) in roots; (g) soluble protein in leaves and (h) in roots. Additional details as in .
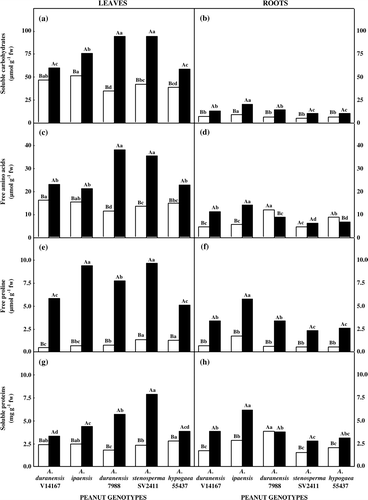
Water stress increased the free amino acids content in the leaves; however, this increase was more pronounced in A. duranensis 7988 (232%) and A. stenosperma SV2411 (162%) genotypes (c). In roots, water deficit increased the amino acids in A. ipaensis (153%) and A. duranensis V14167 (139%), did not alter in A. stenosperma SV2411, and decreased in A. duranensis 7988 (27%) and A. hypogaea 55437 (23%) genotypes (d).
Proline content also increased as a result of water stress for all genotypes. However, this increase was more evident in leaves (e) than in roots (f). In leaves, the highest increases were observed in A. ipaensis (1308%) and A. duranensis V14167 (1167%), and the lowest in A. hypogaea 55437 (298%). In roots, the genotypes presented a medium increment of about 356% in the proline content after the water stress application, however the highest proline contents were verified in stressed roots of A. ipaensis genotype.
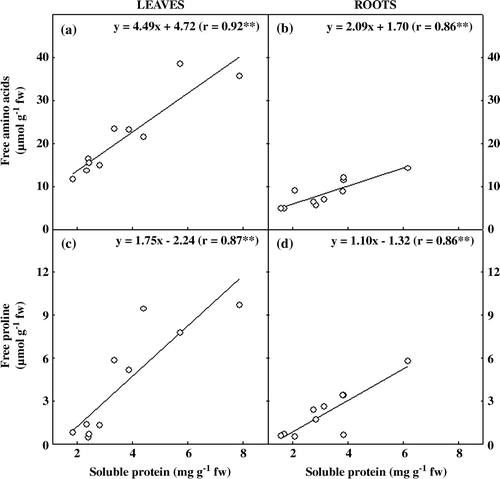
Similarly to observed for carbohydrates and amino acids, the increases in leaf soluble proteins (g) were more conspicuous in A. duranensis 7988 (213%) and A. stenosperma SV2411 (228%). In roots, soluble proteins increased as a result of water stress, except in A. duranensis 7988, which stayed constant (h). It is noteworthy that analogously to other organic solutes, soluble proteins in stressed roots of A. ipaensis genotype were also significantly higher than in the other ones. In addition, comparing the contents of organic solutes in leaves and roots, it was found that there was highly significant correlation between soluble proteins and free amino acids and proline ().
Figure 4. Correlation between soluble protein and amino acids and proline contents in five peanut genotypes after 20 days grown under control or withholding water conditions. (a) Soluble protein vs. amino acids in leaves and (b) in roots; (c) soluble protein vs. proline in leaves and (d) in roots. r = correlation coefficient; ** = statistically significant (p<0.01).
Enzyme activity
Total CAT activity in leaves (a) and roots (b) changed among genotypes, when comparing control and water deficit treatments. Drought did not affect CAT activity in leaves of A. duranensis V14167 genotype. However, CAT activity increased in leaves of A. duranensis 7988 (133%) and A. stenosperma SV2411 (49%), and decreased in A. ipaensis (65%) and A. hypogaea 55437 (52%) genotypes. In roots, drought did not change CAT activity in A. ipaensis and A. stenosperma SV2411, but it decreased in A. duranensis V14167 (57%), A. duranensis 7988 (54%), and A. hypogaea 55437 (51%).
Figure 5. Activity of antioxidative enzymes in five peanut genotypes after 20 days grown under control (open bars) or withholding water (closed bars) conditions. (a) Total CAT activity in leaves and (b) roots; (c) total APX activity in leaves and (d) in roots; (e) total GPX activity in leaves and (f) in roots. Additional details as in .
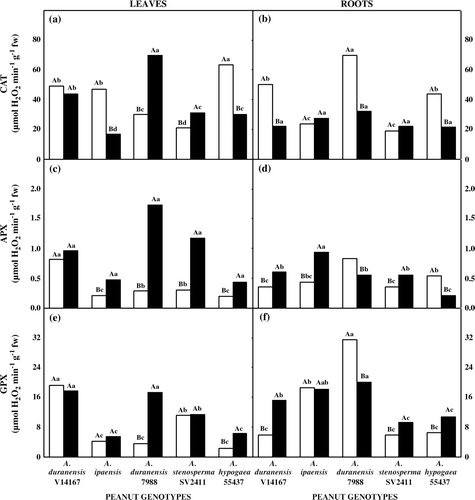
Water stress increased leaf APX activity, except for A. duranensis V14167 genotype (c). However, this increase was more conspicuous in A. duranensis 7988 (500%) than in A. stenosperma SV2411 (295%), A. ipaensis (127%), and A. hypogaea 55437 (132%). In stressed roots, APX activity increased, respectively, 69, 114, and 55% in A. duranensis V14167, A. ipaensis, and A. stenosperma SV2411 genotypes (d). In contrast, drought decreased enzyme activity in A. duranensis 7988 (34%) and A. hypogaea 55437 (62%).
Water stress increased non-specific peroxidase (GPX) activity in leaves of A. duranensis 7988 (392%) and A. hypogaea 55437 (164%) genotypes (e). In roots, water stress response changed among genotypes studied (f). Thus, GPX activity increased in A. duranensis V14167 (155%), A. stenosperma SV2411 (55%), and A. hypogaea 55437 (65%), stayed constant in A. ipaensis, and decreased by 37% in A. duranensis 7988.
Comparing activities of H2O2 scavenging enzymes in leaves of water-stressed plants, CAT activity was 3- and 40-fold greater than GPX and APX activities, respectively. On the other hand, CAT and GPX activities in stressed roots were nearly 40-fold greater than APX activity.
Discussion
Plant growth of all genotypes studied was inhibited by water stress. Genotypes A. duranensis 7988 and A. stenosperma SV2411 suffered the biggest growth reduction, while the smallest reduction was observed for genotype A. ipaensis, suggesting that the former are the most drought-sensitive and the latter the most drought-tolerant.
Water deficit leads to stomatal closure, which reduces CO2 availability in the leaves and inhibits carbon fixation. In control plants, the variation observed in E and gs along the experimental period was the result of environmental changes usually observed under non-controlled conditions. On the other hand, substantial changes were not observed in the time course of E and gs in stressed plants, supporting the statement that the water is the prevalent environmental factor in the stomatal response.
In the present study, water stress led to a significant decrease in E and gs; however, this effect was more pronounced in abaxial than in adaxial surfaces of leaves. Considering that stomatal closure in adaxial surface occurred later than in abaxial surface, reduced transpiration rates observed in abaxial surface did not completely affect the gas exchange in the genotypes studied. At the end of withholding water period, A. ipaensis presented the highest values of E and gs in adaxial surface, in contrast with A. duranensis 7988 and A. stenosperma SV2411 that presented the lowest values. This observation supports the hypothesis that A. ipaensis was more tolerant and A. duranensis 7988 and A. stenosperma SV2411 were more sensitive to drought conditions. This stomatal response has also been reported by Nogueira et al. (Citation1998) and Nogueira and Santos (Citation2000).
Our results showed that, water stressed plants of A. hypogaea 55437 presented the largest reductions in the values of leaf water potential. Although several authors have suggested that the reduction in Ψw due to water shortage should be an indicator of stress adaptation and, consequently, a good morpho-physiological marker for stress tolerance, our data do not support this idea because the genotypes A. duranensis 7988 and A. stenosperma SV2411, which were the more drought-sensitive, showed similar decreases to those observed in A. ipaensis that was the more drought-tolerant. Soluble amino acids and carbohydrates are considered as the main organic solutes involved in osmotic adjustment (Lacerda et al. Citation2001; Azevedo Neto et al. Citation2004). Although drought stress had increased the organic solutes content in leaves of all genotypes, however this increase was more conspicuous in drought-sensitive genotypes. These results suggest that A. duranensis 7988 and A. stenosperma SV2411 genotypes had the highest energetic cost for osmoregulation through the organic solutes synthesis. The occurance of drought-induced disturbances may also have affected the translocation of organic solutes from shoot to root in drought-sensitive genotypes, resulting in the accumulation of these solutes in the shoot (Larsson Citation1992). In addition, considering that the root is the first organ directly exposed to water deficit, and that the root content of all organic solutes was higher in A. ipaensis (most drought-tolerant), the drought-induced organic solutes accumulation in roots may have helped the maintenance of water absorption by the roots and its flux to the shoot. Thus, our results suggest that solutes accumulation in roots may, at least in part, explain the greater tolerance of A. ipaensis to drought stress when compared to other genotypes. Similar results were reported by Azevedo Neto et al. (2004).
It is well known that plants under stress may accumulate small molecular mass proteins that could be used as a source of storage nitrogen that could be mobilized after stress relief or removal (Parida and Das Citation2005). Our data showed that water stress increased soluble proteins, amino acids, and proline contents in the genotypes studied suggesting that drought-induced increase in soluble protein was a result of high amino acids contents. In addition, results also suggest that the increase of de novo synthesis and/or inhibition of amino acids degradation were the prevalent mechanism for the accumulation of most free amino acids. The observation that soluble proteins content was highly correlated with amino acids and proline () supports the hypothesis.
Environmental stresses limiting photosynthesis can also increase oxygen-induced cellular damage due to increased reactive oxygen species (ROS) generation, such as superoxide and hydrogen peroxide (Alscher et al. Citation1997; Mittler Citation2002; Azevedo Neto et al. 2008). On the other hand, an increase in the activity of antioxidative enzymes under abiotic stresses could be indicative of an increased production of ROS and/or a build-up of a protective mechanism to reduce oxidative damage triggered by stress experienced by plants. In plants, a number of enzymes regulate the levels of intracellular hydrogen peroxide, but catalases and peroxidases are considered the most important. In the present study, the activities of these enzymes in response to water stress suggest that oxidative stress may be an important component of environmental stresses in peanut plants.
CAT is one of the ubiquitous enzymes in aerobic organisms and plays a key role in cellular defence mechanisms against H2O2. It is found in peroxisomes, citosol, and mitochondria and dismutates H2O2 into H2O and O2 (McKersie and Leshem Citation1994; Azevedo Neto et al. 2008). Data examinations show a significant increase in CAT activity only in leaves of A. duranensis 7988 and A. stenosperma SV2411 (more drought sensitive) after withholding water period. Although CAT comprises multiple isoforms over the different cell compartments, nearly all CAT is confined to microbodies, particularly peroxisomes (Smirnoff Citation1995). Considering that A. duranensis 7988 and A. stenosperma SV2411 presented the lowest stomatal aperture under stress conditions, it could be hypothesized that the increase in CAT activity is an indirect evidence of an enhanced photorespiration. Water deficit decreased CAT activity in leaves of A. ipaensis and A. hypogaea 55437 genotypes. Several studies have also reported loss in CAT activity as the water deficit progresses (Dhindsa and Matowe Citation1981; Chowdhury and Choudhuri Citation1985; Zhang and Kirkham Citation1994). This may be explained by the continuous enzyme photoinactivation, especially under severe photooxidative conditions (Smirnoff Citation1995). In addition, inhibition of protein synthesis induced by water deficit could impair resynthesis, and partly accounted for the decreased CAT activity, as observed in some species under water deficit in the light (Zhang and Kirkham Citation1994). Apparently, this did not occur in the present experiment, considering that the overall protein content was enhanced under drought conditions.
Peroxidases (APX and GPX) are distributed throughout the cell and are thought to be involved in numerous plant processes, including lignification (Bohm et al. Citation2006), oxidation of phenolic compounds (Bratkovskaja et al. Citation2004), regulation of cell elongation (Lee et al. Citation2007), and detoxification of H2O2 (Azevedo Neto et al. 2008). APX uses ascorbate as an electron donor in the first step of the ascorbate-glutathione cycle and is considered the most important plant peroxidase in H2O2 detoxification (Noctor and Foyer Citation1998). GPX, which is less specific to electron donor substrate, decomposes H2O2 by oxidation of co-substrates such as phenolic compounds and/or ascorbate. Our data show that, similarly to CAT, the more conspicuous increases in APX and GPX activities were observed in leaves of more sensitive genotypes. Although the subcellular distribution of peroxidases suggests that APX and GPX removes H2O2 produced in processes other than photorespiration, considering that H2O2 is relatively stable and diffusible through membranes (Van Breusegem et al. Citation2001), results suggest the significance of the cooperative functions of APX and GPX in the H2O2 scavenging process.
When the activity values of the H2O2 scavenging enzymes were compared () it was observed that CAT and GPX had a higher H2O2 scavenging activity than APX in leaves and roots of both control and water-stressed peanut plants. Therefore, it could be hypothesized that CAT and the non-specific peroxidase (GPX) are the most important among the H2O2 s cavenging enzymes in leaves and roots. Our results are in agreement with those of Azevedo Neto et al. (Citation2005, Citation2006), who suggested that CAT and GPX were the most important H2O2 scavenging enzymes leading to salt tolerance in maize.
The potential contribution of the root toward whole plant ROS detoxification during water stress exposure is not fully understood and some authors have suggested that roots could supply chemical antioxidants such as reduced glutathione to the aerial portions (Rennenberg Citation1982; Pinhero et al. Citation1997). However, like leaves, roots exposed to water deficit are potential producers of ROS, and mitochondria have been suggested as a primary source in non-photosynthetic tissues (Noctor et al. Citation2007). Although in our experiment we have no data on chemical antioxidant levels, we found intrinsically higher enzyme activities in roots, irrespective of water treatments. These results were similar to those observed in maize (Azevedo Neto et al. 2006).
This study indicated that the maintenance of stomatal aperture in adaxial surface and organic solutes accumulation in roots may, at least in part, explain the greater tolerance of A. ipaensis to drought stress, and therefore may be good physiological traits for drought tolerance in peanut genotypes. Furthermore, the obtained results showed a general response of antioxidative enzymes to water deficit and that drought-induced oxidative stress was more conspicuous in the sensitive genotypes.
References
- Alscher , RG , Donahue , JL and Cramer , CL. 1997 . Reactive oxygen species and antioxidants: Relationship in green cells . Physiol Plant. , 100 ( 2 ) : 224 – 233 .
- Azevedo Neto , AD , Gomes-Filho , E and Prisco , JT. 2008 . Abiotic stress and plant responses , 1st ed , 58 – 82 . New Delhi : IK International. Chapter 4, Salinity and oxidative stress .
- Azevedo Neto , AD , Prisco , JT , Enéas-Filho , J , Abreu , CEB and Gomes-Filho , E. 2006 . Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes . Environ Exp Bot. , 56 ( 1 ) : 87 – 94 .
- Azevedo Neto , AD , Prisco , JT , Enéas-Filho , J , Lacerda , CF , Silva , JV , Costa , PHA and Gomes-Filho , E. 2004 . Effects of salt stress on plant growth, stomatal response and solute accumulation of different maize genotypes . Braz J Plant Physiol. , 16 ( 1 ) : 31 – 38 .
- Azevedo Neto , AD , Prisco , JT , Enéas-Filho , J , Medeiros , J-V and Gomes-Filho , E. 2005 . Hydrogen peroxide pre-treatment induces salt stress acclimation in maize plants . J Plant Physiol. , 162 ( 10 ) : 1114 – 1122 .
- Bates , LS , Waldren , RP and Teare , ID. 1973 . Rapid determination of free proline for water stress studies . Plant Soil. , 39 ( 1 ) : 205 – 207 .
- Beers , RF Jr and Sizer , IW. 1952 . A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase . J Biol Chem. , 195 ( 1 ) : 133 – 140 .
- Bohm , P , Zanardo , F , Ferrarese , M and Ferrarese-Filho , O. 2006 . Peroxidase activity and lignification in soybean root growth-inhibition by juglone . Biol Plant. , 50 ( 2 ) : 315 – 317 .
- Bohnert , HJ and Shen , B. 1999 . Transformation and compatible solutes . Sci Hortic. , 78 ( 1 ) : 237 – 260 .
- Bradford , MM. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. , 72 ( 1–2 ) : 248 – 254 .
- Bratkovskaja , I , Vidziunaite , R and Kulys , J. 2004 . Oxidation of phenolic compounds by peroxidase in the presence of soluble polymers . Biochemistry (Moscow) , 69 ( 9 ) : 985 – 992 .
- Bray EA , Bailey-Serres J , Weretilnyk E. 2000 . Biochemistry and molecular biology of plants . Rockville : ASPP . Chapter 22, Responses to abiotic stresses 1158 1203 .
- Chowdhury , SR and Choudhuri , MA. 1985 . Hydrogen peroxide metabolism as an index of water stress tolerance in jute . Physiol Plant. , 65 ( 4 ) : 503 – 507 .
- Dhindsa , RS and Matowe , W. 1981 . Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation . J Exp Bot. , 32 ( 1 ) : 79 – 91 .
- Dubois , M , Gilles , KA , Hamilton , JK , Rebers , PA and Smith , F. 1956 . Colorimetric method for determination of sugars and related substances . Anal Chem. , 28 ( 3 ) : 350 – 356 .
- Gilbert , GA , Gadush , MV , Wilson , C and Madore , MA. 1998 . Amino acid accumulation in sink and source tissues of Coleus blumei Benth. during salinity stress . J Exp Bot. , 49 ( 318 ) : 107 – 114 .
- Hasegawa , PM , Bressan , RA , Zhu , J-K and Bohnert , HJ. 2000 . Plant cellular and molecular responses to high salinity . Annu Rev Plant Physiol Mol Biol. , 51 : 463 – 499 .
- Imlay , JA. 2003 . Pathways of oxidative damage . Annu Rev Microbiol. , 57 : 395 – 418 .
- Lacerda , CF , Cambraia , J , Cano , MAO and Ruiz , HA. 2001 . Plant growth and solute accumulation and distribution in two sorghum genotypes, under NaCl stress . Braz J Plant Physiol. , 13 ( 3 ) : 270 – 284 .
- Larsson , M. 1992 . Translocation of nitrogen in osmotically stressed wheat seedlings . Plant Cell Environ. , 15 ( 4 ) : 447 – 453 .
- Lawlor DH. 1995 . Environment and plant metabolism – flexibility and acclimation . Oxford : BIOS Scientific Publishers . Chapter 8. The effects of water deficit on photosynthesis 129 156
- Lee , SH , Choi , JH , Kim , WS , Park , YS and Gemma , H. 2007 . Effects of calcium chloride spray on peroxidase activity and stone cell development in pear fruit (Pyrus pyrifolia ‘Niitaka’) . J Japan Soc Hort Sci. , 76 ( 3 ) : 191 – 196 .
- Lima , ALS , DaMatta , FM , Pinheiro , HA , Totola , MR and Loureiro , ME. 2002 . Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions . Environ Exp Bot. , 47 ( 3 ) : 239 – 247 .
- McKersie , BD and Leshem , YY. 1994 . Stress and stress coping in cultivated plants , Dordrecht : Kluwer Academic Publishers .
- Mittler , R. 2002 . Oxidative stress, antioxidants and stress tolerance . Trends Plant Sci. , 7 ( 9 ) : 405 – 410 .
- Munns , R. 2002 . Comparative physiology of salt and water stress . Plant Cell Environ. , 25 ( 2 ) : 239 – 250 .
- Munns , R and Tester , M. 2008 . Mechanisms of salinity tolerance . Annu Rev Plant Physiol Plant Mol Biol. , 59 : 651 – 681 .
- Nakano , Y and Asada , K. 1981 . Hydrogen peroxide is scavenged by ascorbate-specific peroxidases in spinach chloroplasts . Plant Cell Physiol. , 22 ( 5 ) : 867 – 880 .
- Noctor , G and Foyer , CH. 1998 . Ascorbate and glutathione: Keeping active oxygen under control . Annu Rev Plant Physiol Plant Mol Biol. , 49 : 249 – 279 .
- Noctor , G , De Paepe , R and Foyer , CH. 2007 . Mitochondrial redox biology and homeostasis in plants . TRENDS Plant Sci. , 12 ( 3 ) : 125 – 134 .
- Nogueira , RJMC and Santos , RC. 2000 . Alteraç[otilde]es fisiológicas no amendoim submetido ao estresse hídrico . R Bras Eng Agric Amb. , 4 ( 1 ) : 41 – 45 .
- Nogueira , RJMC , Santos , RC , Bezerra Neto , E and Santos , VF. 1998 . Comportamento fisiológico de duas cultivares de amendoim submetidas a diferentes regimes hídricos . Pesq Agrop Bras. , 33 ( 12 ) : 1963 – 1969 .
- Parida , AK and Das , AB. 2005 . Salt tolerance and salinity effects on plants: A review . Ecotoxic Environ Saf. , 60 ( 3 ) : 324 – 349 .
- Pinhero , RG , Rao , MV , Paliyath , G , Murr , DP and Fletcher , RA. 1997 . Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings . Plant Physiol. , 114 ( 2 ) : 695 – 704 .
- Plewa , MJ , Smith , SR and Wagner , ED. 1991 . Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase . Mutation Res. , 247 ( 1 ) : 57 – 64 .
- Rennenberg , H. 1982 . Glutathione metabolism and possible biological roles in higher plants . Phytochemistry , 21 ( 12 ) : 2771 – 2781 .
- Santos , RC. 1998 . EMBRAPA releases BRS 151 17, a large-seeded groundnut cultivar for the Northeast region in Brazil . Int Arachis Newsl. , 18 : 11 – 12 .
- Santos RC . 2005 . Estresses ambientais: Danos e benefícios em plantas . Recife : MXM . Chapter 33; Melhoramento genético do amendoim para resistência à seca via hibridização com espécies cultivada e selvagens de Arachis 360 368 .
- Serraj , R and Sinclair , TR. 2002 . Osmolyte accumulation: Can it really help increase crop yield under drought conditions? . Plant Cell Environ. , 25 ( 2 ) : 333 – 341 .
- Smirnoff N. 1995 . Environment and plant metabolism – flexibility and acclimation . Oxford : BIOS Scientific Publishers . Chapter 12; Antioxidant systems and plant response to the environment 217 243 .
- Smirnoff , N and Cumbes , QJ. 1989 . Hydroxyl radical scavenging activity of compatible solutes . Phytochemistry , 28 ( 4 ) : 1057 – 1060 .
- Urbanek , H , Kuzniak-Gebarowska , E and Herka , K. 1991 . Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase . Acta Phys Plant. , 13 ( 1 ) : 43 – 50 .
- Valliyodan , B and Nguyen , HT. 2006 . Understanding regulatory networks and engineering for enhanced drought tolerance in plants . Curr Opin Plant Biol. , 9 ( 2 ) : 189 – 195 .
- Van Breusegem , F , Vranová , E , Dat , JF and Inzé , D. 2001 . The role of active oxygen species in plant signal transduction . Plant Sci. , 161 ( 3 ) : 405 – 414 .
- Yemm , EW and Cocking , EC. 1955 . The determination of amino-acids with ninhydrin . Analyst , 80 ( 948 ) : 209 – 213 .
- Zhang , J and Kirkham , MB. 1994 . Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species . Plant Cell Physiol. , 35 ( 5 ) : 785 – 791 .