Abstract
Biological control involves the use of beneficial organisms such as Trichoderma that promote positive responses by the plant. Trichoderma species produce and/or release a variety of compounds, including cell wall degrading enzymes and secondary metabolites, which enhance root development, crop productivity and resistance to biotic and abiotic stresses. This study examines the effects of the five Trichoderma strains (M7, KHB, G124-1, G46-3 and G46-7) on milk thistle including morphological characteristics and silymarin accumulation. This research indicated that treating milk thistle by M7 strain, significantly increased the highest branch length of plants, while strain KHB induced the production of silychristin, isosilybin and silymarin. Finally, strain G46-7 dramatically increased silydianin content in milk thistle plant. The findings suggested that certain Trichoderma strains are positive elicitors for promoting growth characteristics and silymarin accumulation in Silybum marianum (L.) Gaertn.
Abbreviations
| HBL | = |
highest branch length |
| SL | = |
stem length |
| NC | = |
number of capsules per plant |
| MCD | = |
means of capsules diameter |
| 100SW | = |
weight of 100 seed |
| TXF | = |
taxifolin |
| SC | = |
silychristin |
| SD | = |
silydianin |
| SB | = |
silybin |
| ISB | = |
isosilybin |
| SLM | = |
silymarin |
Introduction
Silymarin is the constitutive natural compound which accumulates in the fruits of the milk thistle (Silybum marianum). It is composed of an isomeric mixture of the flavonolignans, including silychristin (SC), silydianin (SD), silybin (SB), isosilybin (ISB) (Morazzoni and Bombardelli Citation1995; Kim et al. Citation2003; Lee and Liu Citation2003). These natural compounds are synthesized by oxidative coupling between the flavonoid taxifolin (TXF) and a phenylpropanoid, usually coniferyl alcohol (Dewick Citation2002). Moreover, they are of considerable pharmacological interest because of their strong anti-hepatotoxic, hepatoprotective, chemopreventive, hypocholesterolemic, cardioprotective, neuroactive and neuroprotective characteristics (Kren and Walterova Citation2005).
Plant elicitors refer to chemicals of various sources that can induce physiological and morphological responses and phytoalexin accumulation (Namdeo Citation2007). They may include abiotic elicitors such as metal ions and inorganic compounds and biotic elicitors from fungi, bacteria, plant cell wall fragments as well as chemicals that are released at attack site by plants upon pathogen or herbivore attack (Namdeo Citation2007). Treatment of plants with elicitors causes a wide array of defensive reactions, including accumulation of plant defensive secondary metabolites (Cheong and Hahn Citation1991; Hanania and Avni Citation1997). Trichoderma is a fungal genus, first described in 1794, that includes anamorphic fungi, isolated primarily from soil and decomposing organic matter (PersoonCitation1794). The interaction between Trichoderma and plants has been reported to evolve a symbiotic rather than a parasitic relationship, whereby the fungus occupies a nutritional niche and the plant is protected from disease (Vinale et al. Citation2008). Elicitors from Trichoderma spp. activate the expression of genes involved in the plant defensive response system. These fungi also promote the growth of the plant and increase nutrient availability (Yedidia et al. Citation2003; Hanson and Howell Citation2004; Harman et al. Citation2004). Nevertheless, currently no study exists on the effects of use of Trichoderma as an elicitor to increase silymarin accumulation or its impact on growth characteristics of milk thistle. In our current research, we studied the effects of Trichoderma strains on silymarin accumulation and growth characteristics of Silybum marianum by inseminating them at the root of the plant.
Material and methods
Fungal material
Trichoderma harzianum isolates M7, G47-3, KHB, G46-3 and G46-7 were used in this study. Isolate M7 was obtained from soil collected in Mazandaran (Sari), Iran; G47-3 was obtained from soil in a rice field in Gilan (Shaft) Iran; KHB from dead fallen leaf in Mazandaran (Polesefid), Iran; G46-3 from soil in a rice field in Gilan (Rezvanshahr), Iran; and G46-7 was isolated from soil in Gilan (Rezvanshahr), Iran. Trichoderma isolates were grown on potato dextrose agar medium (PDA) for five days at 25°C in the dark and then transferred to light conditions for two days. Following fungus colonies growth on plates, the medium with mycelium were cut into 50 mm plugs (Papavizas Citation1985; Watts et al. Citation1988; Kubicek et al. Citation2003).
Plant material
The seeds of S. marianum were supplied by the Institute of Medicinal Plant, Iranian Academic Center for Education, Culture and Research. The seeds were cultivated at a depth of 2–3 cm in pots (with 9 cm diameter) which were filled with clay-sand-loan sterile soil. The soil was sterilized by autoclave. Afterwards, the pots were placed in a green house for 12 months under partially-controlled temperature and relative humidity. The mean temperature over the year measured at 26±1°C during the day and 17±1°C at night. Relative humidity was approximately 45% during the day, and 70% at night. The light program conformed to the natural and seasonal intensity of the region (about 800 µmol m−2 s−1; 16:8 h light:dark). The plants were watered when required. As the roots of plantlets were approximately 10 cm long (after 60 days), the 90 pieces of plugs of each Trichoderma isolates were placed on the roots of each plantlet. The control pots were filled only with un-inoculated soil (without Trichoderma isolates). The plant material consisted of three samples of each treatment and the fruits were harvested when the capsules of the plants were matured.
Extraction and isolation of flavonolignans from dried fruits of S. marianum
The dried fruits were crushed with a mortar and pestle. The dried fruits powders (3 g) were defatted with ethyl acetate. The flavonolignans were extracted from the dried residue with 50 ml of methanol at 40°C for 8 h. The methanol solution was concentrated to a dry residue. The extract was dissolved in 10 ml of methanol and kept at 4°C in darkness (Cacho et al. Citation1999; Hasanloo et al. Citation2007).
High-performance liquid chromatography (HPLC) quantitative analysis
The amounts of flavonolignans were determined by high-performance liquid chromatography (HPLC) analysis according to Hasanloo et al. (Citation2005). The instrument was equipped with a knauer injector with a 20 µl loop, a Nucleosil C18 5µ (250×4.6 mm) column, a knauer K2600A UV detector and Chromgate software for peak integration (Hasanloo et al. Citation2005). Mobile phase consisted of solvents, acetonitrile: water (40:60) with 10% (V/V) H3PO4, (pH 2.6) through constant gradient. The elution time and flow rate were 30 min and 1 ml min−1 and peaks detected at 288 nm. All solvents and chemicals were of HPLC grade (Merck). Identification was achieved by comparison of retention times (Rt) with standards of SC, SD, SB, TXF and a standard mixture of SLM. Quantification of these metabolites, expressed in mg g−1 of dry weight, was accomplished using a known concentration of standard and peak areas. The data obtained from the analysis of each concentration was taken for the calibration curve with correlation coefficient = 0.999.
Chemicals
Standards of SLM, SB and TXF, were purchased from Sigma Aldrich (Germany); SC and SD from Phytolab (Germany).
Data collection and measurement of growth characteristics
The following growth characteristics were measured for each treatment: Stem length (SL) (cm), number of capsules per plant (NC) (number), means of capsules diameter (MCD) (cm), weight of 100 seed (100SW) (g) and the highest branch length (HBL) (cm).
Statistical analysis
The experiment was arranged in a completely randomized design, which was repeated three times. Data for all characteristics were subject to analysis of variance with the SAS statistical software package (SAS Institute Citation2001). Data for SL, 100SW, HBL, SD, SB and ISB characteristics were transformed to the Johnson transformation scale with the MINITAB release 15 statistical software package (Chou et al. Citation1998; Luh and Guo Citation2001; Mohajeri Naraghi Citation2008), prior to the ANOVA to stabilize the variance. Statistical F-tests were evaluated at p=0.05. Differences among treatments were further analyzed using Duncan's Multiple Range tests.
Results
Effects of Trichoderma strains on growth characteristics
Interactions of Trichoderma-milk thistle had a significant effect on the highest branch length (cm) characteristic, but had no significant effect on the other characteristics (SL, NC, MCD and 100SW) (p=0.05) (). The mean comparison with Duncan's Multiple Range test showed that the M7 strain had a positive effect on HBL (75 cm), which was higher than the control (67 cm) (α = 0.05). But the KHB, G124-1, G46-3 and G46-7 strains had negative effects on HBL, which decreased HBL length (63.7, 46.5, 40.33 and 30.5, respectively) ().
Figure 1. Effects of some Trichoderma strains (including M7, G47-3, KHB, G46-3 and G46-7) and the control group on the highest branch length (HBL) of Silybum marianum L. (Gaertn) (means with the same letter are not significantly different at α = 0.05).
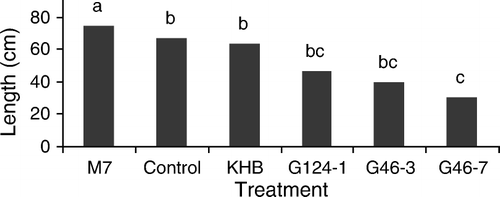
Table 1. Analysis of variance of five growth traits in Silybum marianum which were affected by Trichoderma strains.
Influences of Trichoderma strains on silymarin accumulation
The interaction of Trichoderma-milk thistle had significant effects on SC, SD, ISB and SLM, but had no significant effect on TXF and ISB (). The mean comparisons with Duncan's Multiple Range test demonstrated that the highest SC, ISB and SLM accumulation was obtained from KHB-milk thistle interaction and the highest SD production was acquired in the interaction of G46-7 with the plant (). Studying mean comparison of SC content showed that the KHB strain had a positive and significant effect on the content (1.24 mg g−1 DW), which was 1.46-fold greater than the control; but the other Trichoderma strains were not significantly different from control conditions (a). b shows that the G46-7 strain significantly increased SD production (0.28 mg g−1 DW), which was 2.95-fold of the control, while the other strains had a negative and significant effect on the production. The result indicated that the M7, KHB and G46-7 species dramatically increased ISB content (0.22, 0.28 and 0.23 mg g−1 DW, respectively), which were 2.33, 2.95 and 2.41-fold of that of the control, but the other species were not significantly different from the control condition (c). The mean comparison of SLM accumulation showed that KHB strain had a positive and significant effect on the accumulation (5.28 mg g−1 DW), which was 1.40-fold of the control, but the other strains, with the exception of the M7 strain that dramatically decreased the accumulation, failed to affect accumulation significantly (d).
Figure 2. The influence of different Trichoderma strains (including M7, G47-3, KHB, G46-3 and G46-7) and the control group on silymarin accumulation of milk thistle (Silybum marianum) plant (means with the same letter are not significantly different at α = 0.05). (A) Silychristin content; (B) Silydianin content; (C) Isosilybin content; and (D) Silymarin accumulation.
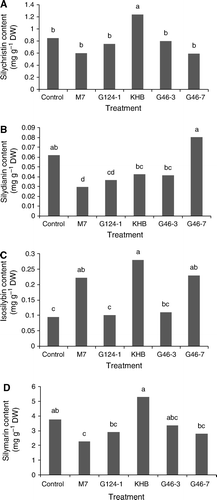
Table 2. Analysis of variance of Silymarin accumulation which was affected by Trichoderma strains.
Discussion
In order to determine the effects of elicitors on the production of valuable metabolites for pharmaceutical industries, it is necessary to evaluate and screen various elicitors with different mechanisms. Recent evidence indicates that many Trichoderma species may have substantial influence on plant growth and development (Harman et al. Citation2004). Mycoparasitism and antibiotic production are the main mechanisms used by Trichoderma spp. to control phytopathogenic agents; also, substantial changes to the plant proteome and metabolism as well as plant growth are the alternative mechanisms (Chet Citation1987; Harman Citation2000; Yedidia et al. Citation2001). An increased yield was also observed when plant seeds were exposed to Trichoderma conidia that were separated from them by cellophane, suggesting that Trichoderma metabolites can influence the plant growth (Benitez et al. Citation2004). Trichoderma spp. also produce organic acids, such as gluconic, citric or fumaric acids, that decrease soil pH and permit the solubilization of phosphates, micronutrients and mineral cations, including iron, manganese and magnesium, that are useful for plant metabolism (Benitez et al. Citation2004; Harman et al. Citation2004). Thus, these fungi induce a variety of effects indicating that these beneficial fungi have multiple modes of action (Harman et al. Citation2004). Based on our observations, Trichoderma-milk thistle interactions were effective on silymarin accumulation of the plant and increased flavonolignans levels such as those of SC, SD, ISB and SLM. Trichoderma exudates contain many classes of chemical components (such as peptides, proteins, simple aromatic compounds, pyrones, butenolides, volatile terpenes and isocyane metabolites) that elicit plant defense responses (Woo et al. Citation2006). However, there has been no effective study on Trichoderma species as elicitors increasing silymarin accumulation in S. marianum. This study showed that Trichoderma-milk thistle interactions affected the growth characteristics such as the highest branch length of the plant. Trichoderma-induced local and systemic defense responses, increased growth response reported for various plant species, including bean (Phaseolus vulgaris L.), cucumber (Cucumis sativus L.), pepper (Capsicum annum L.), periwinkle (Vinca minor), and petunia (Petunia hybrida) (Inbar et al. Citation1994; Yedidia et al. Citation2003; Harman et al. Citation2004). Crop productivity increased by up to 300%, as determined by comparing the treated plots with the untreated controls and measuring fresh/dry root and above ground biomass weights, height of plants, number of leaves and fruits (Vinale et al. 2004). In addition, increased chlorophyll concentration induced by Trichoderma species has also been reported for some plants (Lo and Lin Citation2002).
The results of this work describe a Trichoderma-milk thistle interactive system that is suitable for studying growth characteristics. In addition, this research demonstrated activity levels of defense-related secondary metabolites (e.g. silymarin) in medicinal plants such as S. marianum in response to Trichoderma promoting increased productivity for pharmaceutical industries. This project suggests that the addition of Trichoderma to media may enhance productivity in field process.
Acknowledgements
This work (No. 1-05-05-8702) was supported by the Agricultural Biotechnology Research Institute of Iran (ABRII).
References
- Benitez , T , Rincon , AM , Limon , MC and Codon , AC. 2004 . Biocontrol mechanisms of Trichoderma strains . Int Microbiol. , 7 : 249 – 260 .
- Cacho , M , Moran , M , Corchete , P and Fernandez-Tarrago , J. 1999 . Influence of medium composition on the accumulation of flavonolignans in cultured cells of Silybum marianum (L.) Gaertn . Plant Sci. , 144 ( 22 ) : 63 – 68 .
- Cheong , JJ and Hahn , MG. 1991 . A specific, high-affinity binding site for the heptaglucoside elicitor exists in soybean membranes . Plant Cell. , 3 : 137 – 147 .
- Chet , I. 1987 . “ Trichoderma-application, mode of action, and potential as a biocontrol agent of soil borne plant pathogenic ” . In Innovative approaches to plant disease control , Edited by: Chet , I . 137 – 160 . New York : John Wiley and Sons .
- Chou , Y , Polansky , AM and Mason , RL. 1998 . Transforming non-normal data to normality in statistical process control . J Qual Technol. , 30 ( 2 ) : 133 – 141 .
- Dewick , PM. 2002 . Medicinal natural products. A biosynthetic approach , Chichester : John Wiley & Sons .
- Hanania , U and Avni , A. 1997 . High-affinity binding site for ethylene-inducing xylanaseelicitor on Nicotiana tabacum membranes . Plant J. , 12 ( 1 ) : 113 – 120 .
- Hanson , LE and Howell , CR. 2004 . Elicitors of plant defense responses from biocontrol strains of Trichoderma virens . J Phytopathol. , 94 ( 2 ) : 171 – 176 .
- Harman , GE. 2000 . Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22 . Plant Dis. , 84 ( 44 ) : 377 – 393 .
- Harman , GE , Howell , CR , Viterbo , A , Chet , I and Lorito , M. 2004 . Trichoderma species opportunistic, avirulent plant symbionts . Nature Rev Microbiol. , 2 ( 1 ) : 43 – 56 .
- Hasanloo , T , Khavari Nejad , RA , Majidi , E and Shams Ardakani , MR. 2005 . Analysis of flavonolignans in dried fruits of Silybum marianum (L.) Gaertn from Iran . Pakistan J Biol Sci. , 8 ( 12 ) : 1778 – 1782 .
- Hasanloo , T , Khavari Nejad , RA and Majidi , E. 2007 . Evaluation of phenotypic coefficient and flavonolignan content in dried fruits of cultivated and endemic Silybum marianum (L.) Gaertn . J Medicinal Plants. , 6 ( 22 ) : 77 – 90 .
- Inbar , J , Abramsky , M , Cohen , D and Chet , I. 1994 . Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings grown under commercial conditions . Eur J Plant Pathol. , 100 : 337 – 346 .
- Kim , NC , Graf , TN , Sparacino , CM , Wani , MC and Wall , ME. 2003 . Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum) . Org Biomol Chem. , 1 : 1684 – 1689 .
- Kren , V and Walterova , D. 2005 . Silybin and silymarin new effects and applications . Biomed Papers. , 149 ( 1 ) : 29 – 41 .
- Kubicek , CP , Bissett , J , Druzhinina , I , Kullnig-Gradinger , C and Szakacs , G. 2003 . Genetic and metabolic diversity of Trichoderma: A case study on South East Asian isolates . Fungal Genet Biol. , 38 : 310 – 319 .
- Lee , DYW and Liu , YZJ. 2003 . Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle) . J Nat Prod. , 66 : 1171 – 1174 .
- Lo , CT and Lin , CY. 2002 . Screening strains of Trichoderma spp for plant growth enhancement in Taiwan . Plant Cell. , 11 : 215 – 220 .
- Luh , WM and Guo , JH. 2001 . Using Johnson's transformation and robust estimators with heteroscedastic test statistics: An examination of the effects of non-normality and heterogeneity in the non-orthogonal two-way ANOVA design . Br J Math Stat Psychol. , 54 ( 1 ) : 79 – 94 .
- Mohajeri Naraghi S. 2008 . Study of genetic diversity and agro morphological traits in the samples of Iranian cowpea landraces. MSc Thesis in plant breeding . Islamic Azad University, Karaj Branch , Iran .
- Morazzoni , P and Bombardelli , E. 1995 . Silybum marianum (Carduus marianus) . Fitoterapia , 66 ( 1 ) : 3 – 42 .
- Namdeo , AG. 2007 . Plant cell elicitation for production of secondary metabolites: A review . Pharmacog Rev. , 1 ( 1 ) : 69 – 79 .
- Papavizas , GC. 1985 . Trichoderma and Gliocladium: Biology, ecology and potential for biocontrol . Ann Rev Phytopathol. , 23 : 23 – 54 .
- Persoon CH. 1794 . Dispositio methodica fungorum . Römer's neues Magazin der Botanik . 1 : 81 128 .
- SAS Institute . 2001 . SAS System for Windows. Release 8.2 . SAS Institute, Inc., Cary , NC, , USA .
- Vinale , F , Sivasithamparam , K , Ghisalberti , EL , Marra , R , Woo , SL and Lorito , M. 2008 . Trichoderma-plant-pathogen interactions . Soil Biol Biochem. , 40 : 1 – 10 .
- Vinale , F , D'Ambrosio , G , Abadi , K , Scala , F , Marra , R Turra , D . 2006 . Application of Trichoderma harzianum (T22) and Trichoderma atroviride (P1) as plant growth promoters, and their compatibility with copper oxychloride . J Zhejiang Univ Sci. , 30 : 2 – 8 .
- Watts , R , Dahiya , J , Chaudhary , K and Tauro , P. 1988 . Isolation and characterization of a new antifungal metabolite of Trichoderma reseii . Plant Soil. , 107 : 81 – 84 .
- Woo , SL , Scala , F , Ruocco , M and Lorito , M. 2006 . The molecular biology of the interactions between Trichoderma, phytopathogenic fungi and plants . Phytopathology , 96 ( 2 ) : 181 – 185 .
- Yedidia , I , Shoresh , M , Kerem , Z , Benhamou , N , Kapulnik , Y and Chet , I. 2003 . Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins . Appl Environ Microbiol. , 69 ( 12 ) : 7343 – 7353 .
- Yedidia , I , Srivastva , AK , Kapulink , Y and Chet , I. 2001 . Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants . Plant Soil. , 235 ( 22 ) : 235 – 242 .