Abstract
Artemisia annua L. is an aromatic-antibacterial herb that destroys malarial parasites, lowers fevers and checks bleeding, and of which the secondary compound of interest is artemisinin. Enhanced production of the artemisinin content in the whole plant is highly desirable. Keeping in mind, the importance of this valuable antimalarial plant, field experiments were conducted to investigate the effects of triacontanol alone and in combination with gibberellic acid on growth attributes, photosynthesis, enzymatic activities, essential oil and artemisinin content and yield of Artemisia. The results indicate that combination of triacontanol and gibberellic acid (1.5 mg l−1+75 mg l−1) significantly increased activities of nitrate reductase and carbonic anhydrase by 25.9% and 21.5%, and net photosynthetic rate, stomatal conductance and internal CO2 by 25.4%, 14.1% and 15.4% higher, respectively, when compared to unsprayed plants. This combined treatment also significantly enhanced artemisinin content and yield (29% and 61% higher values).
Introduction
Artemisia annua L. is an aromatic plant which has been used for centuries in Chinese traditional medicine for the treatment of fever and malaria (Klayman Citation1985). It has been introduced into experimental cultivation in India (Singh et al. Citation1986), Thailand, Brazil, Australia, Madagascar and in Europe into The Netherlands, Switzerland and France as far north as Finland (Laughlin Citation2002). Being the world's most severe parasitic infection, malaria causes more than 1 million deaths and 500 million cases annually. Despite tremendous efforts for the control of malaria, the global morbidity and mortality have not been significantly changed in the last 50 years (World Health Organization [WHO] Citation2002). The main problem is the failure to find effective medicines against malaria.
Artemisinin, a sesquiterpene lactone containing an endoperoxide bridge, has become increasingly popular as an effective and safe alternative therapy against malaria (Abdin et al. Citation2003). Artemisinin and its derivatives are effective against multi-drug resistant, Plasmodium falciparum strains mainly in Southeast Asia and more recently in Africa, without any reputed cases of resistance (Kremsner and Krishna Citation2004).
Plant growth regulators (PGRs) stimulate growth and terpenoid biosynthesis in various aromatic plants, which can result in beneficial changes in both quality and quantity of terpenoids (Shukla et al. Citation1992). Biosynthesis of terpenoids is dependent on primary metabolism, e.g., photosynthesis and oxidative pathways for carbon and energy supply (Singh et al. Citation1990). The PGR such as triacontanol (TRIA) increases dry matter production may influence the inter-relation between primary and secondary metabolism leading to increased biosynthesis of secondary products. Gibberellic acid (GA3), a phytohormone exhibits a broad spectrum of physiological effects in plants. It induces growth, photosynthesis, flowering and cell expansion (Yuan and Xu Citation2001; Taiz and Zeiger Citation2006). GA3 also enhances the metabolic activity within pathways leading to stress (defense-related secondary metabolites) and anthocyanin biosynthesis (Ohlsson and Bjork Citation1988).
Several PGRs have been tested for enhancing artemisinin production but only TRIA and GA3 individually proved effective for artemisinin content and yield (Shukla et al. Citation1992; Ferreira et al. Citation1997, Citation2005; Weathers et al. Citation2005; Zhang et al. Citation2005). Taking into account the importance of this enormously important antimalarial drug plant, it was decided to conduct field experiments to test whether the foliar spray of TRIA alone or together with GA3 could improve the crop productivity, photosynthetic capacity, enzymatic activities and artemisinin production in Artemisia annua L.
Materials and methods
Plant material and sampling procedures
The seeds of Artemisia annua L. were initially sown in November in the seedbeds to obtain seedlings. The seedlings were transplanted into the field in January at a spacing of 45×45 cm. Physico-chemical characteristics of the soil were: texture-sandy loam, pH (1:2) 7.2, E.C. (1:2) 0.46 m mhos cm−1, available N, P and K 98.84, 6.83 and 142.9 mg kg−1 soil, respectively. There were 15 Artemisia plants in each plot and the plots were maintained at 1 m distance to nullify the effect of treatments. Each treatment was replicated five times, using randomized block design. Before transplantation, a uniform basal dose of N, P and K applied at the rate of 80, 40 and 40 kg ha−1, respectively, to the field plots. The plants were sprayed with TRIA alone and in combination with different concentrations of GA3. There were four treatments excluding control (double distilled water). The treatments consisted of: (i) 1.5 mg TRIA (T1), (ii) 1.5 mg l−1 TRIA + 50 mg l−1 GA3 (T2), (iii) 1.5 mg l−1 TRIA + 75 mg l−1 GA3 (T3), and (iv) 1.5 mg l−1 TRIA + 100 mg l−1 GA3 (T4). Foliar sprays of both PGRs were given at 10-day intervals starting from 30 days after planting (DAP). The plants were irrigated as and when required. Plants were sampled at 90 DAP. The experiments were conducted in two successive years and the mean of the data of both years was taken into consideration.
Physiological and biochemical analyses
The fresh leaves were used for the analysis of various physiological and biochemical attributes except leaf N, P and K content.
Determination of net photosynthetic rate, stomatal conductance and internal CO2
Net photosynthetic rate (P N), stomatal conductance (gs) and internal CO2 (ci) were measured on sunny days at 11:00 h using fully expanded leaves of Artemisia annua L. with the help of an IRGA (Infra Red Gas Analyzer, LI-COR 6400 Portable Photosynthesis System, Lincoln, Nebraska, USA). Before recording the measurement, the IRGA was calibrated and zero was adjusted approximately every 30 min during the measurement period. Each leaf was enclosed in a 1-litre gas exchange chamber for 60 s. All the attributes measured by IRGA were recorded three times for each treatment.
Estimation of total chlorophyll and carotenoid content
Total chlorophyll and carotenoid contents in fresh leaves were estimated by the method of MacKinney (Citation1941) and MacLachlan and Zalik (Citation1963), respectively. One hundred mg of fresh tissue from interveinal leaf-areas was ground using a mortar and pestle containing 80% acetone. The optical density (OD) of the solution was recorded at 645 and 663 nm for chlorophyll estimation and at 480 and 510 nm for carotenoid estimation using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
Determination of nitrate reductase (NR) activity
Nitrate reductase (E.C. 1.6.6.1) activity in the leaf was determined by the intact tissue assay method of Jaworski (Citation1971). Chopped leaf pieces (200 mg) were incubated for 2 h at 30°C in a 5.5 ml reaction mixture, which contained 2.5 ml of 0.1 M phosphate buffer, 0.5 ml of 0.2 M potassium nitrate, and 2.5 ml of 5% isopropanol. The nitrite formed subsequently was colorometrically determined at 540 nm after azocoupling with sulphanilamide and naphthylene diamine dihydrochloride. The NR activity was expressed as nM NO2 g−1 FW h−1.
Determination of carbonic anhydrase (CA) activity
Carbonic anhydrase (E.C. 4.2.1.1) activity was measured in fresh leaves using the method as described by Dwivedi and Randhawa (Citation1974). Two hundred mg of fresh leaf pieces were weighed and transferred to Petri plates. The leaf pieces were dipped in 10 ml of 0.2 M cystein hydrochloride solution for 20 min at 4°C. To each test tube, 4 ml of 0.2 M sodium bicarbonate solution and 0.2 ml of 0.022% bromothymol blue were added. The reaction mixture was titrated against 0.05 N HCl using methyl red as indicator. The enzyme was expressed as µM CO2 kg−1 leaf FW s−1.
Estimation of nitrogen, phosphorus and potassium contents in leaves
Leaf samples from each treatment were digested for the estimation of leaf N, P and K content. The leaves were dried in a hot air oven at 80°C for 24 h. Dried leaves were powdered using a mortar and pestle and the powder was passed through a 72 mesh. The sieved leaf-powder was used for N, P and K content. One hundred mg of oven-dried leaf powder was carefully transferred to a digestion tube where 2 ml of AR (analytical reagent) grade concentrated sulfuric acid was added also. This solution was heated on a temperature controlled assembly at 80°C temperature for about 2 h and then cooled for about 15 min at room temperature. Afterwards, 0.5 ml of 30% hydrogen peroxide (H2O2) was added to the solution. The addition of H2O2 followed by heating was repeated until the contents of the tube became colorless. The prepared aliquot (peroxide-digested material) was used to estimate per cent N, P and K in the leaves on the dry weight basis.
Estimation of N, P and K contents
Leaf N content was estimated according to the method of Lindner (Citation1944) with slight modification by Novozamsky et al. (Citation1983). The leaf dried powder was digested in H2SO4 in a digestion tube. A 10 ml aliquot (peroxide-digested-material) was poured into a 50 ml volumetric flask where 2 ml of a 2.5 N sodium hydroxide and 1 ml of 10% sodium silicate solutions were added to neutralize the acid excess and prevent turbidity. A 5 ml aliquot of this solution was poured into a 10 ml graduated test tube and a 0.5 ml Nessler’s reagent was added. The OD of the solution was recorded at 525 nm, using the spectrophotometer.
The method of Fiske and Subba Row (Citation1925) with slight modification by Rorison et al. (Citation1993) was used to estimate the leaf-P content in the digested material. The peroxide-digested material was used to determine the leaf-P content. A 5 ml aliquot was poured into a 10 ml graduated test tube where 1 ml of molybdic acid (2.5%) was added, followed by addition of 0.4 ml 1-amino-2-naphthol-4-sulphonic acid. When the color became blue, the volume was increased to 10 ml with the addition of double distilled water. The OD of the solution was recorded at 620 nm using the spectrophotometer.
Potassium content in the leaves was analyzed flame-photometrically (Hald Citation1946). It was estimated in the aliquot with the help of emission spectra using specific filters. In the flame-photometer, the solution (peroxide-digested-material) was discharged through an atomizer in the form of a fine mist into a chamber, where it is drawn into a flame. Combustion of the element produces light of a particular wavelength [λmax for K = 767 nm (violet)]. The light produced was passed through the appropriate filter to impinge upon a photoelectric cell that activates a galvanometer. Readings were recorded with the help of emission spectra using specific filter in a flame-photometer (Model, C150, AIMIL, India).
Yield and quality parameters
For the yield attributes, 15 plants from each treatment were collected at 90 DAP. The dried leaves were weighed for measuring the leaf-yield accordingly. Artemisinin yield was calculated by multiplying corresponding leaf-yield and artemisinin content.
Estimation of essential oil content
The leaf samples were steam distilled for 4 h using a Clevenger's apparatus by which the essential oil was extracted and determined gravimetrically. A total of 100 g of fresh leaves were used for the oil extraction. The essential oil content was calculated in percentage.
Artemisinin extraction and estimation
Dry leaf material (1 g) was used for the estimation of artemisinin by the method as described by Zhao and Zeng (Citation1986). As artemisinin lacks any chromophore for UV detection in HPLC hence, it was chemically modified to a compound Q260. It was then quantified using HPLC method (Zhao and Zeng Citation1986). Standard curve was prepared using 1 mg of standard artemisinin dissolved in 1 ml of HPLC-grade methanol to make the stock solution. It was extracted with 20 ml petroleum ether in shaker at 70 rpm for 24 h. After 24 h, solvent was decanted and pooled and 20 ml of petroleum ether added again and this step was repeated three times. Petroleum ether fractions were pooled and concentrated under reduced pressure and residues defatted with CH3CN (10 ml×3). Precipitated fat was filtered out and filtrate concentrated under reduced pressure. Residues were dissolved in 1 ml of methanol. 100 µl aliquot of each sample of each treatment was taken and to this 4 ml of 0.3% NaOH was added. The samples were incubated in shaking water bath at 50°C for 30 min, thereafter cooled and neutralized with glacial acetic acid (0.1 M in 20% MeOH). The pH of the solution was maintained at 6.8. Derivatized artemisinin was analyzed and quantified through reverse phase column (C18; 5 µm; 4.6 mm; 250 mm) using premix methanol: 10 mM K-Phosphate buffer (pH, 6.5) in the ratio of 60:40 as mobile phase at constant flow rate of 1 ml/min, with the detector set at 260 nm. Artemisinin was quantified against the standard curve of artemisinin, obtained from Sigma-Aldrich, USA, prepared using HPLC.
Statistical analysis
Each plot was treated as one replicate and all the treatments were replicated five times. The experiment was repeated twice in successive years. The mean of the data of both the years were analyzed statistically using SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA). Mean values were statistically compared by Duncan's Multiple Range test (DMRT) at p<0.05 level using different letters.
Results
Foliar spray of TRIA alone and together with different concentrations of GA3 increased the growth of the plants. Of the four concentrations, treatment T3 proved better than the other concentrations. It was effective in increasing the values of all growth attributes over their respective controls. Shoot and root lengths were increased by 29.8% and 21.7%, respectively, in comparison to their respective controls when treated with 1.5 mg l−1 TRIA + 75 mg l−1 GA3 (). The combined effect of TRIA and GA3 treatment (T3) enhanced the fresh weight and dry weight per plant by 22.5% and 19.4%, respectively, when compared to control plants ().
Table 1. Effect of foliar sprays of different concentrations of TRIA and GA3 on growth attributes of Artemisia annua L. Means within a column followed by the same letter are not significantly different (p≤0.05). The data shown are means of five replicates±SE.
Foliar application of TRIA alone accelerated the rate of photosynthesis, stomatal conductance and internal CO2 when compared to non-sprayed plants. However, the combined application of both PGRs was found more effective in enhancing the rate of photosynthesis. The treatment (T3) increased P N, gs and ci by 25.4%, 14.1% and 15.5% higher, respectively, exceeding the control ( a–c).
Figure 1. Effect of foliar application of different concentrations of TRIA and GA3 on photosynthetic rate (a), stomatal conductance (b) and internal CO2 (c) of Artemisia annua L. Bars showing the same letter are not significantly different at p≤0.05 as determined by Duncan's Multiple Range test. Error bars (⊺) show SE.
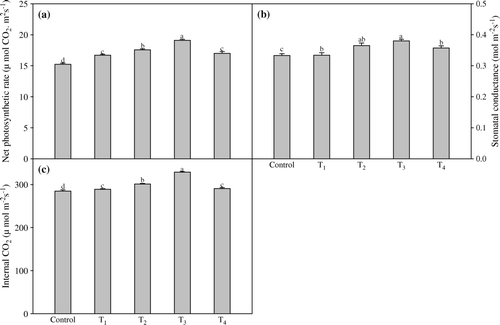
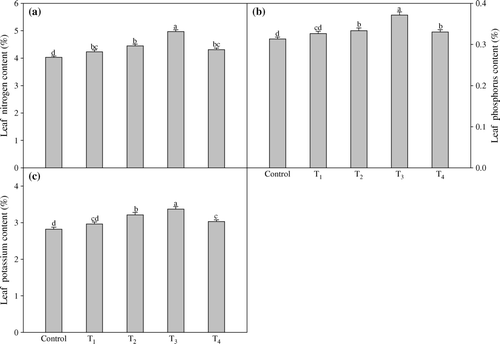
Amongst the biochemical parameters, total chlorophyll and carotenoid contents (18.5% and 11.9% higher values) increased by the treatment T3 (a, b). The combined spray of TRIA and GA3 (1.5 mg l−1 TRIA + 75 mg l−1 GA3) enhanced the activities of nitrate reductase and carbonic anhydrase maximally by 22.5% and 17.8%, respectively, when compared to their controls (c, d). TRIA in combination with GA3 significantly increased the leaf N, P and K contents. The concentrations of leaf nutrients (N, P and K) were found significantly greater at treatment T3 over the control. It significantly increased the nitrogen, phosphorus and potassium contents by 23.7%, 18.4% and 19.4%, respectively, over the control (a–c).
Figure 2. Effect of foliar application of different concentrations of TRIA and GA3 on chlorophyll content (a) and carotenoid content (b), nitrate reductase activity (c) and carbonic anhydrase activity (D) of Artemisia annua L. Bars showing the same letter are not significantly different at p≤0.05 as determined by Duncan's Multiple Range test. Error bars (⊺) show SE.
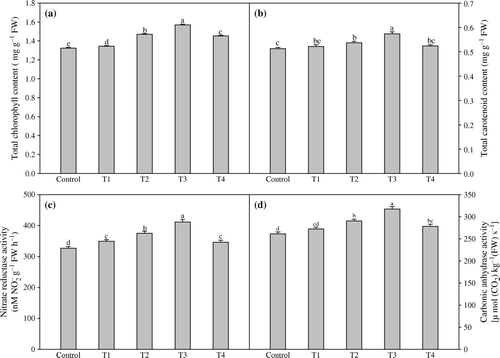
Figure 3. Effect of foliar application of different concentrations of TRIA and GA3 on leaf nitrogen (a), phosphorus (b) and potassium (c) contents of Artemisia annua L. Bars showing the same letter are not significantly different at p≤0.05 as determined by Duncan's Multiple Range test. Error bars (⊺) show SE.
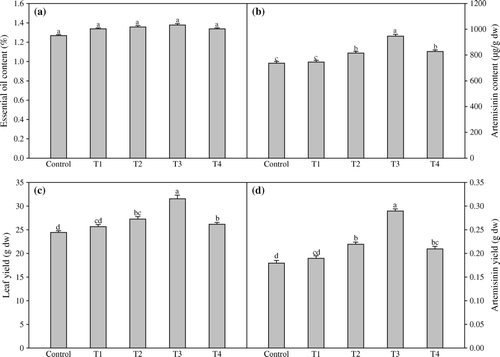
The application of TRIA and GA3 did not increase the essential oil content and result was found non-significant (a). Data indicates that leaf artemisinin content was also found maximum in plants treated with both PGRs. The treatment (1.5 mg l−1 TRIA + 75 mg l−1 GA3) improved the artemisinin content and by 28.4% as compared to non-sprayed plants (b). The combined effect of TRIA and GA3 (1.5 mg l−1 TRIA + 75 mg l−1 GA3) was found more beneficial in increasing leaf-yield when compared to the non-sprayed plants. The application of both PGRs (Treatment T3) resulted in maximum leaf-yield which increased by 28.9% in comparison to control (c). The treatment (1.5 mg l−1 TRIA + 75 mg l−1 GA3) improved the artemisinin yield maximally and registered 61.0% higher value as compared to control (d).
Figure 4. Effect of foliar application of different concentrations of TRIA and GA3 essential oil content (a), artemisinin content (b), leaf yield (c) and artemisinin yield (d) of Artemisia annua L. Bars showing the same letter are not significantly different at p≤0.05 as determined by Duncan's Multiple Range test. Error bars (⊺) show SE.
Discussion
The significant increase in growth attributes (shoot and root lengths, fresh and dry weights per plant) treated with TRIA and in combination with GA3 could be ascribed by the well known roles of TRIA and GA3 in plants (). It is established fact that GA3 promotes cell enlargement and cell division (Buchanan et al. Citation2000; Taiz and Zeiger Citation2006) while TRIA elicits a secondary messenger which moves rapidly throughout the plant resulting in stimulation of growth (dry weight increase) and water uptake (Ries Citation1985; Khan et al. Citation2007; Naeem et al. Citation2009). Earlier studies have reported that gibberellic acid as foliar spray on transplanted cutting increased plant height (Sadowska et al. Citation1984). Srivastava and Srivastava (Citation2007) reported that foliar spray of GA3 increased plant height and leaf length. An increase in growth parameters like shoot and root lengths, fresh and dry weights in plants sprayed with TRIA and GA3 (Treatment T3) is in accordance with the known fact that exogenous application of PGRs evoke the intrinsic genetic potential of the plant causing increase in elongation of internodes as a consequence of cell division and cell wall extensibility (Moore Citation1989; Khan et al. Citation2006; Taiz and Zeiger Citation2006). Similar results were also reported by Srivastava and Sharma (Citation1990) and Naeem et al. (Citation2009) in case of Papaver somniferum and Lablab purpureus L., respectively, by TRIA application. On the other hand, Srivastava and Srivastava (Citation2007) and Shah et al. (Citation2006) reported growth promotion by GA3 in Catharanthus roseus and Nigella sativa, respectively.
The present work reveals that a significant improvement was found in chlorophyll and carotenoid concentrations when TRIA and GA3 were applied together (a, b). An increase in total chlorophyll and photosynthetic CO2 assimilation and specific activity of Rubisco by TRIA (Trewavas and Gilory Citation1991) and by GA3 (Taiz and Zeiger Citation2006) has already been reported. The increased contents of photosynthetic pigments (chlorophyll and carotenoid) could be attributed to the increase in number and size of chloroplasts by TRIA spray as revealed by Ivanov and Angelov (Citation1997), and to the enhancement of ultrastructural morphogenesis of plastids by GA3 (Arteca Citation1996). Increase in photosynthesis has already been reported as an important response to TRIA which could be associated with increased chlorophyll content in leaves (Ivanov and Angelov Citation1997; Naeem et al. Citation2009). In fact, a number of studies have demonstrated an increased rate of CO2 fixation in a variety of plant species by the application of nanomolar concentrations of TRIA and GA3 (Srivastava and Sharma Citation1990; Guoping Citation1997; Kumaravelu et al. Citation2000; Muthuchelian et al. Citation2003).
Foliar application of TRIA and GA3 increased CA activity, with 1.5 mg l−1 TRIA + 75 mg l−1 GA3 treatment proving the best. In the present study, TRIA sprayed leaves showed a greater CA activity than the control (d). Such a response of the plants to the applied TRIA or in combination with GA3 is expected because both tested PGRs have diverse role in the physiological processes and consequently improved the stomatal conductance (b) that might have facilitated the diffusion of carbon dioxide into the stomata. In turn, the CO2 might have been acted upon by CA. Finally, the CO2 could be reduced by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in the chloroplast stroma. A probable reason for the enhancement of CA activity due to TRIA alone or by application of both PGRs might be the de novo synthesis of CA, which involves translation/transcription of the genes associated (Okabe et al. Citation1980). Enhancement in the CA activity in treated plants might have responsible for the enhanced rate of CO2 fixation and hence have resulted in significant increase in fresh and dry weight of treated plants.
Nitrate reductase (NR) is the key enzyme in nitrogen metabolism and is responsible for the initiation of nitrate assimilation and hence protein synthesis. An increase in NR activity by combined application of TRIA and GA3 may have exerted a pivotal role in enhancement of photosynthetic rate. The ultimate culmination of enhancement of NR activity has increased overall growth and yield of treated plant. Nitrate reductase activity in leaves was influenced by different concentrations of TRIA + GA3 as revealed in the present study. The treatment T3 proved to be the best concentration resulting in a significant increase in NR activity (27.6%) compared to the control (c). Such a response at higher concentrations of TRIA and GA3 has also been reported by Misra and Srivastava (Citation1991), Muthuchelian et al. (2003), Shah et al. (Citation2006) and Naeem et al. (Citation2009).
Application of TRIA spray alone and in combination with GA3 proved effective for leaf N, P and K content. The concentrations of leaf nutrients were found significantly greater at 1.5 mg l−1 TRIA + 75 mg l−1 GA3 over the control. However, 1.5 mg l−1 TRIA + 100 mg l−1 GA3 concentration did not further increase nutrient elements as compared to treatment T3 (a–c). Enhancement in leaf-nutrients, particularly nitrogen, due to TRIA application could be attributed to the compositional or chemical change in plants leading to alterations in nitrogen concentration (Knowles and Ries Citation1981). Presumably, increased uptake of nutrients enhanced photosynthesis and improved translocation of photosynthates and other metabolites to the sinks that might have contributed to the improved yield of TRIA treated plants. These findings are in accordance with data on TRIA effects reported regarding plant nutrient elements (Kumaravelu et al. Citation2000; Khan et al. Citation2006; Khan et al. Citation2007; Naeem et al. Citation2009). GA3 increase membrane permeability (Crozier and Turnbull Citation1984; Al Wakeel et al. Citation1995). An increase in membrane permeability would facilitate absorption and utilization of mineral nutrient (Khan et al. Citation1998) and also transport of assimilate. Moreover, it contributes towards enhancing the capacity of the treated plants for biomass production as reflected in shoot and root dry mass of the plants. This enhancement could be the result of increased uptake of nutrients, enhanced photosynthesis and improved translocation of photosynthates and other metabolites to the reproductive parts (Miniraj and Shanmugavelu Citation1987). This sustained increase in the above mentioned parameters of the treated plants which is expected to culminate the maximization of artemisinin content and its yield. The concentration of artemisinin in the dry leaves was significantly higher in all PGRs sprayed plants as compared to control. The artemisinin concentration was highest in treatment T3 (b). It has already been reported that TRIA significantly enhanced the artemisinin yield in the leaves (Shukla et al. Citation1992). On the other hand, GA3 also improved the level of artemisinin content as studied by Ferreira and Janick (Citation2002), Weathers et al. (Citation2005) and Zhang et al. (Citation2005).
No doubt, it is the first report of the combined application of these two tested PGRs on this particular antimalarial plant, which significantly alters artemisinin biosynthesis. Terpenoid biosynthesis through mevalonate-isoprenoid pathway occurs in oil glands which are pr esent in the leaves of the many aromatic plants. The PGRs which can improve leaf development and herbage have been used to increase terpenoid yield (Ries and Wert Citation1977). In fact, the leaves are the major site of trichomes in which biosynthesis of artemisinin occurs; the increase in artemisinin content by application of PGRs is obvious.
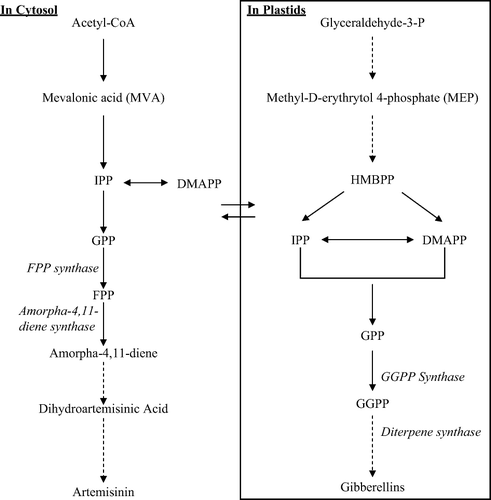
Now it is unequivocally proved that two distinct and independent biosynthetic routes exist to isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP), the two building blocks for isoprenoids in plants. The cytosolic pathway is triggered by Acetyl Coenzyme A where classical intermediate mevalonic acid (MVA) is formed which, in turn, converts into IPP and DMAPP (). These further combine to elongate into sesquiterpenes and triterpenes; whereas the plastidial, i.e., MEP pathway provides precursors for the biosynthesis of gibberellins and others terpenes (Akhila Citation2007). Although this subcellular compartmentation allows both pathways to operate independently in plants, there is evidence that they cooperate in the biosynthesis of certain metabolites (Laule et al. Citation2003; Kloer et al. Citation2006). Artemisinin is synthesized from FPP via amorpha-4,11-diene and dihydroartemisinic acid involving many steps (). It was tempting to suppose that the addition of GA3 might contribute to some mechanism for stimulating the artemisinin biosynthetic pathway.
Figure 5. The biosynthetic pathway of gibberellins and artemisinin (dashed arrow means more than one step) (Laule et al. Citation2003; Akhila Citation2007). DMAPP, dimethylallyl pyrophosphate; FPP, farnesyl pyrophosphate; GGPP, geranyl geranyl pyrophosphate; GPP, geranyl pyrophosphate; HMBPP, 1-Hydroxy-2-methyl-(E)-butenyl 4-diphosphate; IPP, Iso pentenyl pyrophosphate; MEP, Methyl-D-erythrytol 4-phosphate; MVA, Mevalonic acid.
Conclusions
Therefore, it may be suggested that the combined foliar spray of TRIA + GA3 (1.5 mg l−1 TRIA + 75 mg l−1 GA3) was highly effective for production of biomass and artemisinin content and improved the overall performance of the crop. It proved considerably important for increasing photosynthesis, crop productivity, activities of NR, CA and artemisinin content and yield of Artemisia annua L. Thus (1.5 mg l−1 TRIA + 75 mg l−1 GA3) concentration, in the form of foliar spray, might presumably be recommended for maximizing the productivity and quality of Artemisia annua L.
Acknowledgements
The authors would like to thank Prof. J.F.S. Ferreira, USDA, USA, for his valuable suggestions for the cultivation of Artemisia plant in the Indian subcontinent.
References
- Abdin , MZ , Israr , M , Rehman , RU and Jain , SK. 2003 . Artemisinin, a novel antimalarial drug: Biochemical and molecular approaches for enhanced production . Planta Med. , 69 : 289 – 299 .
- Akhila , A. 2007 . Metabolic engineering of biosynthetic pathways leading to isoprenoids: Mono- and sesquiterpenes in plastids and cytosol . J Plant Interact. , 2 : 195 – 204 .
- Al Wakeel , SAM , Hamed , AA and Dadoura , SS. 1995 . Interactive effects of water stress and gibberellic acid on mineral composition of fenugreek plant . Egyptian J Physiol Sci. , 18 : 269 – 282 .
- Arteca , RN. 1996 . Plant growth substances: Principles and applications , New York : Chapman and Hall Inc .
- Buchanan , BB , Gruissem , W and Jones , RL. 2000 . Biochemistry and molecular biology of plants , Rockville, Maryland : American Society of Plant Physiologists .
- Crozier , A and Turnbull , CGN. 1984 . Gibberellins: Biochemistry and action in extension growth . What's New Plant Physiol. , 15 : 9 – 12 .
- Dwivedi , RS and Randhawa , NS. 1974 . Evaluation of rapid test for hidden hunger of zinc in plants . Plant Soil. , 40 : 445 – 451 .
- Ferreira , JFS and Janick , J. 2002 . Production of artemisinin from in vitro cultures of Artemisia annua L . Biotechnol Agric For. , 51 : 1 – 12 .
- Ferreira , JFS , Laughlin , JC , Delabays , N , Magalhães , PM and de Magalhães , PM. . 2005 . Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin . Plant Genetic Res. , 3 : 206 – 229 .
- Ferreira , JFS , Simon , JE and Janick , J. 1997 . Artemisia annua: Botany, horticulture and pharmacology . Hort Rev. , 19 : 319 – 371 .
- Fiske , CH and Subba Row , Y. 1925 . The colorimetric determination of phosphorus . J Biol Chem. , 66 : 375 – 400 .
- Guoping , Z. 1997 . Gibberellic acid modifies some growth and physiological effects of paclobutrazol on wheat . J Plant Growth Regul. , 16 : 21 – 25 .
- Hald , PM. 1946 . The flame photometer for the measurement of sodium and potassium in biological materials . J Biol Chem. , 163 : 499 – 510 .
- Ivanov , AG and Angelov , MN. 1997 . Photosynthesis response to triacontanol correlates with increased dynamics of mesophyll protoplast and chloroplast membranes . Plant Growth Regul. , 21 : 145 – 152 .
- Jaworski , EG. 1971 . Nitrate reductase assay in intact plant tissue . Biochem Biophys Res Comm. , 43 : 1274 – 1279 .
- Khan , MMA , Gautam , C , Mohammad , F , Siddiqui , MH , Naeem , M and Khan , MN. 2006 . Effect of gibberellic acid spray on performance of tomato . Turk J Biol. , 30 : 11 – 16 .
- Khan NA , Ansari HR , Samiullah 1998 . Effect of gibberellic acid spray during ontogeny of mustard on growth, nutrient uptake and yield characteristics . J Agron Crop Sci . 181 : 61 73 .
- Khan , R , Khan , MMA , Singh , M , Nasir , S , Naeem , M , Siddiqui , MH and Mohammad , F. 2007 . Gibberellic acid and triacontanol can ameliorate the opium yield and morphine production in opium poppy (Papaver somniferum L.) . Acta Agric Scand B Soil Plant Sci. , 57 : 307 – 312 .
- Klayman , DL. 1985 . Qinghaosu (artemisimin): An antimalarial drug from China . Science. , 228 : 1049 – 1055 .
- Kloer , DP , Welsch , R , Beyer , P and Schulz , GE. 2006 . Structure and reaction geometry of geranylgeranyl diphosphate synthase from Sinapis alba . Biochemistry. , 45 : 15197 – 15204 .
- Knowles , NR and Ries , SK. 1981 . Rapid growth and apparent total nitrogen increases in rice and corn plants following applications of triacontanol . Plant Physiol. , 68 : 1279 – 1284 .
- Kremsner , PG and Krishna , S. 2004 . Antimalarial combinations . The Lancet. , 364 : 285 – 294 .
- Kumaravelu , G , Livingstone , VD and Ramanujam , MP. 2000 . Triacontanol-induced changes in the growth, photosynthetic pigments, cell metabolites, flowering and yield of green gram . Biol Plant. , 43 : 287 – 290 .
- Laughlin , JC. 2002 . Post harvest drying treatment effects on antimalarial constituents of Artemisia annua L . Acta Hort. , 576 : 315 – 320 .
- Laule , O , Furholz , A , Chang , HS , Zhu , T , Wang , X , Heifetz , PB , Gruissem , W and Lange , BM. 2003 . Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana . Proc Natl Acad Sci USA. , 100 : 6866 – 6871 .
- Lindner , RC. 1944 . Rapid analytical methods for some of the common inorganic constitutes of plant tissues . Plant Physiol. , 19 : 76 – 89 .
- MacKinney , G. 1941 . Absorption of light by chlorophyll solutions . J Biol Chem. , 140 : 315 – 322 .
- MacLachlan , S and Zalik , S. 1963 . Plastid structure, chlorophyll concentration, free amino acid composition of chlorophyll mutant of barley . Can J Bot. , 41 : 1053 – 1062 .
- Miniraj , N and Shanmugavelu , KG. 1987 . Studies on the effect of triacontanol on growth, flowering, yield, quality and nutrient uptake in chillies . South Indian Hort. , 35 : 362 – 366 .
- Misra , A and Srivastava , NK. 1991 . Effects of the triacontanol formulations “Miraculan” on photosynthesis, growth, nutrient uptake, and essential oil yield of lemongrass (Cymbopogon flexuosus) Steud, Watts . Plant Growth Regul. , 10 : 57 – 63 .
- Moore , TC. 1989 . Biochemistry and physiology of plant hormones , New York : Springer-Verlag Inc .
- Muthuchelian , K , Velayutham , M and Nedunchezhian , N. 2003 . Ameliorating effect of triacontanol on acidic mist-treated Erythrina variegata seedings. Changes in growth and photosynthetic activities . Plant Sci. , 165 : 1253 – 1257 .
- Naeem M , Khan MMA , Moinuddin , Siddiqui MH. 2009 . Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.) Sci Hort . 121 : 389 396 .
- Novozamsky , I , Houba , VJG , Eck , RV and Vark , VW. 1983 . A noval digestion technique for multi-element plant analysis . Comm Soil Sci Plant Anal. , 14 : 239 – 248 .
- Ohlsson , AB and Bjork , L. 1988 . Effects of gibberellic acid on cardenolide accumulation by Digitalis lanata tissue cultures grown in light and darkness . J Plant Physiol. , 133 : 535 – 538 .
- Okabe , K , Lindlar , A , Tsuzuki , M and Miyachi , S. 1980 . Effects of carbonic anhydrase on ribulose 1,5-biphosphate carboxylase and oxygenenase . FEBS Lett. , 114 : 142 – 144 .
- Ries , SK. 1985 . Regulation of plant growth with triacontanol . Crit Rev Plant Sci. , 2 : 239 – 285 .
- Ries , SK and Wert , V. 1977 . Growth responses of rice seedlings to triacontanol in light and dark . Planta. , 135 : 77 – 82 .
- Rorison IH , Spencer RE , Gupta PL. 1993 . Chemical analysis . In : Hendry GAE , Grime JP Methods in Comparative Plant Ecology . New York : Chapman and Hall . 156 161 .
- Sadowska , A , Racka , M , Staszkiewicz , J and Sadowska , M. 1984 . Effect of some growth substances upon rooting of cuttings, yield and alkaloid content of Catharanthus roseus . Acta Hort. , 144 : 99 – 102 .
- Shah SH , Ahmad I , Samiullah 2006 . Effect gibberellic acid spray on growth, nutrient uptake and yield attributes during various growth stages of black cumin (Nigella sativa) . Asian J Plant Sci . 5 : 881 884 .
- Shukla , A , Farooqi , AHA , Shukla , YN and Sharma , S. 1992 . Effect of triacontanol and chlormequat on growth, plant hormones and artemisinin yield in Artemisia annua L . Plant Growth Regul. , 11 : 165 – 171 .
- Singh , A , Kaul , VK , Mahajan , VP , Singh , A , Mishra , LM , Thakur , RS and Husain , A. 1986 . Introduction of Artemisia annua in India and isolation of artemisinin, a promising antimalarial drug . Indian J Pharm Sci. , 48 : 137 – 138 .
- Singh , N , Luthra , R and Sangwan , RS. 1990 . Oxidative pathway of essential oil biosynthesis in the developing Cymbopogon flexuosus leaf . Plant Physiol Biochem. , 28 : 703 – 710 .
- Srivastava , NK and Sharma , S. 1990 . Effect of triacontanol on photosynthesis, alkaloid content and growth in opium poppy (Papaver somniferum L) . Plant Growth Regul. , 9 : 65 – 71 .
- Srivastava , NK and Srivastava , AK. 2007 . Influence of gibberellic acid on 14CO2 metabolism, growth, and production of alkaloids in Catharanthus roseus . Photosynthetica. , 45 : 156 – 160 .
- Taiz L , Zeiger E. 2006 . Plant Physiology , 4th ed Sunderland, MA : Sinauer Associates Inc., Publishers .
- Trewavas , AJ and Gilory , S. 1991 . Signal transduction in plant cells . Trends Genet. , 7 : 356 – 361 .
- Weathers , PJ , Bunk , G and McCoy , MC. 2005 . The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots . In Vitro Cell Dev Biol Plant. , 41 : 47 – 53 .
- World Health Organization (WHO) . 2002 . Report: Meeting on antimalarial drug development, Shanghai, China, 16–17 November 2001; Manila, The Philippines .
- Yuan , L and Xu , DQ. 2001 . Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean . Photosynthesis Res. , 68 : 39 – 47 .
- Zhang , YS , Ye , HC , Liu , BY , Wang , H and Li , GF. 2005 . Exogenous GA3 and flowering induce the conversion of artemisinic acid to artemisinin in Artemisia annua plants . Russian J Plant Physiol. , 52 : 58 – 62 .
- Zhao , SS and Zeng , MY. 1986 . Determination of qinghaosuin in Artemisia annua L. by high performance liquid chromatography . Chinese J Pharma Anal. , 6 : 3 – 5 .