Abstract
Compounds which are able to reduce the damaging effects of various stresses such as drought should be of great importance. In this research we have used arginine pretreatment and the effect of this compound on alleviation of oxidative damages under drought stress has been investigated. Our findings showed that arginine pretreatment reduced the lipid peroxidation when water stress was imposed. In drought stressed plants, H2O2 increased and the activity of antioxidative enzymes were elevated over the controls, while glutathione reductase (GR) activity decreased. When plants pretreated with arginine, activity of catalase and guaiacol peroxidase decreased while the activity of superoxide dismutase (SOD), ascorbate peroxidase, and GR increased. Drought stress decreased ascorbate and reduced glutathione and increased dehydroascorbate. Opposite results were obtained after arginine pretreatment. When arginine was used as a precursor of nitric oxide (NO), the amelioration of the drought effects which was observed could well be the indication that these effects may be related to NO production. To prove that, we applied arginine + Nw-nitro-l-arginine methyl ester (LNAM) and on many parameters, arginine and arginine + LNAM pretreatment had the same effects and it seems that in these situations other pathways of arginine metabolism rather than nitric oxide synthase may be activated
1. Introduction
Water deficit is one of the most common environmental stresses limiting plant productivity. Understanding the cellular processes that ameliorate the consequences of water loss is clearly important (Neill et al. Citation2003b). One of the biochemical changes occurring when plants are subjected to this harmful stress condition is the accumulation of reactive oxygen species (ROS) (Smirnoff Citation1993). When plants are subjected to water deficit, a variety of ROS, such as superoxide (O2 –) hydrogen peroxide (H2O2) and hydroxyl radicals (•OH), which cause oxidative damage in plants are generated (Lei et al. Citation1998). Free radicals are toxic to living organisms unless removed rapidly, destroyed or inactivated by various cellular components. In the absence of effective mechanisms which remove or scavenge free radicals, they can seriously damage plant by lipid peroxidation, protein degradation, breaking of DNA, and cell death (Tian and Li Citation2006). To control the level of ROS, plants have evolved an antioxidant defense system comprising of enzymes such as superoxide dismutase (SOD), Catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), glutathione reductase (GR) as well as non-enzymatic constituents such as ascorbate (ASA) and glutathione (GSH), which are responsible for scavenging excessively accumulated ROS in plants under stress conditions (Shi et al. Citation2007). The regulation of these antioxidant constituents by an exogenous substance might mediate the plant tolerance to drought stress.
l-arginine is one of the most functionally diverse amino acids in living cells. In addition to serving as a constituent of proteins, arginine is a precursor for biosynthesis of polyamines (PAs), Agmatine, and proline as well as the cell signaling molecules glutamine and nitric oxide (NO) (Chen et al. Citation2004; Liu et al. Citation2006). Two of the most intensive pathways of arginine metabolism are those catalyzed by arginase (ARG) and nitric oxide synthase (NOS). ARG hydrolyzes arginine to urea and ornithine, the latter is a precursor for PA and proline synthesis and the NOS pathway products, are NO and citrulline (Chen et al. Citation2004). Most studies of plant ARG have focused on its role in mobilizing arginine as a nitrogen source during post-germinative growth (Kang and Cho Citation1990; Carvajal et al. Citation1996; Hwang et al. Citation2001). However, the molecular mechanism by which ARG expression in plants is regulated by developmental or stress-related cues remain to be determined. NO has been proven to be a functional metabolite in plants and arginine-dependent NOS activity has been detected along with inhibition of NO production by the NOS inhibitor (Arasimowicz & Floryszak-Wieczorek Citation2007). Over the past 20 years, the fact that NO plays an important role as a signaling molecule for a variety of response in animals has been clearly established (Vital et al. Citation2008), and it is now becoming increasingly evident that NO plays a similar role in plant biology. It has been suggested that NO is involved in plant response to environmental stress such as drought, low, and high temperatures, UV and ozone exposure (Neill et al. Citation2003a).
In previous research, we applied Sodium nitroprosside (SNP), an NO donor to counteract the effect of drought stresses on tomato plants (Nasibi and Kalantari Citation2009). However not any data are available on the effect of exogenous arginine as a precursor of NO in the possible antioxidative responses of plants against water stress. In this research we have used arginine as pretreatment and the effect of this compound on alleviation of oxidative damages under drought stress were investigated. Comparing these responses can be useful in understanding the physiological and biochemical mechanisms of these compounds in plants which have to cope with drought stress.
2. Materials and methods
2.1. Plant material
Tomato plants (Lycopersicon esculentum Mill cv Alicante) were grown from seeds (provided from Thomson and Morgan company, UK) in trays of compost until the seeds were germinated. After germination, the seedlings were transferred to growth chamber with day/night temperature of 22°C/18°C and a 16 h photoperiod with a relative humidity of 50%. The seedlings were irrigated with water once a day and half-strength Long Ashton nutrient solution once a week. After 4 weeks, the seedlings were transferred to bottles containing Long Ashton nutrient solution aerated with air, then the plants were divided in to eight groups with three replicates. Four groups of plant were sprayed either with (10 ml) 1 mM arginine or (10 ml) 1 Mm arginine + 2 mM Nw-nitro-l-arginine methyl ester (LNAM) (NOS inhibitor) solutions, other four groups were sprayed either with (10 ml) distilled water or (10 ml) 2 mM LNAM, for 2 days 0.1% Tween-20 (V/V) was used as a surfactant and the pH of solution was 6.5. In third day, after spraying the solutions, plants were subjected to in vitro water stress for 24 h. For this purpose three seedlings were placed in aerated bottle containing distilled water served as a control and polyethylene glycol (PEG-6000) of 11.2% (W/V) strengths to achieve water (osmotic) stress level of –0.2 MPa. After 24 h of root osmotic stress the second leaves (counting from the bottom) were harvested and immediately frozen in liquid nitrogen and stored at –80°C for future analysis.
2.2. Hydrogen peroxide content
Hydrogen peroxide content was measured spectrophotometrically after reaction with potassium iodide (KI) according to the method of Alexieva et al. (Citation2001). Leaf tissues (500 mg) were homogenized in ice bath with 5 ml 0.1% TCA (W/V). The homogenate was centrifuged at 12,000×g for 15 min. The reaction mixture consisted of 0.5 ml of supernatant, 0.5 ml of 100 mM K-phosphate buffer (pH 7.0), and 2 ml reagent (1 M KI in fresh double-distilled water). The blank probe consisted of 0.1% TCA (W/V) in the absence of leaf extract. The reaction was carried out for 1 h in darkness and absorbance was measured at 390 nm. The amount of hydrogen peroxide was calculated using a standard curve prepared with known concentration of H2O2.
2.3. Thiobarbituric acid reactive substance (TBRS)
One hundred mg of the leaf tissue of plants were homogenized in 10 ml of 0.1% TCA (W/V), then centrifuged at 10,000×g for 15 min. One ml of supernatant was then swirled with 4 ml of 20% TCA (W/V) containing 0.5% 2-thiobarbituric acid (TBA) (W/V), and the solution was heated for 30 min at 90°C. Samples were cooled on ice for 5 min and then re-centrifuged for 10 min at 10,000×g. For malondealdehyde (MDA) measurement, the non-specific absorbance of supernatant at 600 nm was subtracted from the maximum absorbance at 532 nm and an extinction coefficient (ϵ) of 1.55×105 M–1 cm–1 was used for determination of MDA concentration (Heath and Packer Citation1968). For other aldehydes measurement, absorbance of 600 nm was subtracted from maximum absorbance of 455 nm and the extinction coefficient of 0.457×105 M–1 cm–1 was used for calculation (Meirs et al. Citation1992).
2.4. Enzyme extraction and activity determination
Five hundred mg leaves were homogenized in cool 50 mM potassium phosphate buffer (pH 7.0) containing 1% soluble polyvinilpyrolidone (PVP) (W/V), 1 mM ethylene diamine tetra acetic acid (EDTA) and 1 mM phenylmethylsulfonyl fluoride (PMSF) with the addition of 10 mM ascorbic acid in the case of the APX assay. All of the procedures were done at 4°C. The homogenate was centrifuged at 20,000×g for 20 min and the supernatant was used for assay of the activity of enzymes.
2.4.1. Superoxide dismutase (SOD) (EC 1.15.1.1)
Total SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitrobluetetrazolium (NBT) according to the method of Giannopolitis and Ries (Citation1977). One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT as monitored at 560 nm.
2.4.2. Catalase (CAT) activity (EC 1.11.1.6)
CAT activity was determined spectrophotometrically by following the decrease of absorbance of H2O2 within 30 s at 240 nm (Dhindsa et al. Citation1981). The 3 ml reaction solution consisted of 50 mM potassium phosphate buffer (pH 7.0), 15 mM H2O2, and 100 µl of enzyme extract. Addition of H2O2 started the reaction and the decrease in absorbance was recorded after 30 s.
2.4.3. Guaiacol peroxidase (GPX) (EC1.11.1.7)
GPX activity was measured using Guaiacol as a substrate. Reaction mixture (3 ml) contained 25 µl of enzyme extract, 2.77 ml of 50 mM phosphate buffer (pH 7.0), 0.1 ml of 1% H2O2 (V/V), and 0.1 ml of 4% guaiacol (V/V). The increase in absorbance at 470 nm due to the guaiacol oxidation was recorded for 3 min. One unit of enzyme activity was defined as the amount that causes a change of 0.01 in absorbance per minute (Zhang et al. Citation2005)
2.4.4. Ascorbate peroxidase (APX) (EC 1.11.1.11)
APX was determined spectrophotometrically according to the oxidation of ASA. The reaction solution contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ASA, 0.1 mM H2O2, and 150 µl enzyme extract. APX activity was calculated by following the decrease in absorbance of ASA within 1 min at 290 nm (Nakano and Asada Citation1981).
2.4.5. Glutathione reductase (GR) (EC 1.6.4.2)
GR activity was determined by following the oxidation of NADPH at 340 nm (extinction coefficient 6.2 mM–1 cm–1) for 3 min in 1 ml of an assay mixture containing 50 mM potassium phosphate buffer (pH 7.8), 2 mM EDTA, 0.15 mM NADPH, 0.5 mm Oxidized glutathione (GSSG), and 100 µl of enzyme extract. The reaction was initiated by adding NADPH. Correction was made for the background absorbance at 340 nm without NADPH (Schaedle and Bassham Citation1977).
2.5. Total soluble proteins
Protein content was determined according to the method of Bradford (Citation1976) using Bovine serum albumin as standard.
2.6. Estimation of ascorbate (ASA) and dehydroascorbate (DHASA)
For ASA, 200 mg of leaf tissue were homogenized in 5% 10 ml meta-phosphoric acid (W/V) and centrifuged for 15 min at 10,000×g. The concentration of ASA and dehydroascorbate (DHASA) were determined spectrophotometrically according to the method of De Pinto et al. (Citation1999).
2.7. Measurement of reduced glutathione (GSH)
Reduced GSH was estimated following the modified method of Ellman (Citation1959). About 200 mg of the leaves were homogenized in 4 ml of 15% meta-phosphoric acid (W/V) and centrifuged at 10,000×g for 30 min at 4°C. Aliquots of 0.2 ml of the supernatant were mixed with 2.6 ml 150 mM K-phosphate buffer (pH 7.7) and 0.2 ml 5,5-Dithio-bis (2-Nitrobenzoic acid) (DTNB). The color was allowed to develop for 30 min at room temperature, and the absorbance of the clear supernatant was recorded at 412 nm.
2.8. Statistical analyses
Data are means±SE of three replicates. Statistical analyses were carried out by one-way ANOVA using Duncan test to evaluate whether the means were significantly different, taking p<0.05 as significant.
3. Results
3.1. Lipid peroxidation
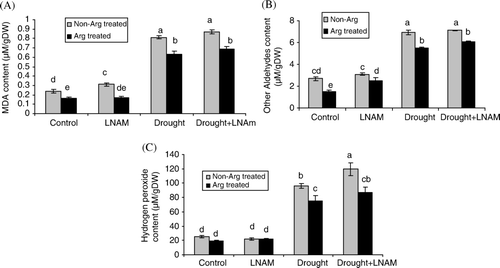
MDA and other aldehydes were measured as an indicator of lipid peroxidation. The data showed that drought stress increased the amount of MDA and other aldehydes (Figure 1A and B). Lipid peroxidation decreased in control and drought stressed plants which were pretreated with arginine in comparison with non-pretreated plants. Application of arginine + LNAM as pretreatment showed that the pattern for lipid peroxidation was the same as arginine pretreatment.
3.2. H2O2 accumulation
Water deficit caused an increase in H2O2 content (Figure 2). Arginine pretreatment had no significant effect in decreasing of H2O2 in control condition. However in those plants which were under drought stress, arginine pretreatment decreased the amount of hydrogen peroxide.
3.3. Antioxidant enzyme activities
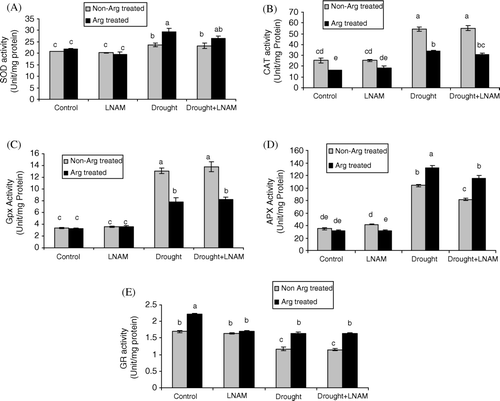
The effect of drought stress on SOD, CAT, GPX, APX, and GR in tomato plant leaves, either with or without arginine pretreatment was assayed. As shown in , the activity of SOD (A), CAT (B), GPX (C), and APX (D) was higher in stressed plants than those of the control groups, which may be a reflection of the oxidative burst under drought stress. Drought stress reduced GR activity in tomato seedlings (E). arginineinine pretreatment decreased the activity of CAT and GPX either when arginine was used separately or applied with LNAM in those plants which were under drought stress (B and C). Application of arginine or arginine +LNAM pretreatment increased the activity of APX and GR in drought stressed plants and may be related to the key role of these enzymes in ROS detoxification under these conditions. However the activity of APX in arginine + LNAM pretreatment was much lower than that of the arginine pretreated plants (D).
3.4. Ascorbate (ASA), dehydroascorbate (DHASA), and reduced glutathione (GSH)
As it is shown in , under drought stress, ASA and GSH content declined significantly in comparison with control plants, however the content of DHASA increased in drought stressed plants. In plants which were under drought stress, arginine or arginine + LNAM as pretreatments, decreased the amount of DHASA while ASA and GSH increased significantly. Application of arginine or arginine + LNAM did not have any significant effects on ASA, DHASA, and GSH under control condition.
Table 1. Changes in the ascorbate (A), dehydroascorbate (C), and reduced glutathione (E) in tomato seedlings pretreated with arginine or arginine + LNAM under drought stress.
4. Discussion
Two main pathways of arginine metabolism have been reported which are catalyzed either by ARG or NOS so that the end product will be ornithine or NO, respectively. Ornithine is a precursor for PAs or proline biosynthesis. In this study LNAM was used as an inhibitor of NOS, to study the role of NO in some physiological parameters under drought stress. One of the described damages provoked by water deficit stress is the membrane injury and liberation of ions from the cell to extra cellular space (Halliwel and Gutteridge Citation1984). This is a consequence of an oxidative burst leading to lipid peroxidation, membrane permeability, and cell injury (Scandalios Citation1993). As shown in A, MDA content increased in drought stressed plants. These results corresponded well with the results of other aldehydes and H2O2 content. When plants were pretreated with arginine or arginine + LNAM, the amounts of MDA, other aldehydes and H2O2 decreased and this effect is very important for drought stress tolerance (A–C). In previous study on tomato plants, our findings showed that decrease of lipid peroxidation under SNP pretreatment related to NO release because in the presence of 1- 4,4,5,5,tetramethylimidazoline-1oxyl-3-oxide (PTIO), lipid peroxidation increased when compared with those plants which were pretreated with SNP (Nasibi and Kalantari Citation2009). It has been reported that the role of NO in prevention of lipid peroxidation is related to its ability to react with lipid alcoxyl (LO•) and lipid peroxyl (LOO•) radicals and stop the chain of peroxidation (Beligni & Lamattina Citation1999), however our findings showed that in drought stressed plants, both arginine and arginine + LNAM treatment decreased the amount of MDA and other aldehydes indicating that the other metabolism pathway of arginine is probably more effective than the NOS pathway. Under normal conditions, the total amount of ROS formed in the plants is determined by the balance between the multiple ROS producing pathways and the ability of the enzymatic and non-enzymatic mechanism to deal with them. Under stress conditions, ROS formation is higher than the ability of plants to remove it and this could result in oxidative damages (Laspina et al. Citation2005). In tomato plants under drought stress, SOD, APX, GPX, and CAT activities were elevated over the controls, however GR activity decreased in comparison with control plants. So we can assume that the plant antioxidant machinery was effectively struggling against stress (A–E). In the present study, application of arginine, significantly increased the SOD activity, and suggested that application of this compound could promote the conversion of O•2 into H2O2 and O2, which is an important step in protecting the cell. The same result observed in SNP treated plants under drought stress (Nasibi and Kalantari Citation2009). If the produced H2O2 cannot be scavenged efficiently, it can interact with O•2 to form highly reactive hydroxyl radicals that are thought to be primarily responsible for oxygen toxicity in the cell. Therefore, the efficient scavenging of H2O2 is very important for normal metabolism of plant. CAT, GPX, and APX enzymes are important H2O2 scavenger enzymes. Our results indicated that the activity of CAT and GPX were declined under arginine pretreatment, while the H2O2 content decreased in arginine pretreated plants. However, APX activity increased in drought stress and treatment of plants with arginine increased the activity of this enzyme. APX prevents the accumulation of excess H2O2 in cells via ASA–GSH pathway (Foyer & Halliwell Citation1976). An increase in APX activity as observed in arginine pretreated plants under drought stress with a concomitant decrease in H2O2 concentration suggests the key role of APX in detoxification of H2O2 under drought stress. In previous work with SNP pretreatment it has been observed that APX have the key role in H2O2 detoxification (Nasibi and Kalantari Citation2009). APX reduction of H2O2 requires ASA as substrate, and DHASA conversion back to ASA needs GSH substrate, so higher activities of DHAR and GR are very important for keeping higher substrate concentration for APX. Decrease of ASA and increase of DHASA content and GR activity under drought stress confirmed the role of ASA–GSH cycle against oxidative stress in tomato seedlings under this condition and protective role of arginine pretreatment probably related to this cycle. When arginine was used as a precursor of NO, the amelioration of the drought effects on tomato plants which was observed could well be the indication that these effects may be related to NOS activity and NO production. To prove that, we applied arginine + LNAM and in almost all parameters which we measured in this study, arginine and arginine + LNAM pretreatment had the same effects and it seems that in these situations other pathways of arginine metabolism rather than NOS may be activated. Therefore it seems that under these situation protective effects of arginine are related to PAs or indirect synthesis of NO from PAs which has been reported more recently (Tun et al. Citation2006, Yamasaki and Cohen Citation2006) and proline biosynthesis.
References
- Alexieva , V , Sergiev , I , Mapelli , S and Karanov , E. 2001 . The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat . Plant Cell Environ. , 24 : 1337 – 1344 .
- Arasimowicz , M and Floryszak-Wieczorek , J. 2007 . Nitric oxide as a bioactive signaling molecule in plant stress responses . Plant Sci. , 172 : 876 – 887 .
- Beligni , MV and Lamattina , L. 1999 . Is nitric oxide toxic or protective? . Trends in Plant Sci. , 4 : 299 – 300 .
- Bradford , MM. 1976 . A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. , 72 : 248 – 254 .
- Carvajal , N , Olave , N , Salas , M , Uribe , E and Enriquez , S. 1996 . Properties of an arginase from the cotyledons of Phaseolus vulgaris . Phytochemistry , 41 : 373 – 376 .
- Chen , H , Mc Carig , B , Melotto , M , Yang He , S and Howe , GA. 2004 . Regulation of plant arginase by wounding, Jasmonate and the phytotoxin coronatine . J Biol Chem. , 279 : 45998 – 46007 .
- De Pinto , D , Francis , L and Gara , D. 1999 . The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells . Protoplasma , 209 : 90 – 95 .
- Dhindsa , RS , Dhindsa , P and Thorpe , A. 1981 . Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase . J Exp Bot. , 32 : 93 – 101 .
- Ellman , Gl. 1959 . Tissue sulfydryl groups . Arch Biochem Biophys. , 82 : 70 – 77 .
- Foyer , CH and Halliwell , B. 1976 . The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism . Planta , 133 : 21 – 25 .
- Giannopolitis , CN and Ries , SK. 1977 . Superoxide dismutase. I. Occurrence in higher plants . Plant Physiol. , 59 : 309 – 314 .
- Halliwel , B and Gutteridge , JM. 1984 . Oxygen toxicity, oxygen radicals, transition metal and disease . Biochem J. , 219 : 1 – 14 .
- Heath , RL and Packer , L. 1968 . Photoperoxidation in isolated chloroplast, kinetics and stoichiometry of fatty acid peroxidation . Arch Biochem Biophys. , 125 : 189 – 198 .
- Hwang , HJ , Kim , EH and Cho , YD. 2001 . Isolation and properties of arginase from a shade plant, ginseng (Panax ginseng C.A. Meyer) roots . Phytochemistry , 58 : 1015 – 1024 .
- Kang , JH and Cho , YD. 1990 . Purification and properties of arginase from Soybean, Glycine max, Axes . Plant Physiol. , 93 : 1230 – 1234 .
- Laspina , NV , Groppa , MD , Tomaro , ML and Benavides , MP. 2005 . Nitric oxide protects sunflower leaves against Cd-induced oxidative stress . Plant Sci. , 169 : 323 – 330 .
- Lei , L , Staden , JV and Jager , AK. 1998 . Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress . Plant Growth Regul. , 25 : 81 – 87 .
- Liu , JH , Nada , K , Honda , C , Kitashiba , H and Wen , XP. 2006 . Polyamine biosynthesis of apple callus under salt stress. Importance of the arginine decarboxylase pathway in stress responses . J Exp Bot. , 57 : 2589 – 2599 .
- Meirs , P , Hada , S and Aharoni , N. 1992 . Ethylene increased accumulation of fluorescent lipid peroxidation products detected during parsly by a newly developed method . J Am Soc Hort Sci. , 117 : 128 – 132 .
- Nakano , Y and Asada , K. 1981 . Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach choloroplast . Plant Cell Physiol. , 22 : 867 – 880 .
- Nasibi , F and Kalantari , Kh. 2009 . Influence of nitric oxide in protection of tomato seedling against oxidative stress induced by osmotic stress . Acta Physiol Plant. , 31 : 1037 – 1044 .
- Neill , JS , Radhika , D and Hancock , JT. 2003a . Nitric oxide signaling in plant . New Phytol. , 159 : 11 – 35 .
- Neill JS , Desikan R , Hancock J. 2003b Nitric oxide as a mediator of ABA signaling instomatal guard cells Bul J Plant Physiol. Special Issue : 124 – 132
- Scandalios , JG. 1993 . Oxygen stress and superoxide dismutase . Plant Physiol. , 101 : 7 – 12 .
- Schaedle , M and Bassham , JA. 1977 . Chloroplast glutathione reductase . Plant Physiol. , 59 : 1011 – 1012 .
- Shi , Q , Ding , F , Wang , X and Wei , M . 2007 . Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress . Plant Physiol Biochem. , 45 : 542 – 550 .
- Smirnoff , N. 1993 . The role of active oxygen in the response of plants to water deficit and desiccation . New Phytol. , 125 : 27 – 58 .
- Tian , X and Li , Y. 2006 . Nitric oxide treatment alleviates drought stress in wheat seedlings . Biol Plant. , 50 ( 4 ) : 775 – 778 .
- Tun , NN , Santa-Catarina , C , Begum , T , Silveira , V , Handro , W , Floh , E and Scherer , G. 2006 . Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings . Plant Cell Physiol. , 47 : 346 – 354 .
- Vital , SA , Fowler , RW , Virgen , A , Gossett , DR , Banks , SW and Rodriguez , J. 2008 . Opposing role for superoxide and nitric oxide in NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue . Plant Cell Physiol. , 62 : 60 – 68 .
- Yamasaki , H and Cohen , MF. 2006 . No signal at the crossroads: polyamine-induced nitric oxide synthesis in plant . Trends Plant Sci. , 11 : 522 – 523 .
- Zhang , Z , Pang , X , Duan , X , Ji , ZL and Jiang , Y. 2005 . Role of peroxidase in anthocyanine degradation in litchi fruit pericarp . Food Chem. , 90 : 47 – 52 .